Abstract
Evidence regarding the long-term risk of infections in preterm infants is lacking. In this study, we examined whether preterm infants developed various common childhood infections more frequently than full-term infants by the age of 2 years by analyzing data from a questionnaire completed by 67,282 mother–toddler pairs in a nationwide birth cohort study. Of the target population, 2885 (4.3%) were born prematurely. After covariate adjustment for maternal and children factors, lower respiratory tract infections appeared more frequent in preterm than in full-term infants at both 1 and 2 years (adjusted odds ratio [aOR] 1.21, 95% confidence interval [CI] 1.05–1.41, and aOR 1.27, 95% CI 1.11–1.46, respectively). However, there was no significant difference in the frequencies of lower respiratory tract infection between preterm and full-term infants after Palivizumab administration. The risk of other common infections, such as in the upper respiratory tract infection, otitis media, urinary tract infection, gastroenteritis, herpangina, hand-foot-and-mouth disease, chickenpox, influenza virus, and adenovirus infections, was not higher in preterm than in full-term infants after covariates adjustment for maternal and children factors. These findings suggest Palivizumab prophylaxis could reduce the frequencies of lower respiratory tract infection in preterm to the same level as in full-term infants.
Similar content being viewed by others
Introduction
In Western and developed countries, approximately 5–12% of all pregnancies result in preterm birth and this rate has not decreased over the past two to three decades1. In Japan, the preterm birth rate has remained at 5.4–5.7% since 20002. Although survival has significantly improved for extremely preterm infants, severe bacterial infectious diseases, such as sepsis, bacterial meningitis, and pneumonia, remain a cause of neonatal death and long-term complications during neonatal intensive care unit (NICU) admission3. Infections can also contribute to a lower quality of life for preterm infants after hospitalization from the NICU to home; infection-related deaths and hospitalizations increased significantly in extremely preterm infants4,5 who show persistently higher rates of infection-related hospital admissions until 18 years of age6,7. However, differences in the frequency of various common childhood infections between preterm and full-term infants have not yet been fully investigated. Several studies have reported that the frequency of viral respiratory infections in preterm infants is comparable to that of full-term infants after hospitalization8,9. Respiratory syncytial virus (RSV) is considered the most common cause of lower respiratory tract infection in infants. Similar to the American Academy of Pediatrics indications, Palivizumab, a humanized monoclonal antibody, has been used widely in Japan since 2002 against RSV F glycoprotein10. However, population-based data investigating the effect of Palivizumab on RSV infections after its widespread use are still lacking.
In this research, we compared the frequency of the different common childhood infections between preterm and full-term infants by the age of 2 years, after hospitalization, using data extracted from the Japan Environment and Children’s Study (JECS). Furthermore, we assessed the effects of Palivizumab on RSV-associated infectious diseases on post-hospitalized preterm infants.
Results
The characteristics of the participants in preterm and full-term births are presented in Table 1; out of 67,282 mothers, 2885 (4.3%) delivered prematurely, whereas 64,397 (95.7%) had a full-term delivery. There were significant differences in maternal age at delivery, pre-pregnancy body mass index (BMI), history of maternal allergy, physical activity, alcohol intake, maternal active and passive smoking history, marital status, Cesarean section, child sex, major anomaly, feeding methods during the first month after birth, and anti-RSV vaccine received before 1 and 2 years old. In contrast, no significant differences were detected regarding parity, energy intake during pregnancy, annual household income, highest education level, employment during early pregnancy, birth season, and attending a childcare facility.
Table 2 shows crude and adjusted odds ratios (aOR) with 95% confidence intervals (CI) of the association between preterm birth and infections at 1 and 2 years of age; in the crude model, preterm birth was associated significantly with increased risks of lower respiratory tract and urinary tract infections at 1 year, and lower respiratory tract infection at 2 years in the offspring compared with full-term infants (OR 1.24, 95% CI 1.08–1.44, p = 0.0028; OR 1.57, 95% CI 1.10–2.24, p = 0.0131; OR 1.28, 95% CI 1.12–1.46, p = 0.0004, respectively). In contrast, preterm birth was associated with decreased risks of otitis media and exanthema subitum at 1 year of age compared with full-term birth (OR 0.87, 95% CI 0.76–0.98, p = 0.0225; OR 0.86, 95% CI 0.78–0.95, p = 0.0024, respectively). However, after covariates adjustment by adding maternal factors (Model 1), as well as children factors such as feeding methods and attending a childcare facility (Model 2), only lower respiratory tract infections were more frequent in preterm than full-term infants at both years 1 and 2 (aOR 1.21, 95% CI 1.05–1.41, p = 0.0102; aOR 1.27, 95% CI 1.11–1.46, p = 0.0007, respectively), whereas the urinary tract infections did not show any significant difference between preterm and full-term infants at 2 years of age. In contrast, preterm birth was associated inversely with otitis media and exanthema subitem risk at 1 year of age compared with full-term birth in both Models 1 and 2, similar to the results of crude OR (Model 1 [otitis media; aOR 0.85, 95% CI 0.75–0.97, p = 0.0134, exanthema subitum; aOR 0.89, 95% CI 0.80–0.98, p = 0.0170] and Model 2 [otitis media; aOR 0.87, 95% CI 0.77–0.99, p = 0.0336, exanthema subitum; aOR 0.90, 95% CI 0.81–0.995, p = 0.0397]).
To determine the mediating effects of RSV and lower respiratory tract infections in preterm birth, we included Palivizumab as a covariate (Table 3); after adjusting Model 2 and having received Palivizumab (Model 3), there was no significant difference in the frequency of lower respiratory tract infection between preterm and full-term infants (1 year; aORs 1.11, 95% CI 0.92–1.32, p = 0.2731, 2 years; aORs 1.10, 95% CI 0.93–1.30, p = 0.2859). The frequency of RSV infections in preterm and full-term infants did not differ in Models 3, with no significant difference.
Discussion
This study found that preterm infants developed lower respiratory tract infections more frequently than full-term infants by 1 and 2 years of age, however, the risks were comparable after receiving Palivizumab. The occurrence of other common childhood infections in preterm infants after hospitalization, however, was not significantly higher than that in full-term infants.
In Japan, the preterm birth rate was 5.6% in 20152, although it counted for 4.6% in our current study, in which approximately 80% of the preterm infants were late preterm (34–36 weeks of gestational age), similar to the provided data on preterm births in Japan2. Although the no response or missing data rate for extremely preterm infants might be higher, we assume that our study represents the current situation of preterm and full-term birth infants in Japan. Several studies have reported that preterm infants might have poor protection against infections owing to an overactive or poorly responsive innate immune system, suppressed chemokine production, and immature neutrophil function11,12. Owing to these immunological mechanisms, the vaccine response in preterm infants could be inferior to that in full-term infants11,13,14. In addition, serum immunoglobulin G (IgG) levels are lower in preterm infants because less IgG is transferred through the placenta15. Consequently, we believe that healthcare providers should be able to segregate the infections according to their higher risk in compromising the health of preterm infants, not only during NICU admission, but also after discharge.
In our study, only the frequency of lower respiratory tract infection remained higher in both age groups in preterm infants, after covariates adjustment for maternal and children factors. Concerning the mechanism, preterm infants might be more susceptible to lower respiratory tract inflammation owing to either bronchopulmonary dysplasia or airway hyper-responsiveness. In this context, several studies have reported that preterm infants have a higher incidence of lower respiratory tract infections, which might contribute to more treatments requiring hospitalization and higher medical costs compared with full-term infants5,7,13,16. However, these studies did not show any association between the incidence of lower respiratory tract infection and Palivizumab prophylaxis. RSV is considered the most common cause of lower respiratory tract infection in children < 1 year of age17. Although the efficacy of Palivizumab in reducing RSV-related hospitalizations was well established18,19,20,21, our study suggests that Palivizumab administration reduced the incidence of lower respiratory tract infection in preterm infants to the same level as in full-term infants. Recently, some studies have reported that Nirsevimab, an Fc-modified monoclonal antibody against RSV with an extended half-life, was effective in preventing RSV-associated lower respiratory tract infection in healthy preterm and full-term infants22,23. Once Nirsevimab is approved for use, further studies on its effectiveness will be needed.
Our data show that the incidence of otitis media and exanthema subitum by 1-year of age was lower in preterm than in full-term infants, even after adjustment for maternal and postnatal factors (Table 2). This is because preterm infants spent more days in the hospital, therefore, it is expected that they spent less time at home before 1 year. In this context, Caserta et al. showed that the daily risk of acquiring a respiratory viral infection in preterm infants in the NICU is significantly lower than in full-term infants living in a normal community8. Consequently, preterm infants who spent less time in the community before the age of one might have less opportunity to develop otitis media and exanthema subitum.
In our data, there was no difference in the incidence of other infections between preterm and full-term infants up to 1 and 2 years of age even after adjustment for confounding factors (Table 2). Van den Berg showed that transplacental IgG transport for measles, mumps, rubella, and varicella zoster were significantly lower in preterm infants than in full-term infants24. Other studies revealed that preterm infants mounted antibody responses that were similar to those of full-term infants after vaccination25. Although there is limited information about the associations between other viral infections and preterm infants, hospitalizations due to the influenza virus infection (H1N1) were similar between preterm and full-term infants9. Studies on adenovirus, herpangina, and hand-foot-mouth disease are still lacking. However, although gastroenteritis is considered the second most common reason of postneonatal hospitalization by infection for preterm infants26, in our cohort study, there was no notable difference concerning this infection between preterm and full-term infants.
The strengths of our study are as follows; (1) large size of the sample (> 60,000 mother–child pairs), (2) we recruited this sample from 15 regional centers in Japan within the past decade. Therefore, we trust that our records are illustrative of the recent Japanese mother–child pairs, and (3) we added several variables that probably impact the development of infectious diseases in children, including not only maternal characteristics, socioeconomic, and smoking history, but also maternal parity, feeding methods, and attending a childcare facility. Finally, to our knowledge, our study is the first report to present the occurrence of various common infections in preterm infants compared with full-term infants after discharge from a neonatal care facility.
Several limitations also need to be considered; (1) the questionnaire regarding infections depended on the mother’s memory, and data were not obtained from medical records. In addition, the questionnaire missed information on multiple infections during the period, infection severity, or whether hospitalization was needed; and (2) the no response or missing data rate might have been higher in more preterm infants because questions about infants start after birth.
In conclusion, our data show that preterm infants are at a higher risk of lower respiratory tract infection than full-term infants by 1 and 2 years of age, however, the risk was comparable after adjustment for Palivizumab administration. Nevertheless, the occurrence of other common childhood infections in preterm infants after discharge from a neonatal care facility was not significantly higher than for full-term infants.
Methods
Study design and participants
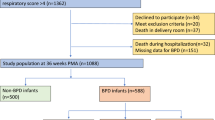
Features of JECS have been published comprehensively in terms of model and design elsewhere27,28,29. In brief, the JECS is a cohort study about birth, funded by the Japan government to reach all of Japan, and it includes many elements that influence the wellbeing and growth of children. The participants were enrolled in person in 15 Regional Centres throughout Japan between January 2011 and March 2014. The current study analyzed the set of data referenced by jecs-qa-20210401 (jecs-ta-20190930), which was available in April 2021. Data from 103,057 pregnancies until 3 years postnatal were included, from which we omitted records with inappropriate data for analysis, such as those with numerous participation, multiple births, miscarriages/still births, post-term delivery, missing data on gestational week, no response to or missing data on the questionnaire regarding infectious diseases, or missing data on covariates. Therefore, 67,282 mother–child pairs remained for the last examination (Fig. 1).
The authors declared that ethical standards complied with all processes defining this research from relevant national and institutional committees on studies comprising participation from human as per the declaration of Helsinki. The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies (no. 100910001), the Ethics Committees of all participating institutions, and the Committee of Ethics at Toyama University (no. R2022055). Finally, we obtained written informed consent from all participants.
Data collection
First, we gave the questionnaire for every participant autonomously to be auto-completed at trimesters one, two or three, and at 1 month, 6 months, 1 year, 1.5 year and 2 years after delivery. The questionnaire had many inquiries regarding demographic factors, socioeconomic status, lifestyle, occupation, medical history, physical and mental health, and housing conditions. We transcribed perinatal medical records from each cooperating health care provider, including gestational age and infant physical examinations of the infant at birth and at 1 month of age.
Outcome measures and covariates
The frequency of infection development in infants is regarded as the first outcome. Infections included upper and lower respiratory tract infections, otitis media, urinary tract infection, gastroenteritis, exanthema subitum, herpangina, hand-foot-mouth disease, chickenpox, influenza virus, RSV infection, and adenovirus infections. We assessed the data when the infants were at 1 and 2 years of age, through the following question: “Has your child been diagnosed with any of these infections by a physician since the last survey until the present time?”.
The covariates involved in the analysis were maternal age, prepregnancy BMI, parity, history of maternal allergy, history of any physical disease, marital status, maternal employment, maternal highest education level, annual household income, maternal alcohol intake, maternal smoking history, maternal physical activity, feeding methods, attending a childcare facility at 6 months of age, and receiving Palivizumab prophylaxis.
Statistical analysis
The participants were classified into preterm (< 37 weeks) and full-term (37 till < 42 weeks) birth groups according to the gestational weeks for the statistical analysis. We used t-test and chi-square to evaluate the significant difference between the two groups. We conducted multivariable logistic regression analysis to identify the relationship between preterm birth and the frequency of infant infections by the age of 1 and 2 years.
We used the compulsory entry method to comprise covariates in the multivariable analysis. We adjusted the regression in Model 1 for maternal age, pre-pregnancy BMI, parity, history of maternal allergy, history of any physical disease, marital status, employment, highest education level, annual household income, total energy intake assessed using Food Frequency Questionnaire30, alcohol intake, smoking history, and physical activity31,32. Next, we adjusted Model 2 by adding to the first model, feeding methods, and attending a childcare facility. Finally, we adjusted Model 3 by adding the inquiry regarding Palivizumab administration to the Model 2.
We present the results as crude and aORs with 95% CI, we set the significance at p < 0.05, and used SAS 9.4 (SAS Institute Inc., Cary, North Carolina) for statistical analyses.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and the legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare also restrict the open sharing of the epidemiological data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling inquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
References
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. Lancet 371, 75–84. https://doi.org/10.1016/S0140-6736(08)60074-4 (2008).
Vital statistics, Ministry of Health, Labour and Welfare, Japan. https://www.mhlw.go.jp/toukei/list/81-1a.html (2022)
Shane, A. L., Sánchez, P. J. & Stoll, B. J. Neonatal sepsis. Lancet 390, 1770–1780. https://doi.org/10.1016/s0140-6736(17)31002-4 (2017).
Kramer, M. S. et al. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA 284, 843–849. https://doi.org/10.1001/jama.284.7.843 (2000).
Houweling, L. M. et al. First year of life medication use and hospital admission rates: premature compared with term infants. J. Pediatr. 163, 61–66. https://doi.org/10.1016/j.jpeds.2012.12.014 (2013).
Miller, J. E. et al. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: a population-based, data-linkage study from Western Australia. Lancet. Infect. Dis 16, 952–961. https://doi.org/10.1016/s1473-3099(16)00150-x (2016).
Steiner, L., Diesner, S. C. & Voitl, P. Risk of infection in the first year of life in preterm children: An Austrian observational study. PLoS ONE 14, e0224766. https://doi.org/10.1371/journal.pone.0224766 (2019).
Caserta, M. T., Yang, H., Gill, S. R., Holden-Wiltse, J. & Pryhuber, G. Viral respiratory infections in preterm infants during and after hospitalization. J. Pediatr. 182, 53–58. https://doi.org/10.1016/j.jpeds.2016.11.077 (2017).
Drysdale, S. B. et al. Pandemic influenza A (H1N1) virus 2009 in a prospectively followed cohort of prematurely born infants. Pediatr. Infect. Dis. J. 31, 91–92. https://doi.org/10.1097/INF.0b013e3182324a1f (2012).
American Academy of Pediatrics Committee on Infectious, D., Committee on, F. & Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 112, 1442–1446 (2003).
Levy, O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7, 379–390. https://doi.org/10.1038/nri2075 (2007).
Bjorkqvist, M., Jurstrand, M., Bodin, L., Fredlund, H. & Schollin, J. Defective neutrophil oxidative burst in preterm newborns on exposure to coagulase-negative staphylococci. Pediatr. Res. 55, 966–971. https://doi.org/10.1203/01.pdr.0000127018.44938.89 (2004).
Fortmann, I. et al. Five year follow up of extremely low gestational age infants after timely or delayed administration of routine vaccinations. Vaccines 9, 493. https://doi.org/10.3390/vaccines9050493 (2021).
Rouers, E. D. M. et al. Association of routine infant vaccinations with antibody levels among preterm infants. JAMA 324, 1068–1077. https://doi.org/10.1001/jama.2020.12316 (2020).
Sandberg, K. et al. Preterm infants with low immunoglobulin G levels have increased risk of neonatal sepsis but do not benefit from prophylactic immunoglobulin G. J. Pediatr. 137, 623–628. https://doi.org/10.1067/mpd.2000.109791 (2000).
Bérard, A., Le Tiec, M. & De Vera, M. A. Study of the costs and morbidities of late-preterm birth. Arch. Dis. Child Fetal Neonatal. Ed 97, F329-334. https://doi.org/10.1136/fetalneonatal-2011-300969 (2012).
Hall, C. B. et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360, 588–598. https://doi.org/10.1056/NEJMoa0804877 (2009).
Meissner, H. C., Long, S. S., American Academy of Pediatrics Committee on Infectious, D., Committee on, F. & Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 112, 1447–1452, https://doi.org/10.1542/peds.112.6.1447 (2003).
Frogel, M. et al. Prevention of hospitalization due to respiratory syncytial virus: Results from the Palivizumab Outcomes Registry. J. Perinatol. 28, 511–517. https://doi.org/10.1038/jp.2008.28 (2008).
Sorrentino, M. & Powers, T. Effectiveness of palivizumab: Evaluation of outcomes from the 1998 to 1999 respiratory syncytial virus season. The Palivizumab Outcomes Study Group. Pediatr. Infect. Dis. J. 19, 1068–1071. https://doi.org/10.1097/00006454-200011000-00007 (2000).
Oh, P. I. et al. Palivizumab prophylaxis for respiratory syncytial virus in Canada: Utilization and outcomes. Pediatr. Infect. Dis. J. 21, 512–518. https://doi.org/10.1097/00006454-200206000-00007 (2002).
Griffin, M. P. et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N. Engl. J. Med. 383, 415–425. https://doi.org/10.1056/NEJMoa1913556 (2020).
Hammitt, L. L. et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 386, 837–846. https://doi.org/10.1056/NEJMoa2110275 (2022).
van den Berg, J. P. et al. Lower transplacental antibody transport for measles, mumps, rubella and varicella zoster in very preterm infants. PLoS ONE 9, e94714. https://doi.org/10.1371/journal.pone.0094714 (2014).
D’Angio, C. T. et al. Measles-mumps-rubella and varicella vaccine responses in extremely preterm infants. Pediatrics 119, e574-579. https://doi.org/10.1542/peds.2006-2241 (2007).
Iacobelli, S. et al. Gestational age and 1-year hospital admission or mortality: A nation-wide population-based study. BMC Pediatr. 17, 28. https://doi.org/10.1186/s12887-017-0787-y (2017).
Iwai-Shimada, M. et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 23, 45. https://doi.org/10.1186/s12199-018-0733-0 (2018).
Kawamoto, T. et al. Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health 14, 25. https://doi.org/10.1186/1471-2458-14-25 (2014).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28, 99–104. https://doi.org/10.2188/jea.JE20170018 (2018).
Yokoyama, Y. et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 26, 420–432. https://doi.org/10.2188/jea.JE20150064 (2016).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports. Exerc. 35, 1381–1395 (2003).
Murase, N. et al. International standardization of physical activity level: reliability and validity study of the Japanese version of the International Physical Activity Questionnaire (IPAQ). J. Health Welfare Statistics (Kosei no Shihyo) 49, 1–9 (2003) ([in Japanese]).
Acknowledgements
We are grateful to the JECS participants and to the individuals who performed the data collection. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese government.
Funding
The JECS is funded by the Ministry of the Environment, Japan. This funding source played no role in the study’s design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit this paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
K.T. drafted the paper. K.T. and K.M. analysed the data. K.T., K.M. and T.Y. conceived and designed the study. A.T., H.I., and the JECS group critically reviewed the draft and checked the analysis. The JECS group collected the data and obtained the funding. H.I. provided administrative, technical, and material support. All authors approved the submission of the manuscript in its current form.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamura, K., Matsumura, K., Tsuchida, A. et al. Prevalence of infectious diseases in preterm infants: a 2-year follow-up from the Japan Environment and Children’s Study. Sci Rep 12, 22488 (2022). https://doi.org/10.1038/s41598-022-26748-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-022-26748-0