Abstract
Iatrogenic injury to endometrial tissue is the main cause of intrauterine adhesions (IUA) and infection can also damage the endometrium. The microbiota plays an important role in the health of the female reproductive tract. However, the mechanism is still unclear. In total, 908 patients with IUA and 11,389 healthy individuals were retrospectively selected for this clinical study. Participant information including vaginal microecological results and human papillomavirus (HPV) status were collected. Univariate and multivariate logistic regression analyses were used to identify the factors related to IUA. Next, animal experiments were performed in a curettage-induced IUA rat model. After the procedure, rats in the experimental group received a vaginal infusion of a Candida albicans (C. albicans) fungal solution. On days 3, 7, and 14 after curettage and infusion, the expression levels of IL-6, fibrotic pathway-related factors (TGF-β1, Smad 2, and COL1), and estrogen receptor (ER) and progesterone receptor (PR) in rat endometrial tissues were assessed. Fungal infection of the reproductive tract was found to be an independent risk factor for IUA (P < 0.05). The inflammatory response and degree of fibrosis were greater in rats infected with C. albicans than in the controls. The levels of IL-6, TGF-β1, Smad 2, and COL1 expression in endometrial tissues were significantly higher in the experimental group than in the control group (P < 0.05). However, the ER and PR levels were lower in the IUA group than in the non-IUA group (P < 0.05). C. albicans infection may be related to IUA. C. albicans elicits a strong inflammatory response that can lead to more severe endometrial fibrosis.
Similar content being viewed by others
Introduction
Intrauterine adhesions (IUA) have many causes, the most common of which is iatrogenic endometrial injury1. Typical clinical manifestations of IUA are decreased menstruation, amenorrhea, recurrent miscarriage, and secondary infertility2. A common clinical observation is that some patients develop IUA after uterine cavity surgery, whereas others do not, and that the adhesions range from severe to very mild. These differences suggest that a single iatrogenic injury does not constitute the entire mechanism behind the occurrence and development of IUA, and that other pathogenic factors may be involved.
Many types of microorganisms can be found in the reproductive tracts of healthy women. A fine and balanced relationship exists between the host and microorganisms, which is conducive to maintaining the steady state of the microbiota and resisting invasion by pathogenic microorganisms. The microbiome plays an important role in disease and the health of the female reproductive tract3,4. Studies have confirmed that the main cause of IUA is injury to the basal layer of the endometrium, and that any event resulting in such injury can potentially lead to IUA1. However, in the clinical setting, patients with IUA rarely exhibit typical signs of reproductive tract infection. The only definite infection-related cause of IUA is genital tuberculosis5, and the etiological role of other uterine inflammatory infections in the formation of IUA remains contentious6.
In recent decades, the incidence of human fungal infections has increased significantly7. Many factors influence the occurrence of fungal diseases, including receipt of immunosuppressive therapy, invasive surgery, or broad-spectrum antibiotics. Reproductive tract fungal infections can have serious effects on women's professional and personal lives8,9. Harnessing 16S and ITS2 rDNA sequencing analysis, our previous study found that the fungal genera were enriched in the lower genital tract of IUA patients10. Moreover, site-specific fungal-bacterial correlation networks were discovered in those patients10. Vaginal candidiasis is the most common vaginal fungal infection11. The main pathogenic agent of vaginal candidiasis is the polymorphic fungal pathogen Candida albicans (C. albicans)12. Only 30% of recurrent vulvovaginal candidiasis cases are caused by non-C. albicans infections13.
The specific host immune response to C. albicans is generally considered to play a key role in protection by preventing symbiotic Candida from turning into conditional pathogens at the mucus membrane–Candida interface. Transforming growth factor (TGF)-β may be involved in the immune response to fungal infection in the reproductive tract. Taylor et al.14 found that TGF-β1 expression was significantly increased in the vaginal tissue of mice with candidiasis. Furthermore, TGF-β1 was found to be dominant in draining lymph nodes, but not in non-draining lymph nodes. These findings suggest that TGF-β1 may be involved in the immune regulation of vaginal Candida infections14. Other studies have found that TGF-β plays a specific role in the process of fungal morphology recognition by dendritic cells15. Drug therapy has been reported to downregulate the ratio of proinflammatory cytokine interleukin (IL)-1β to anti-inflammatory cytokine TGF-β, thereby reducing the number of neutrophils in the vagina16. TGF-β is a key cytokine in human tissue and animal models of fibrotic diseases, such as IUA17,18,19. It has been shown to have a significant effect on fibroblast proliferation20,21 and extracellular matrix production22,23 in vitro. The interplay between Smad proteins and other proteins in TGF-β signaling pathway mediate regulatory signals that control expression of target genes24. So they are mediators and essential components of TGF-β signaling pathway. We speculated that C. albicans infection may lead to IUA and that the immune regulation mechanism may be related to TGF-β/Smad signaling pathway activation by C. albicans.
As a fibrosis-promoting inflammatory cytokine25, IL-6 has been detected at significantly increased levels in patients with pulmonary fibrosis26, as well as in a fibrosis mouse model27. Recent studies have shown that IL-6 can promote fibrosis by mediating chronic inflammation28 and activating the TGF-β/Smad pathway29,30,31,32,33. Studies have further suggested that Smad 2/3 may be the target of IL-6 trans-signal transduction29,34,35. The present study took IL-6 as a starting point for further exploration of the mechanism by which C. albicans activates the TGF-β signal transduction pathway.
We speculated that some patients with IUA who have "asymptomatic" genital tract infections may have abnormal changes in reproductive tract microorganisms and that this vaginal microecological imbalance may be involved in the formation of fibrosis after endometrial injury. Furthermore, we posited that the imbalance of immune regulation caused by C. albicans infection may be an important link in the mechanism of IUA and that IL-6 may be a key regulatory factor. In this research we first retrospectively analyzed the relationship between vaginal microecology and IUA, and preliminarily investigated the etiology of IUA using logistic regression analysis. To further explore the mechanism of endometrial injury caused by fungi, C. albicans was used to stimulate primary intimal cells in a pregnant rat uterine curettage model, and the levels of IL-6, TGF-β1, Smad 2, collagen 1 (COLl), estrogen receptor (ER), and progesterone receptor (PR) were subsequently evaluated. By exploring the mechanism underlying C. albicans-mediated IL-6 upregulation in the reproductive tract and its role in IUA formation, we aimed to reveal the various roles of C. albicans in the progression of IUA and provide new ideas and targets for the discovery of effective preventative measures and treatments for this condition.
Methods
Study population
In this study, 908 patients with IUA who were diagnosed in the Gynecology Clinic of Xiangya Third Hospital of Central South University between August 2017 and September 2018 were retrospectively enrolled as the study group. A further 11,389 healthy people who attended the Health Management Center of Xiangya Third Hospital of Central South University during the same period were retrospectively selected as the control group. The clinical data of the study participants were carefully recorded and reviewed by two research assistants.
All 12,297 study participants provided written informed consent, and the study was approved by the Ethics Committee of Xiangya Third Hospital of Central South University (ethics no. I 22031). All methods were performed in accordance with the relevant guidelines and regulations of the Ethics Committee.
The inclusion criteria for participants were as follows: (i) had not used drugs or other means to treat vaginitis in the 3 months before sampling; (ii) had not engaged in sexual intercourse or other vaginal operations in the 1 day before sampling; (iii) had the ability to understand the advantages and disadvantages of participating in this study and willingness to cooperate with the sampling and testing work required; and (iv) had provided written informed consent. Participants were excluded from the study if they had not been sexually active before, had symptoms or signs of reproductive tract infection, were pregnant or lactating, were unable to cooperate with examinations, or withdrew from the study.
Collection of clinical samples
Samples of vaginal secretions were obtained by gynecologists trained to collect secretions from the upper third of the vaginal sidewall. The swabs with vaginal secretions were inserted into aseptic test tubes and sealed. Then, the cervical mucus was wiped away with a large cotton swab, and a human papillomavirus (HPV) sampling brush was extended into the cervical tube and gently rotated clockwise five times. The front segment of the HPV sampling brush was then snapped off and stored in preservation solution. A test barcode was affixed to the specimen bottle, which was then immediately sent to the laboratory.
Testing of vaginal secretions
The pH of vaginal secretions was detected using pH 3.8–5.4 precision pH test paper, as described previously36. The cleanliness of vaginal secretions was checked directly under a high-power microscope using the saltwater glass film method37. Samples were stained with Gram staining solution (Baso Diagnostics, Guangdong, China) following standard steps. Nugent scores were determined based on the bacterial morphology of Lactobacillus, Campylobacter, Gardnerella, and Bacteroides. Fungal infection was evaluated by observing fungal hyphae, budding spores, and spores. A vaginitis detection kit (Jiangsu Bioperfectus Technologies, Jiangsu, China) was used to evaluate the vaginal ecology. All tests on vaginal secretions were performed by experienced technicians.
HPV test
HPV DNA was amplified by polymerase chain reaction (PCR) and detected using an HPV gene chip detection kit (HybriBio, Guangdong, China). Twenty-one types of HPV were detected, of which 14 were high risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), 5 were low risk (6, 11, 42, 43 and 44), and 2 were of unknown risk (53 and CP8304)38. If any of these HPV types were detected, a sample was considered HPV positive,if none of these HPV types were detected, a sample was considered HPV negative.
Diagnostic information
Vaginal secretions with a pH of < 4.5 were regarded as normal, whereas those with a pH of ≥ 4.5 were regarded as abnormal36. Bacteria observed in the highest numbers under the microscope were defined as the dominant bacteria. Bacteria were divided into two groups: Gram-positive bacteria and Gram-negative bacteria (2016). The cleanliness of vaginal secretions was graded into four categories: grade I, mainly vaginal bacilli, with a large number of epithelial cells,grade II, some vaginal bacilli, epithelial cells, white blood cells, and miscellaneous bacteria; grade III, only a small amount of vaginal bacilli and epithelial cells, but a large number of white blood cells and miscellaneous bacteria; and grade IV, full of white blood cells and miscellaneous bacteria, with no vaginal bacilli. Grades I and II were considered normal, whereas grades III to IV was considered abnormal (2016). The Nugent score of vaginal secretions was calculated according to the Gram staining score standard39. Samples were considered positive for fungi if spores, budding spores, or hyphae were observed under the microscope.
Animals
A total of 12 female Sprague Dawley (SD) rats (13–15 days pregnant, weight 400–450 g) were obtained from the Hunan SJA Laboratory Animal Co. Ltd (Hunan, China). The rats were housed in a standard environment under a 12-h light–dark cycle with 60–70% relative humidity at 22–24 °C. All animal experiments were approved by the Ethics Committee for Animal Research of Central South University and strictly complied with the guidelines and requirements of the National Institutes of Health (NIH) for the care and use of laboratory animals (Animal Ethics Committee Review no. 2020sydw0713). The study is reported in accordance with ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments).
Reagents
A Masson’s trichrome stain kit was purchased from Solarbio (Shanghai, China). Neutral balsam, a hematoxylin and eosin (H&E) staining system, and a rabbit anti-sheep immunohistochemistry (IHC) kit were obtained from Auragene (Hunan, China). Rat TGF-β1 and rat IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Shanghai Enzyme-linked Biotechnology (Shanghai, China). Primary anti-IL-6, anti-TGF-β1, anti-Smad 2, anti-COLl, anti-ER, and anti-PR antibodies, and secondary goat anti-rabbit antibodies were purchased from Abcam (Cambridge, MA, USA). C. albicans was obtained from the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China), and cultured according to the instructions.
Establishing an IUA model in pregnant rats
The rats were purchased at 13 days of gestation, and the experiment was carried out after 2–3 days of adaptive feeding in the laboratory. The rats were anesthetized by intraperitoneal injection of 40 mg/kg of 2% pentobarbital sodium. A median straight incision was made in the lower abdomen, and a 2–3 cm incision was made through the skin, subcutaneous tissue, rectus abdominis, and peritoneum to enter the abdominal cavity. The Y-shaped uterus was located and gently removed from the abdominal cavity and spread on the surgical hole towel. A 0.5-cm longitudinal incision was made at the proximal end of the bilateral uterus; the fetus, placenta, and other gestational tissues were removed in turn. Curettage was performed using a custom-made curette. The length of the curettage was approximately 5–10 cm from the incision. The strength of the curette was tailored to allow a large amount of tissue to be scraped out and the uterine cavity to become rough, while avoiding penetration of the uterus. The uterus was then sutured, and the abdomen closed with 5-0 silk thread.
C. albicans infection assay
On day 1 after the procedure, the rats were randomly divided into two groups: the experimental group and the control group (n = 6 in each group). In the experimental group, 50 μL C. albicans culture fluid (with a fungal content of ~ 3 × 107) was injected into the vagina of each rat using a gastric perfusion needle inserted approximately 2 cm inside the vagina (approximately at the junction of the cervical canal and the uterus). Then the C. albicans migrated from the vagina to the uterine cavity. In the control group, 50 μL phosphate-buffered saline (PBS) solution was administered into the vagina of each rat. There was no difference in the timing of administration between the two groups. Uterine samples were obtained from rats in each group on postoperative days 3, 7, and 14. Specimens were stored in a refrigerator at 4 °C for 24 h, then frozen at –80 °C until use.
For histological analysis, specimens were stained with H&E and Masson’s trichrome. IHC was used to detect the levels of IL-6, TGF-β1, Smad 2, COLl, ER, and PR in the endometrium, and the levels of IL-6 and TGF-β1 were measured by ELISA.
Histological examination with H&E staining
For H&E staining, three uterus sections were randomly selected from each rat. The samples were first dewaxed and hydrated and then stained with hematoxylin for 10 min. Next, the samples were rinsed under running water for 2 min, differentiated in 1% saline alcohol for 2 s, rinsed again for 15 min, washed with distilled water for 1–2 s, and then stained with eosin for 10 min. The samples were next differentiated in an 80% ethanol solution according to their color before being dehydrated, first with 85% ethanol for 5 min, then with 95% ethanol for 5 min, and finally with anhydrous ethanol for 10 min, twice. Xylene was used to achieve transparency. After sealing with neutral gum, morphological changes in the endometrium were observed under an inverted microscope (40×).
Masson’s trichrome staining
Samples were first dewaxed and hydrated, and then stained with a pre-prepared Weigert’s hematoxylin staining solution for 8 min. Next, the samples were differentiated with 1% acidic ethanol differentiation buffer for 10 s, before being washed with water and transferred to Masson’s blue solution for 5 min to be blued. After that, they were dyed with Masson’s magenta dye for 5 min, washed for 1 min, and then transferred directly to aniline blue dye for 2 min. Finally, the specimens were dehydrated, rendered transparent, and sealed in neutral resins.
IHC staining
Samples were dewaxed and hydrated. After high-temperature and high-pressure antigen repair, the samples were incubated with 3% H2O2 at room temperature for 10 min to block endogenous peroxidase activity. Then, primary antibodies for IL-6, TGF-β1, Smad 2, COIL1, ER, and PR were added at a dilution of 1:200, and the samples were incubated at 4 °C overnight. PBS was used as the negative control solution. Following overnight incubation in a wet box, the samples were rewarmed to 37 °C for 30 min. Horseradish peroxidase-labeled polymer (ready-to-use; P009IH; Auragene, Hunan, China) was added to the samples, which were subsequently subjected to incubation at room temperature for 30 min, followed by washing with PBS.
Four high-power visual fields were selected in each section, and there were no blank areas in any field. Images of the IHC-stained endometrial sections were captured. Brown areas or granules in the endometrium were taken as positive. The integrated optical density (IOD) of each image was measured using Image-ProPlus 6.0 image analysis software (Media Cybernetics, Bethesda, MD, USA). The IOD values of the endometrial proteins were observed, and the mean IOD value was taken as the IOD value for each uterine specimen. The higher the IOD value, the stronger the positive expression of the proteins.
ELISA
The enzyme-linked immunosorbent assay (ELISA) of endometrial tissues in the two groups were conducted according to the IL-6 and TGF-β1 assay kits. Absorbance was measured at 450 nm with an enzyme-labeling instrument, and the levels of IL-6 and TGF-β1 were calculated using a standard curve.
Evaluation of the number of endometrial glands and the fibrotic area in rats
In each H&E-stained section of rat endometrial tissue, four visual fields were randomly selected for analysis under a microscope (50×). The number of endometrial glands in each visual field was counted, and the mean was calculated. Four visual fields (100×) in each Masson’s trichrome-stained section were also randomly selected for microscopic analysis. The area of endometrial interstitial fibrosis, the area of the endometrial stroma, and the glandular density in each visual field were calculated using Image-ProPlus 6.0. The fibrosis ratio was calculated as the mean of the area of endometrial interstitial fibrosis divided by the total area of the endometrial stroma and glands in each visual field.
Statistical analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Numerical variables were expressed as the mean ± SD. Data were compared using t-tests for count data and one-way analysis of variance (ANOVA) or Fisher’s exact probability method for measurement data. Logistic regression analysis was used to determine the dominant variables for establishing IUA prediction models. A two-sided P < 0.05 was considered significant.
Results
Clinical baseline characteristics of the IUA and non-IUA groups
In total, 12,297 participants were included in this study including 908 patients with IUA (7.38%; the IUA group) and 11,389 healthy controls (92.62%; the non-IUA group). Clinical baseline parameters of the study population are presented in Table 1. Six cases (0.7%) in the IUA group were HPV positive, whereas none of the 11,389 cases in the non-IUA group had HPV infection. The proportion of patients with a large population of Lactobacilli was significantly higher in the IUA group than in the non-IUA group (39.6% vs. 0.2%, respectively; P = 0.0000). Compared to the non-IUA group, the IUA group had a higher proportion of cases with abnormal microbiota (56.4% vs. 30%, respectively; P = 0.0000) and poorer vaginal cleanliness (P = 0.0000). Interestingly, the proportion of cases with fungal infection was significantly higher in the IUA group than in the non-IUA group (18.2% vs. 9.1%, respectively; P = 0.0000), as was the proportion of cases with pH > 4.6 (43.2% vs. 3.2%, respectively; P = 0.0000). There was no significant difference in the dominant bacteria or acetylglucosaminidase between the two groups (P > 0.05).
Univariate and multivariate logistic regression analysis of factors influencing IUA occurrence
To identify factors that influence IUA, we first conducted univariate logistic regression analysis (Table 2). The amount of Lactobacillus, vaginal microecology, fungal infection, Nugent score, leukocyte esterase, H2O2, pH, and vaginal cleanliness were all identified as variates that may affect the occurrence of IUA. Compared with the non-IUA group, the IUA group was more likely to have vaginal fungal infection [P < 0.0001; odds ratio (OR) 0.451, 95% confidence interval (CI): 0.377 to 0.540] and higher vaginal pH (P < 0.0001; OR 9.817, 95% CI: 8.456 to 11.399). Multivariate logistic regression analysis further showed that fungal infection, pH, the amount of Lactobacillus, Nugent score, H2O2, and vaginal cleanliness were all significantly correlated with IUA (P < 0.05; Table 2).
General appearance of uterine samples after C. albicans perfusion in rats
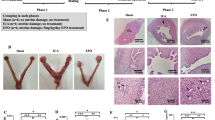
The uterine samples from the C. albicans and control groups were not fully contracted on the 3rd day postoperatively. However, the inflammatory edema in the C. albicans group was slightly more severe than that in the control group (Fig. 1).
Changes in inflammation and fibrosis of the reproductive tract after C. albicans perfusion
In the control group, on postoperative day 3, large areas of necrosis, coagulative necrosis, suppurative necrosis, bleeding foci, and local edema were observed. A small number of glandular structures and more collagen fibers could also be seen in local tissues (Fig. 2A). On postoperative day 7, a small number of glands, a small number of neutrophils in the lamina propria, and more collagen fiber hyperplasia were observed (Fig. 2B). On day 14 after the procedure, there were no obvious glandular structures, but we did observe a small amount of cytoplasmic loss and nuclear pyknosis in the uterine luminal epithelium, scattered neutrophils in the lamina propria, and more collagen fibers (Fig. 2C).
H&E staining of rat endometrial tissues on postoperative days 3, 7, and 14 in the Candida albicans and control groups. (A) On day 3, H&E staining results in the control group show suppurative necrosis (black arrows) and local edema (green arrow). (B,C) H&E staining results in the control group on the day 7 and 14. (D) On day 3, H&E staining results in the C. albicans group show neutrophils (black arrow) and lamina propria edema (green arrows). (E) On day 7, H&E staining results in the C. albicans group show local uterine epithelial cytoplasmic vacuolation (blue arrow), a small number of lymphocytes (yellow arrow), and more collagen fiber hyperplasia (green arrow) in the lamina propria. (F) On day 14, H&E staining results in the C. albicans group show inflammatory exfoliation and deletion of the uterine epithelium (black arrow). H&E hematoxylin and eosin.
In the C. albicans group, we observed a small number of glandular structures in local tissues, dilated glands, and neutrophils in some glandular cavities on postoperative day 3, as well as more neutrophil infiltration in the lamina propria, necrotic foci and eosinophilia in tissues, lamina propria edema, and multiple local hemorrhagic foci (Fig. 2D). On postoperative day 7, we observed a small number of glandular structures in the lamina propria, necrosis and nuclear fragmentation in some glandular epithelial cells, local uterine epithelial cytoplasmic vacuolation, a small number of lymphocytes, and more collagen fiber hyperplasia in the lamina propria (Fig. 2E). Furthermore, a hematoma had formed in the local tissue, which was surrounded by connective tissue, forming a capsule, and there was phagocytosis of hemosiderin-laden macrophages in the outer layer (Fig. 2E). On day 14 after the procedure, we observed inflammatory exfoliation and deletion of the uterine epithelium, exfoliated epithelial cells, necrotic tissue fragments, neutrophils, hyperemia, dilatation of the subepithelial capillaries, no obvious glandular structures, more inflammatory cell infiltration, and local fibroblast hyperplasia in the lamina propria (Fig. 2F).
Changes in inflammation and fibrosis of the reproductive tract after C. albicans perfusion
In the control group, mild to moderate collagen fiber hyperplasia was observed on day 3 (Fig. 3A), and there was slight collagen fiber proliferation on days 7 (Fig. 3B) and 14 (Fig. 3C).
Masson’s trichrome staining of rat endometrial tissues in the control and C. albicans groups on postoperative days 3, 7, and 14. (A,B) Masson trichrome staining results in the control group on the day 3 and 7. (C) On day 14, staining results in the control group show slight, collagen fiber proliferation (black arrow). (D) On day 3, staining results in the C. albicans group show severe collagen fiber hyperplasia (black arrow). (E) On day 7, staining results in the C. albicans group show extremely severe hyperplasia of the collagen fibers, with some of the fibers forming strips (black arrow). (F) On day 14, staining results in the C. albicans group show severe collagen fiber proliferation can be seen, with some of the fibers forming strips.
In the C. albicans group, severe collagen fiber hyperplasia was observed on postoperative day 3 (Fig. 3D). On day 7, extremely severe collagen fiber hyperplasia was observed, with some of the fibers forming strips (Fig. 3E). On day 14, there was severe collagen fiber proliferation, with some of the fibers forming strips (Fig. 3F).
The mean number of endometrial glands in the control and C. albicans groups was 36.230 ± 20.630 and 19.320 ± 12.780 /mm2, respectively. The endometrial gland density in the C. albicans group was significantly lower than that in the control group (P < 0.001, Table 3). On postoperative days 3, 7, and 14, the ratio of the endometrial fibrosis area to the the total endometrial area in the control group was 0.128 ± 0.058, 0.106 ± 0.038, and 0.069 ± 0.024, respectively; in the C. albicans group, it was 0.211 ± 0.142, 0.371 ± 0.059, and 0.413 ± 0.131, respectively. These results show that the degree of endometrial fibrosis stimulated by C. albicans in the IUA model rats was significantly greater than that in the control group at three different time points (postoperative days 3, 7, and 14) (P < 0.001; Table 3).
Expression of IL-6, TGF-β1, Smad 2, COLl, ER, and PR in the endometrium of IUA model rats
The expression levels of the IL-6, TGF-β1, Smad 2, COLl, ER and PR proteins in the endometrium, as assessed by IHC, were significantly higher in the C. albicans group than in the control group, whereas the levels of ER and PR were significantly lower in the C. albicans group than in the control group (Fig. 4, Table 4). The expression levels of IL-6 and TGF-β1 in the endometrium, as detected by ELISA, were found to be significantly higher in the C. albicans group than in the control group (Table 5).
Discussion
The reproductive tract microbiome is an extensive and diverse system of pathogenic and non-pathogenic microorganisms that play an important role in female reproductive health40,41. In one study, endometrial samples from 46 patients with IUA and 21 infertile patients without IUA were analyzed. The results showed that the proportions of Klebsiella, Shewanella, and Lactobacillus at the genus level were higher in patients with IUA than those without IUA, whereas the proportion of Acinetobacter was significantly lower in patients with IUA42. Liu et al.43 collected samples of lower genital tract secretions from 50 patients with IUA and 30 healthy women for microbiological evaluation. In their study, patients with IUA had a significantly lower percentage of Firmicutes and a higher percentage of Actinobacteria at the phylum level than did women in the healthy vaginal secretion group43. At the genus level, nearly 50% of patients with IUA were found to have markedly reduced levels of the probiotic Lactobacillus, accompanied by an excess of pathogenic Gardnerella and Prevotella43. Their study also pointed out that patients with IUA lacked some normal bacteria, which may impair the endometrium’s repair functions43.
Through a retrospective analysis of a large clinical sample, we found that the fungal infection rate in patients with IUA was higher than that in healthy women and was accompanied by an increased vaginal pH, and fungal infection was further revealed to be an independent risk factor for IUA. It was also found that vaginal fungal infection in patients with IUA occurred simultaneously with an increase in Lactobacillus. This finding is in contrast with the results of a previous study which compared the vaginal microbiota of 50 patients with IUA and 30 healthy women and found that the amount of Lactobacillus was significantly decreased in approximately 50% of the IUA group43. It has been confirmed that vaginal fungal infections can be accompanied by an increase in pH44. We speculate that the reason for this increase may be that fungal infection elevates the pH of the vagina, which cannot be offset by Lactobacillus. It remains unclear whether this serves as a compensatory mechanism by which the vaginal microecosystem in patients with IUA can maintain a normal vaginal pH so as to resist fungal infection, and whether the increase in vaginal Lactobacillus in patients with IUA results from fungal infection. Further investigation is required to address these issues.
In recent years, fungal vaginitis has been considered to be an immunopathological reaction45. A study has shown that vaginal epithelial cells can distinguish colonized yeast from invasive hyphae by activating pathways such as the oxidative stress pathway46. Furthermore, invasive hyphae have been associated with cell damage46. It has also been shown that C. albicans with mycelial deletion strains can colonize as well as, or even better than, wild-type strains but cannot induce immunopathological markers or mucosal damage46. Fungal infection of the reproductive tract is not necessarily accompanied by clinical symptoms47. These results suggest that in fungal vaginitis, the transition from spores to hyphae or downstream mycelium-related effectors may be the key to tissue damage and subsequent immunopathological processes47.
It has been reported that C. albicans can cause a strong proinflammatory response, upregulate the expression of cytokines, and activate the nuclear factor (NF)-κB signaling pathway48. The increase in inflammatory cytokines actively promotes the occurrence and development of IUA49. The NF-κB pathway plays an important role in the production of cytokines and cell survival, and is an important part of the immune response, having been found to actively promote the pathogenesis of many inflammatory diseases50. Researchers have found significantly increased mRNA and protein levels of NF-κB in patients with IUA and in IUA rat models51. Through IHC studies, activation of the NF-κB signal in IUA has been further confirmed, and a close relationship has been detected between the NF-κB signaling pathway and the pathogenesis of IUA, as well as cytokines involved in the pathogenesis of IUA, such as TGF-β1, tumor necrosis factor (TNF)-α, and IL-651. Research has also shown that the activation and expression of the NF-κB signaling pathway in IUA tissue plays an important role in the pathogenesis of the condition51.
IL-6 can promote fibrosis and activate the TGF-β/Smad pathway. The IL-6 receptor (IL-6R) is usually membrane bound, although it also exists in a soluble form (sIL-6R). When IL-6 binds to sIL-6R, a complex is formed which activates membrane-bound glycoprotein 130. This complex is constitutively expressed in most cell types. IL-6 binding to SIL-6R also leads to the activation of signal transducers and activators of transcription (STAT) signaling pathways, known as IL-6 trans-signals48. Importantly, unlike other soluble cytokine receptors, sIL-6R does not antagonize the activity of IL-6,instead, it has the opposite effect. While sIL-6R can be formed by limited proteolysis of membrane-bound receptors, it can also be secreted directly from cells through selective mRNA splicing29. Fibrotic diseases are characterized by the activation of fibroblasts. In the present study, we found that fibroblast activation was attributable to increases in the levels of the inflammatory cytokines TNF-α and IL-6. We also found that IL-6 upregulated the factors related to the TGF-β signaling pathway.
As a key mediator of tissue and organ fibrosis, TGF-β1 has a significant effect on fibroblast proliferation and the production of extracellular matrix in vitro52. A major process in TGF-β1 signal transduction is the recognition of Smad proteins22. Receptor-Smads (R-Smads,including Smad 2 and Smad 3) are the main downstream effectors of the TGF-β signaling pathway53. Considering the key role of IL-6 in the production of TGF-β1, we speculate that IL-6 may activate the Smad signaling pathway. After C. albicans perfusion, we observed that the expression of Smad 2 in endometrial sections was upregulated relative to that in the control group, which indicates that the activation of Smad 2 may be one of the intracellular mechanisms of IL-6-mediated endometrial fibrosis. Recent data have provided new evidence that the Smad protein is mainly activated by the classical TGF-β1 trigger mechanism22. Our results also show that IL-6-induced Smad activation was mediated by TGF-β1 in our rat fibrosis model.
The current study has several limitations that should be noted. First, we identified a problem based on retrospective clinical study of a large sample, and then conducted preliminary verification through an animal experiment. We identified an C. albicans-TGF-β/Smad-IL-6 axis, but did not confirm its regulatory relationship through further experiments. The C. albicans was migrated from vagina to uterine cavity, but not colonized in the uterine cavity directly. Therefore, inflammation in the uterus may be affected by inflammation in the vagina, and the increase in inflammatory cytokines might be associated to the occurrence and development of IUA. A more effective and valuable animal model of IUA needs further exploration. Despite these limitations, this is the first study to explore the pathogenesis of IUA from the perspective of reproductive tract fungal infection. Our findings provide a new insight for understanding the pathogenesis of IUA and a new direction for the treatment and prevention of this condition. In future research, the regulation of C. albicans-TGF-β/Smad-IL-6 axis needs to be verified in vivo or in vitro.
In conclusion, this study has found that vaginal microecological disorders exist in patients with IUA, and that vaginal fungal infection may be one of the risk factors for IUA. C. albicans may cause a strong inflammatory response involving IL-6 upregulation and COL1 deposition, which aggravates endometrial fibrosis. C. albicans infection may therefore be related to IUA.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Sugimoto, O. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions. Am. J. Obstet. Gynecol. 131(5), 539–547. https://doi.org/10.1016/0002-9378(78)90116-3 (1978).
Frey, C., Chanelles, O. & Poncelet, C. Synéchies utérines postopératoires: quels moyens de prévention? [How to prevent postoperative intrauterine adhesions?]. Gynecol. Obstet. Fertil. 38(9), 550–552. https://doi.org/10.1016/j.gyobfe.2010.07.004 (2010).
Nunn, K. L. & Forney, L. J. Unraveling the dynamics of the human vaginal microbiome. Yale J. Biol. Med. 89(3), 331–337 (2016).
Wiesenfeld, H. C., Hillier, S. L., Krohn, M. A., Landers, D. V. & Sweet, R. L. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 36(5), 663–668. https://doi.org/10.1086/367658 (2003).
Evans-Hoeker, E. A. & Young, S. L. Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 32(5), 392–401. https://doi.org/10.1055/s-0034-1376358 (2014).
Bosteels, J. et al. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane Database Syst. Rev. 11, CD11110. https://doi.org/10.1002/14651858.CD011110.pub2 (2015).
Rüping, M. J., Vehreschild, J. J. & Cornely, O. A. Patients at high risk of invasive fungal infections: When and how to treat. Drugs 68(14), 1941–1962. https://doi.org/10.2165/00003495-200868140-00002 (2008).
Kojic, E. M. & Darouiche, R. O. Candida infections of medical devices. Clin. Microbiol. Rev. 17(2), 255–267. https://doi.org/10.1128/CMR.17.2.255-267.2004 (2004).
Sobel, J. D. & Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Exp. Opin. Pharmacother. 19(9), 971–977. https://doi.org/10.1080/14656566.2018.1476490 (2018).
Liu, N. N. et al. Mycobiome dysbiosis in women with intrauterine adhesions. Microbiol. Spectr. 10(4), e0132422. https://doi.org/10.1128/spectrum.01324-22 (2022).
Sobel, J. D. Vulvovaginal candidosis. Lancet (London, England) 369(9577), 1961–1971. https://doi.org/10.1016/S0140-6736(07)60917-9 (2007).
Achkar, J. M. & Fries, B. C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 23(2), 253–273. https://doi.org/10.1128/CMR.00076-09 (2010).
Nyirjesy, P., Seeney, S. M., Grody, M. H., Jordan, C. A. & Buckley, H. R. Chronic fungal vaginitis: The value of cultures. Am. J. Obstet. Gynecol. 173(3 Pt 1), 820–823. https://doi.org/10.1016/0002-9378(95)90347-x (1995).
Taylor, B. N., Saavedra, M. & Fidel, P. L. Jr. Local Th1/Th2 cytokine production during experimental vaginal candidiasis: Potential importance of transforming growth factor-beta. Med. Mycol. 38(6), 419–431. https://doi.org/10.1080/mmy.38.6.419.431 (2000).
Raska, M., Bĕláková, J., Krupka, M. & Weigl, E. Candidiasis—Do we need to fight or to tolerate the Candida fungus?. Folia Microbiol. 52(3), 297–312. https://doi.org/10.1007/BF02931313 (2007).
Rodero, C. F. et al. Curcumin-loaded liquid crystalline systems for controlled drug release and improved treatment of vulvovaginal candidiasis. Mol. Pharm. 15(10), 4491–4504. https://doi.org/10.1021/acs.molpharmaceut.8b00507 (2018).
Okada, H. et al. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation 111(19), 2430–2437. https://doi.org/10.1161/01.CIR.0000165066.71481.8E (2005).
Khan, R. & Sheppard, R. Fibrosis in heart disease: Understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118(1), 10–24. https://doi.org/10.1111/j.1365-2567.2006.02336.x (2006).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 12(6), 325–338. https://doi.org/10.1038/nrneph.2016.48 (2016).
Akiyama-Uchida, Y. et al. Norepinephrine enhances fibrosis mediated by TGF-beta in cardiac fibroblasts. Hypertension (Dallas, Tex. : 1979) 40(2), 148–154. https://doi.org/10.1161/01.hyp.0000025443.61926.12 (2002).
Kapoun, A. M. et al. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: Fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ. Res. 94(4), 453–461. https://doi.org/10.1161/01.RES.0000117070.86556.9F (2004).
Petrov, V. V., Fagard, R. H. & Lijnen, P. J. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension (Dallas, Tex. : 1979) 39(2), 258–263. https://doi.org/10.1161/hy0202.103268 (2002).
Lijnen, P. J., Petrov, V. V. & Fagard, R. H. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol. Genet. Metab. 71(1–2), 418–435. https://doi.org/10.1006/mgme.2000.3032 (2000).
Tzavlaki, K. & Moustakas, A. TGF-β signaling. Biomolecules 10(3), 487. https://doi.org/10.3390/biom10030487 (2020).
Larsson, O. et al. Fibrotic myofibroblasts manifest genome-wide derangements of translational control. PLoS ONE 3(9), e3220. https://doi.org/10.1371/journal.pone.0003220 (2008).
Zhou, Y., Murthy, J. N., Zeng, D., Belardinelli, L. & Blackburn, M. R. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS ONE 5(2), e9224. https://doi.org/10.1371/journal.pone.0009224 (2010).
Pedroza, M. et al. Interleukin-6 contributes to inflammation and remodeling in a model of adenosine mediated lung injury. PLoS ONE 6(7), e22667. https://doi.org/10.1371/journal.pone.0022667 (2011).
Fielding, C. A. et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40(1), 40–50. https://doi.org/10.1016/j.immuni.2013.10.022 (2014).
O’Reilly, S., Ciechomska, M., Cant, R. & van Laar, J. M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J. Biol. Chem. 289(14), 9952–9960. https://doi.org/10.1074/jbc.M113.545822 (2014).
Wang, J. H. et al. Hypoxia-stimulated cardiac fibroblast production of IL-6 promotes myocardial fibrosis via the TGF-β1 signaling pathway. Lab. Invest. 96(8), 839–852. https://doi.org/10.1038/labinvest.2016.65 (2016).
Sheppard, D. Transforming growth factor beta: A central modulator of pulmonary and airway inflammation and fibrosis. Proc. Am. Thorac. Soc. 3(5), 413–417. https://doi.org/10.1513/pats.200601-008AW (2006).
Gauldie, J., Bonniaud, P., Sime, P., Ask, K. & Kolb, M. TGF-beta, Smad3 and the process of progressive fibrosis. Biochem. Soc. Trans. 35(Pt 4), 661–664. https://doi.org/10.1042/BST0350661 (2007).
Hinz, B. Mechanical aspects of lung fibrosis: A spotlight on the myofibroblast. Proc. Am. Thorac. Soc. 9(3), 137–147. https://doi.org/10.1513/pats.201202-017AW (2012).
Milara, J. et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: An experimental study. Thorax 73(6), 519–529. https://doi.org/10.1136/thoraxjnl-2017-210728 (2018).
Pedroza, M. et al. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J. 30(1), 129–140. https://doi.org/10.1096/fj.15-273953 (2016).
Sha, B. E. et al. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J. Clin. Microbiol. 43(9), 4607–4612. https://doi.org/10.1128/JCM.43.9.4607-4612.2005 (2005).
Chinese Medical Association Obstetrics and Gynecology Branch Infectious Diseases Collaborative Group. Expert consensus on clinical application of vaginal microecosystem assessment. Chin. J. Obstet. Gynecol. 51, 721–723 (2016).
Tao, P., Zheng, W., Wang, Y. & Bian, M. L. Sensitive HPV genotyping based on the flow-through hybridization and gene chip. J. Biomed. Biotechnol. 2012, 938780. https://doi.org/10.1155/2012/938780 (2012).
Nugent, R. P., Krohn, M. A. & Hillier, S. L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29(2), 297–301. https://doi.org/10.1128/jcm.29.2.297-301.1991 (1991).
Wolf, M., Müller, T., Dandekar, T. & Pollack, J. D. Phylogeny of Firmicutes with special reference to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int. J. Syst. Evol. Microbiol. 54(Pt 3), 871–875. https://doi.org/10.1099/ijs.0.02868-0 (2004).
Champer, M. et al. The role of the vaginal microbiome in gynaecological cancer. BJOG Int. J. Obstet. Gynaecol. 125(3), 309–315. https://doi.org/10.1111/1471-0528.14631 (2018).
Qiu, T. et al. Analysis of endometrial microbiota in intrauterine adhesion by high-throughput sequencing. Ann. Transl. Med. 9(3), 195. https://doi.org/10.21037/atm-20-2813 (2021).
Liu, Z. et al. Revealing the interaction between intrauterine adhesion and vaginal microbiota using high-throughput sequencing. Mol. Med. Rep. 19(5), 4167–4174. https://doi.org/10.3892/mmr.2019.10092 (2019).
Gonçalves, B. et al. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 42(6), 905–927. https://doi.org/10.3109/1040841X.2015.1091805 (2016).
Peters, B. M. et al. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect. Immun. 82(2), 532–543. https://doi.org/10.1128/IAI.01417-13 (2014).
Moyes, D. L. et al. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med. Microbiol. Immunol. 201(1), 93–101. https://doi.org/10.1007/s00430-011-0209-y (2012).
Fidel, P. L. Jr. et al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect. Immun. 72(5), 2939–2946. https://doi.org/10.1128/IAI.72.5.2939-2946.2004 (2004).
Freudlsperger, C. et al. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene 32(12), 1549–1559. https://doi.org/10.1038/onc.2012.171 (2013).
Attisano, L. & Wrana, J. L. Signal transduction by the TGF-beta superfamily. Science (New York, N.Y.) 296(5573), 1646–1647. https://doi.org/10.1126/science.1071809 (2002).
Jones, R. L., Stoikos, C., Findlay, J. K. & Salamonsen, L. A. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction (Cambridge, England) 132(2), 217–232. https://doi.org/10.1530/rep.1.01076 (2006).
Baker, R. G., Hayden, M. S. & Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 13(1), 11–22. https://doi.org/10.1016/j.cmet.2010.12.008 (2011).
Garbers, C. & Rose-John, S. Dissecting interleukin-6 classic- and trans-signaling in inflammation and cancer. Methods Mol. Biol. (Clifton, N.J.) 1725, 127–140. https://doi.org/10.1007/978-1-4939-7568-6_11 (2018).
Euler-Taimor, G. & Heger, J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc. Res. 69(1), 15–25. https://doi.org/10.1016/j.cardiores.2005.07.007 (2006).
Funding
This study was supported by the Natural Science Foundation of Hunan Province (2021JJ40956, 2021JJ40593 and 2020JJ5859), the National Key Research and Development Program of China (2018YFC1004800), the Hunan Provincial Clinical Medical Technology Innovation Guiding Project (2020SK53605 and 2020SK53606), the Key Research and Development Program of Hunan province (2022SK2033), the Hunan Science and Technology Department (2020 SK4017), the Natural Science Foundation of Guangxi Province (2020GXNSFBA297081) and the Self-funded project of Guangxi Health Commission (Z20200232).
Author information
Authors and Affiliations
Contributions
D.B.X., Y.L. and X.P.Z. conceived and designed the study. D.S. drafted the manuscript and analyzed the data. X.P.Z. and A.Q.Z. were responsible for the picture and article format. H.H. and Y.L. reviewed the data. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, X., Sun, D., Zhang, A. et al. Candida albicans-induced activation of the TGF-β/Smad pathway and upregulation of IL-6 may contribute to intrauterine adhesion. Sci Rep 13, 579 (2023). https://doi.org/10.1038/s41598-022-25471-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-25471-0
This article is cited by
-
Exploration of Perturbed Liver Fibrosis-Related Factors and Collagen Type I in Animal Model of Non-Alcoholic Fatty Liver Disease
Applied Biochemistry and Biotechnology (2024)
-
Apelin-13 alleviates intrauterine adhesion by inhibiting epithelial–mesenchymal transition of endometrial epithelial cells and promoting angiogenesis
Human Cell (2024)
-
HPV infection and vaginal microecological disorders in women with intrauterine adhesion: cross-sectional study in a Chinese population
BMC Infectious Diseases (2023)