Abstract
Exposure to prenatal stress (PS) leads to the offspring's vulnerability towards the development of cognitive and behavioral disorders. Laterodorsal tegmentum (LDT) is a part of the brainstem cholinergic system that is believed to play a pivotal role in the stress-associated progression of anxiety, memory impairment, and addictive behaviors. In this study, we aimed to investigate the electrophysiological alterations of LDT cholinergic neurons and its accompanied behavioral and cognitive outcomes in the offspring of mice exposed to physical or psychological PS. Swiss Webster mice were exposed to physical or psychological stress on the tenth day of gestation. Ex vivo investigations in LDT brain slices of adolescent male offspring were performed to evaluate the effects of two stressor types on the activity of cholinergic neurons. Open field test, elevated plus maze, passive avoidance test, and conditioned place preference were conducted to assess behavioral and cognitive alterations in the offspring. The offspring of both physical and psychological PS-exposed mice exhibited increased locomotor activity, anxiety-like behavior, memory impairment, and preference to morphine. In both early- and late-firing cholinergic neurons of the LDT, stressed groups demonstrated higher firing frequency, lower adaptation ratio, decreased action potential threshold, and therefore increased excitability compared to the control group. The findings of the present study suggest that the hyperexcitability of the cholinergic neurons of LDT might be involved in the development of PS-associated anxiety-like behaviors, drug seeking, and memory impairment.
Similar content being viewed by others
Introduction
Prenatal stress (PS) is generally referred to as the stress, both physical or psychological, experienced by a mother during pregnancy. Accumulating clinical and animal studies have demonstrated that PS raises the risk of neurodevelopmental disorders in later life periods in the offspring. For instance, offspring born to mothers who experienced stress during pregnancy have been shown to demonstrate higher anxiety levels, depression-like behaviors, and cognitive disorders in the adolescence and adulthood1,2,3,4. In addition, it has been shown that stressful events during pregnancy could affect the vulnerability of the offspring to addiction and drug abuse in later life5,6. Cognitive dysfunctions, including the persistence of aversive memories7,8, and impaired spatial learning and memory9,10,11 of the offspring, are other significant consequences of PS.
Various mechanisms have been put forth as to how PS could promote the incidence and development of psychiatric disorders in newborn. The hypothalamic–pituitary–adrenal (HPA) axis is the main stress response pathway, activated through the stimulation of stress hormones' release, which include corticotropin-releasing factor (CRF), adrenocorticotrophic hormone (ACTH), and cortisol (or corticosterone in rodents)12. On the other hand, chronic stress alters acetylcholine (Ach) release in different regions of the brain and the hyperactivity of the cholinergic system is known to be associated with the development of psychiatric disorders, including anxiety, depression and substance use disorder (SUD)13,14.
The laterodorsal tegmentum (LDT) nucleus is located in the pontine central gray, beneath the caudal part of the aqueduct, and connects the third and fourth ventricles. The LDT contains predominantly cholinergic, glutamatergic, and GABAergic neurons and is known as a part of brainstem cholinergic system15. It has been indicated that the LDT contains CRF- and urocortin (Ucn1)-containing neurons16,17,18, which are involved in the stress-associated progression of anxiety, depression, SUD, inappropriate arousal, and control of the sleep and wakefulness19,20,21,22,23. These neurons contribute to the elevated risk of psychiatric disorders linked to chronic stress by modulating the cholinergic transmission of LDT24. In addition, LDT is involved in controlling mood through cholinergic excitatory projections to the ventral tegmental area (VTA), which is a fundamental regulator of emotional behaviors25,26,27. Furthermore, LDT activates VTA dopaminergic neurons via glutamatergic and cholinergic pathways, increasing the dopamine (DA) release in the nucleus accumbens (NAc)28,29. Therefore, it is not surprising that LDT plays an important role in drug-dependent behaviors, as previous studies have indicated that this nucleus is associated with drug-seeking behaviors and increased risk of addiction induced by amphetamine, nicotine, and morphine30,31,32.
Previously, we have reported that prenatal exposure to both physical and psychological stress during fetal development changes drug-seeking and anxiety-like behaviors in the offspring, which is associated with the hyperexcitability of VTA dopaminergic neurons33. These neurons receive inputs from LDT and are tightly modulated by cholinergic projections. LDT-VTA projections are vital for the activity of dopaminergic neurons in the VTA and control DA-related behaviors28,31. Based on these findings, the present study was carried out to examine the effects of physical and psychological stress on the electrophysiological properties of LDT cholinergic neurons in Swiss Webster mice. In addition, changes in passive avoidance learning and memory, locomotor activity, anxiety-like behavior and addiction were examined in offspring (Fig. 1).
Results
Effect of PS on locomotor activity and anxiety-like behavior of the offspring
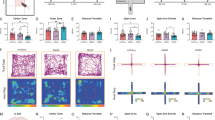
In order to evaluate the locomotor activity and anxiety-like behavior, the total time spent in the center zone, total distance moved, mobility duration, velocity, and the number of grooming and rearing were assessed in the open field test (Fig. 2).
Comparison of the effect of PS on locomotor activity and anxiety-like behavior of the offspring in the open field test. (A) Frequency of grooming per session. (B) Frequency of rearing per session. (C) Total distance moved. (D) Duration of mobility. (E) Mean velocity. (F) Time spent in the center zone. Data are displayed as Mean ± SEM. Phy s: physical stress, Psy s: psychological stress, * (p < 0.05), ** (p < 0.01), *** (p < 0.001) compared to the control group.
The number of grooming in the offspring of dams exposed to physical and psychological stress was significantly increased compared to the control group (F 2, 27 = 2.34; p = 0.014, Fig. 2A). However, no significant difference was observed in the number of rearing between the groups (Fig. 2B).
The total distance moved (F 2, 27 = 7.2; p = 0.003) in the two groups of physical stress (p < 0.01) and psychological stress (p < 0.001) was significantly higher compared to the control group (Fig. 2C). No significant difference was observed in the mobility duration among the groups (F 2, 27 = 0.6; p = 0.54, Fig. 2D). In terms of anxiety-like behavior, there was no significant difference in movement velocity between the study groups (F2, 27 = 0.4; p = 0.6, Fig. 2E), however, time spent in the center zone was significantly lower in the two prenatally stressed groups compared to the control group (F2, 27 = 5.4; p = 0.018, Fig. 2F).
The EPM test revealed that there was no significant difference in terms of the frequency of entries into open and closed arms between any of the groups (Fig. 3A, B). However, the offspring of both physical and psychological PS-exposed groups, demonstrated a significant shorter time spent in the open arms compared to the control group (F 2, 27 = 6.1; p = 0.013, Fig. 3C). Accordingly, both stressed groups had significantly spent more time in the closed arms in comparison to the control group (F 2, 27 = 3.5; p = 0.02, Fig. 3D).
Assessment of the effect of PS on anxiety-like behavior of the offspring in EPM. (A, B) Frequency of entries into open and closed arms. (C, D) Time spent in open and closed arms. Data are displayed as Mean ± SEM. Phy s: physical stress, Psy s: psychological stress, * (p < 0.05) compared to the control group.
Effect of PS on passive avoidance learning and memory of the offspring
In the passive avoidance memory test using shuttle-box, no difference was observed between the prenatally stressed and the control group in terms of the number of shocks (F 2, 27 = 2.2; p = 0.12, Fig. 4A), indicating that the stress applied did not affect avoidance learning. On the other hand, a significant decrease in STL was observed in both the physically and psychologically stressed groups compared to the control group (F2, 27 = 7.2; p = 0.019, Fig. 4-B), demonstrating that stress, despite not affecting learning, impaired the memory of mice exposed to prenatal physical and psychological stress.
Effect of PS on morphine-induced place preference of the offspring
Analyzing the CPP score in the offspring of both stressed groups demonstrated a higher preference for the morphine-paired compartment compared to the control group (F 2, 27 = 3.7, p = 0.03; Fig. 5), indicating that physical and psychological PS, results in an increased preference for morphine in the offspring.
Assessment of the effect of PS on conditioned place preference (CPP) score of the offspring. CPP score is defined as the difference in time spent in the non-preferred chamber on post-conditioning day and the time spent in the non-preferred chamber on pre-conditioning day. Data are displayed as Mean ± SEM. Phy s: physical stress, Psy s: psychological stress, * (p < 0.05) compared to the control group.
Recording of acetyl cholinergic neurons of lateral dorsal tegmentum
No significant differences were observed between the groups in terms of the passive properties of the LTD cell membranes. The results are shown as membrane resting potential, internal resistance, series resistance and membrane capacitance in Table 1.
To determine the electrophysiological properties of cholinergic neurons, 100 pA positive current was injected for 500 ms, while the cell was held at a negative 70 mV voltage, and the cell response voltage was recorded. The first group of identified cells, consisted of neurons that responded rapidly, with the first spike spaced less than 42 ms apart. The second group of cells, exhibited less adaptation ratio and the manifestation of first spike in response to the positive injection current was more than 75 ms apart (Fig. 6B).
Injection of 100 pA positive current for 500 ms after clamping at − 70 Mv in offspring of physical and psychological prenatal stressed mothers. (A) Firing frequency of early-firing neurons. (B) Firing pattern of early- and late- firing neurons. (C) Firing frequency of late-firing neurons. (D) Adaptation ratio of early-firing neurons. (E) Adaptation ratio of late-firing neurons. (F) AP threshold of early-firing neurons. (G) AP pattern of early- and late- firing neurons. (H) AP threshold of late-firing neurons. Data are represented as Mean ± SEM. * (p < 0.05), ** (p < 0.01) shows a significant difference compared to the control group. Phy S: Physical stress, Psy S: Psychological stress.
In response to a positive current of 100 pA in mice born to physical and psychological PS-exposed mothers, the frequency of spikes was significantly higher compared to the control group, both in early-firing (F 2, 24 = 7.2, p = 0.002) and late firing (F 2, 24 = 4.8, p = 0.017) neurons (Fig. 6A, C).
Compared to the control group, the offspring of physical PS-exposed mice, showed a significantly lower adaptation ratio in early-firing neurons (F 2, 24 = 5.8, p = 0.018). However, no significant difference was observed in the late-firing neurons (F 2, 24 = 3.3, p = 0.06; Fig. 6-D, E).
Pups born to physical and psychological PS-exposed mothers had significant decreased action potential (AP) threshold compared to control group, both in early- and late-firing neurons [(F 2, 24 = 18.1, p = 0.002); (F 2,24 = 11.2, p = 0.018); Fig. 6F, G, and H]. The alteration of electrophysiological properties in the two groups of neurons, was recorded in response to 100 pA positive current injection. In terms of the number of APs and the first AP latency, a significant difference was observed within the stressed and control groups, both in early- and late-firing neurons (Table 2).
Additionally, the response of early-firing neurons to positive current injection pulses was recorded at intervals of 500 ms with intensities of 60 to 140 pA at a 20 pA increasing pace. Only at the lowest stimulation intensity of 60 pA, neurons in both PS groups showed a significant increase in the number of APs compared to the control group (Fig. 7). Using the same protocol in late-firing neurons, no significant difference was observed between any of the groups.
In order to evaluate the degree of excitability and rheobase activity of early- and late-firing neurons, injection of an increasing ramp current (0 pA to 2nA lasting 1000 ms) was implied in both early-firing (Fig. 8A–C) and late-firing neurons (Fig. 8D, F) of the control and stressed groups. The results demonstrated that prenatal physical and psychological stress decreases the voltage required to reach the threshold and firing AP and increases the excitability in both cell types [(F 2, 24 = 42.6, p = 0.0008); (F 2, 24 = 9.2, p = 0.013); Fig. 8-G-H). In other words, mice with stress conditions in both cell types were stimulated at more negative voltages and had lower thresholds than the control group.
Response of early-firing neurons (A–C) and late-firing neurons (D, F) to Ramp flow injection pulses (0 pA to 2nA lasting 1000 ms) and their corresponding early (G) and late (H) rheobase. Data are represented as Mean ± SEM. *** (p < 0.001) * (p < 0.05) shows a significant difference compared to the control group. Phy S: Physical stress, Psy S: Psychological stress.
Discussion
Clinical and animal studies have demonstrated that PS can lead to aberrant alterations in the behavior and physiological function of the offspring. In our previous study, we showed that the hyperexcitability of VTA dopaminergic neurons of mice whose dams were exposed to prenatal physical or psychological stress was associated with alterations in drug dependency and anxiety-like behaviors through mesocortical and mesoaccumbal pathways33. The LDT-VTA pathway plays a critical role in regulating the activity of VTA DA neurons and the development of motivated behaviors28,31. It has been indicated that LDT regulates stress through CRF- and Ucn1-containing neurons16,17,18, which are involved in the development of stress-associated disorders, including SUD, inappropriate arousal, anxiety, and depression19,20,21. The high activity of cholinergic neurotransmission has also been shown to be involved in the progression of psychiatric disorders34,35. Interestingly, stress-related neuropeptides exert neuronal excitation in LDT cholinergic neurons, which are associated with an increased risk of psychiatric disorders24. In addition, it has been shown that cognitive functions are extremely dependent on the cholinergic system, where deficiency in cholinergic neurotransmission results in disturbances in learning and memory36,37. Pharmacological studies have also demonstrated that cholinergic antagonists induce amnesia in experimental subjects38,39. In light of these findings, the present study was designed to evaluate the effects of PS stressor type on synaptic and membrane properties of LDT cholinergic neurons in addition to the changes in the motivated behaviors of the offspring.
Our findings demonstrated that LDT cholinergic neurons were differentiated into two subtypes, early- and late-firing neurons, based on their firing delays in response to depolarizing current injections. Early-firing neurons are more excitable and have prominent spike frequency adaptation, while late-firing neurons are less excitable and maintain a tonic firing pattern at low frequencies40. We further highlighted the role of LDT cholinergic transmission in PS-associated behavioral outcomes. Our novel findings showed that PS leads to the hyperexcitability of both early- and late-firing cholinergic neurons of the LDT by increasing the firing frequency and decreasing the voltage required to reach the threshold and firing AP. Previous evidence has shown that LDT stimulates the DA neurons in the VTA, increasing the amount of DA released in the NAc26,28,31. Interestingly, it has been reported that PS affects the expression of DA receptors and the electrophysiological activity of DA neurons in NAc and VTA, respectively33,41. Moreover, PS or prenatal exposure to high levels of glucocorticoids (GCs) induce long-lasting changes in the expression of choline acetyltransferase (ChAT) and acetylcholine esterase (AChE) as cholinergic markers in the LDT. These molecular changes in the LDT-VTA circuit, which accompany prominent motivational deficits, could be rescued by optogenetic activation of the LDT-VTA terminals42. Therefore, LDT may be involved in mood control and addictive behavior through the regulation of DA release in mesolimbic regions. According to these findings, our results raise the possibility that physical and psychological PS may lead to the hyperexcitability of LDT cholinergic neurons, which further results in alterations in the LDT-VTA pathway and the regulation of DA.
We also demonstrated that pups born to physical and psychological PS-exposed mice exhibited a significantly increased tendency to morphine compared to the control group. This finding underscores the importance of stressors during fetal development on the incidence of addiction in the offspring. As previously indicated, there is growing evidence reporting that offspring born to PS-exposed mothers elicit a predisposition to dependency on various types of drugs, including cocaine43, amphetamines44, and morphine45. In reward-related circuits, stress-related neurotransmitters and neuromodulators, such as CRF, the κ-opioid agonist dynorphin, and noradrenaline (NA), contribute to addictive behaviors46,47,48,49. Neurobiological studies have shown that LDT is involved in the dependency on a wide range of drugs of abuse30,31,32. Based on these findings, LDT might play a pivotal role in mediating the effects of PS on the drug-seeking behavior of the offspring.
Studies have indicated that both physical and psychological stress during pregnancy increase the risk of depression and anxiety in the newborn50,51. In this regard, we have previously reported that exposure to physical and psychological stress during fetal development produces anxiety-like behavior in offspring, which is accompanied by the hyperexcitability of dopaminergic neurons in the VTA33. Similarly, our findings in this study revealed that both stressor types resulted in increased locomotion and anxiety-like behavior of the offspring. In line with previous studies, we further demonstrated that these stressors resulted in impaired passive memory of the offspring as well52. Building on this evidence, we propose that behavioral and memory deficits due to PS are accompanied by the hyperexcitability of cholinergic neurons in LDT. Therefore, in addition to VTA, cholinergic neurons of LDT might be other pieces of the puzzle contributing to the mechanisms underlying the behavioral outcomes of PS in the offspring.
Conclusion
Overall, the findings of the present study demonstrated that exposure to physical or psychological stress during fetal development elicits behavioral and cognitive deficits in the offspring, which are accompanied by the hyperexcitability of LDT cholinergic neurons. Future endeavors could provide further explanation on how the VTA-LDT circuit is implicated in the outcomes of PS. This may offer novel therapeutic targets aiming to reduce the burden of PS on the offspring, therefore ameliorating the resulting behavioral and cognitive disorders.
Material and methods
Subjects
Swiss Webster mice were prepared from the Kerman University of Medical Sciences. The animals were caged in a photo period-controlled room (12-h light/dark cycle) with a temperature of 25 ± 2 °C, and prior to the introduction of the male, females were housed in cages of three. Food and water were available ad libitum. All experimental procedures were approved by the Animal Research Ethics Committee of the Kerman Neuroscience Research Center (Ethics code: IR.KMU.REC.1397.579). Successful mating was verified by the detection of a vaginal plug and pregnant females were randomly assigned to the control, physical stress, and psychological stress groups (n = 10 in each group), and housed two per cage.
Drugs
Salts that have been used to prepare the intra- and extracellular solutions were purchased from Sigma-Aldrich (St. Louis, MO).
Induction of prenatal stress
A previously established method was used to confront pregnant animals with physical or psychological stress. The stress procedure commenced on tenth day of gestation and continued for the next 7 days using the following protocol52,53. The Communication Box, a 3 × 3 glass house aquarium (25 × 25 × 60 cm3 in volume) containing 5 + 4 cells (nine cells in total), was used to expose animals to stressors. The cells were separated from each other by transparent Plexiglas, which allowed mice in different cells to see one another. Cells housing the pregnant dams exposed to physical stress were connected to an electric pump that filled the cells with water (20 ± 2 °C). Animals were placed in the houses of the aquarium that were filled with water, and animals were forced to swim in the water for 5 min. Afterward, the house drained and the animals could rest for 10 min. Subsequently, the house was filled with water again. All parts of procedure lasted for 1 h and animals were then dried and moved to their cages.
The mice in the psychological stress group were placed in the houses of aquarium that were not connected to the pump and just observed the other animals receiving the physical stress of swimming. Control mice were brought to the laboratory and remained there for the same duration as stress-exposed groups.
Pups were delivered, and the number of offspring, mortality rate and birth weight were recorded for each animal. Two male offspring were selected from each litter and were weaned from their mothers on the 23rd day and were then caged in groups of four (n = 20 for each group; 10 for behavioral tests and 10 for electrophysiological assays). As several previous studies have demonstrated that male and female offspring are differentially affected by prenatal stress52,54,55, to avoid any potential conflicting results that might be attributed to hormonal changes seen in the female mice, in the current study we used males only. The behavioral tests and electrophysiological assays were performed on postnatal days (PND) 40–45. Figure 1 provides an overview of the study's timeline (Fig. 1).
Behavioral procedures
The following behavioral tests were conducted in order on each group of offspring mice with a 15-min rest period in between the tests:
Open field test
The apparatus consisted of an arena made of opaque Plexiglas (90 × 90 × 45 [H] cm). The arena was divided into central and peripheral regions, which contained 16 squares. Each mouse was placed in the center of the arena. The horizontal and vertical activities were recorded for 5 min, and were analyzed with offline analysis (Ethovision7.1, Noldus Information Technology, The Netherlands). Total time spent in the center or periphery, total distance moved, mobility duration, velocity, and the number of grooming and rearing were recorded for each animal. At the end of each trial, the maze was cleaned with a cotton cloth and 70% ethanol56.
Elevated plus maze (EPM)
The EPM was made of wood and was located 38 cm above the ground. The maze consisted of two open arms and two closed arms, each 50 cm in length. The illumination at the center was adjusted to 100 lx (red light)57,58. The animals were placed individually at the center of the platform facing one of the open arms and allowed to move freely on the maze for 5 min. During the test, the percentage of the time spent in open arms and the number of entries into the open arms were recorded using a video camera installed above the maze. The apparatus was carefully cleaned up with cotton and alcohol between the tests.
Passive avoidance test
A shuttle-box apparatus was used to evaluate associative learning and memory, which consisted of one lighted and one dark chamber. The two chambers were separated by a grid door, and a shock generator was linked to the floor rods of the dark chamber56. Before the test, the mice were placed individually in the light arena of the apparatus. After 10 s, the door was opened and the animal was allowed to go to the dark arena without electric shock for 30 s. One hour later, the acquisition phase was performed, and each mouse was placed into the light chamber. The door was then opened and the animal was allowed to enter the dark compartment. The animal received an electrical shock (0.5 mA, 50 Hz) upon entrance to the dark chamber via the stainless steel rods for 2 s. This part was repeated up to five times at 30 min intervals until the learning of the animal to avoid entering the dark compartment occurred. The number of shocks required for learning to happen was recorded. Memory retrieval was examined 24 h after the learning phase. The mouse was placed in the light compartment (door closed), and 10 s later, the door was opened. The latency to enter the dark chamber (step-through latency; STL) was recorded within 5 min.
Conditioned place preference (CPP)
The day after other behavioral assays, CPP was performed to evaluate drug-induced addictive (drug-seeking) behavior59. The CPP apparatus was a two-compartment box (30 × 30 × 40 cm), which were connected through a neutral chamber in the middle. One compartment was painted in white with vertical white stripes with a textured floor, and the other was painted in black with horizontal white stripes and a smooth floor. Each room had gates allowing the animal to access all three rooms freely.
Animals were placed in the neutral part of the apparatus on day one and the doors were opened to allow the animal to explore the compartments for 10 min. The time spent in each compartment was recorded and if the animals spent a relatively equal time in the three chambers (not more than 60% of time spent in one compartment), they were included in the study. For CPP conditioning, the mice were injected with saline or morphine (10 mg/kg, i.p.) for the next 4 days. The two distinct compartments were paired repeatedly, one with the morphine injections and the other with the saline injections. After the injection of morphine or saline, mice were kept in the respective compartment for 30 min with the doors closed. On the sixth day, animals were again put in the neutral compartment, and all doors were opened with access to all chambers. Time spent in each compartment was recorded, and CPP score was calculated using the following formula:
Non-preferred chamber was defined on the first day when the animals spent less than 50% of the time in that compartment.
Electrophysiological assays
Slice preparation
Mice were decapitated under deep anesthesia and the brain was rapidly removed from the skull. All procedures were performed as previously described33. The brain was then submerged in artificial cerebrospinal fluid (aCSF) composition containing (in mM) 124 NaCl, 25 NaHCO3, 10 d-glucose, 4.4 KCl, 2 MgCl2, 1.25 NaH2PO4 and 2 CaCl2. (oxygenated with 5%CO2–95%O2, pH 7.4 ± 0.05 and osmolarity was adjusted to 300 ± 10 mOsm). The brainstem was then blocked in a coronal plane and sectioned at 250 μm thicknesses on a Vibroslicer (Campden Instrument, NVSLM1, Sarasota, FL, USA). The LDT was identified on the basis of its anatomical location relative to the fourth ventricle. From each animal, two slices including the LDT were selected. Slices containing the LDT were incubated for 45 min at 34 ± 2 °C and then stored at room temperature. The LDT slices were continuously superfused at 1 ml/min with aCSF.
Whole-cell patch clamp recording
The effects of PS exposure on the electrophysiological properties of LDT neurons in the male offspring were evaluated by whole-cell patch clamp recordings in LDT brain slices in PND 40–45.
LDT neurons were monitored on a television screen through an infrared charge-coupled device camera (Hamamatsu, Japan) and video microscopy (BX-51, Olympus, Tokyo, Japan). Due to the lack of access to immunohistochemistry and fluorescent microscopy in the intracellular recording laboratory, in order to record the activity of the acetylcholinergic neurons, we first attempted to identify the neurons with the highest physical similarity to acetylcholinergic neurons in the LDT. According to previous studies, cholinergic neurons are characterized by polygonal-shaped large soma, while GABAergic neurons are small round-shaped neurons and glutamatergic neurons are intermittent in size and smaller than cholinergic neurons. Accordingly, neurons with polygonal soma size of larger than 30 mm were selected for whole-cell patch clamp recording60,61.
Patch pipettes were filled with internal solution containing (in mM) 140 potassium gluconate, 5 KCl, 10 HEPES, 2 MgCl2, 0.2 EGTA, 2 Na2ATP and 0.4 Na2GTP. The pH and the osmolarity of the internal solution were adjusted to 7.2 (by KOH) and 285 ± 5 mOsm, respectively.
Current clamp whole-cell patch clamp recordings were conducted on LDT neurons as identified by a well-established criteria defined below using a Multiclamp 700B amplifier (Axon Instruments) and signals were digitized by a Digidata 1440 A/D converter (Axon Instruments). Electrophysiological records were filtered at 20 kHz and sampled at 10 kHz. LDT neurons were visualized with a 60 × water immersion objective using Nomarski-type differential interference contrast (DIC) imaging with infrared illumination. After establishment of a giga seal, brief suction was applied to break through the cell membrane for whole-cell configuration. Cells with a seal < 1 GΩ before rupture of the membrane were discarded and the test seal function was constantly employed and monitored throughout the recording to ensure that the seal was stable62.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.0 software for windows. Kolmogorov–Smirnov test was used to determine the normal distribution of the data. To compare the mean level of variables between the groups while accounting for the impacts of the different types of stressors, one-way ANOVA was employed, followed by Tukey's post-hoc analysis. The time spent in the drug-paired compartment before and after conditioning days was compared using repeated measure ANOVA. P < 0.05 was considered statistically significant.
Ethical approval and informed consent
All procedures performed in this study were in accordance with the ethical standards of the ethical committee of Kerman University of Medical Sciences. All experimental procedures were established in accordance with ARRIVE guidelines and were approved by the Animal Research Ethics Committee of the Kerman Neuroscience Research Center (Ethics code: IR.KMU.REC.1397.579).
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
All software applications used are included in this article.
References
Schmidt, M. et al. Maternal stress during pregnancy induces depressive-like behavior only in female offspring and correlates to their hippocampal Avp and Oxt receptor expression. Behav. Brain Res. 353, 1–10 (2018).
Roshan-Milani, S., Seyyedabadi, B., Saboory, E., Parsamanesh, N. & Mehranfard, N. Prenatal stress and increased susceptibility to anxiety-like behaviors: role of neuroinflammation and balance between GABAergic and glutamatergic transmission. Stress 24, 481–495 (2021).
Wang, Y. et al. Sexual differences in long-term effects of prenatal chronic mild stress on anxiety-like behavior and stress-induced regional glutamate receptor expression in rat offspring. Int. J. Dev. Neurosci. 41, 80–91 (2015).
Yao, D. et al. Prolactin and glucocorticoid receptors in the prefrontal cortex are associated with anxiety-like behavior in prenatally stressed adolescent offspring rats. J. Neuroendocrinol. 35, e13231 (2023).
Said, N. et al. Prenatal stress induces vulnerability to nicotine addiction and alters D2 receptors’ expression in the nucleus accumbens in adult rats. Neuroscience 304, 279–285 (2015).
Stratilov, V., Tyulkova, E. & Vetrovoy, O. Prenatal stress as a factor of the development of addictive states. J. Evol. Biochem. Physiol. 56, 471–490 (2020).
Negrón-Oyarzo, I., Neira, D., Espinosa, N., Fuentealba, P. & Aboitiz, F. Prenatal stress produces persistence of remote memory and disrupts functional connectivity in the hippocampal–prefrontal cortex axis. Cereb. Cortex 25, 3132–3143 (2015).
Parsons, R. G. & Ressler, K. J. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 16, 146–153 (2013).
Li, Y.-J. et al. Prenatal stress impairs postnatal learning and memory development via disturbance of the cGMP–PKG pathway and oxidative phosphorylation in the hippocampus of rats. Front. Mol. Neurosci. 13, 158 (2020).
Guan, S.-Z. et al. Effects of maternal stress during pregnancy on learning and memory via hippocampal BDNF, Arc (Arg3. 1) expression in offspring. Environ. Toxicol. Pharmacol. 46, 158–167 (2016).
Yao, D. et al. Hippocampal AMPA receptors mediate the impairment of spatial learning and memory in prenatally stressed offspring rats. J. Psychiatr. Res. 151, 17–24 (2022).
Smith, S. M. & Vale, W. W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. (2022).
Fernandes, S. S. et al. Enhanced cholinergic-tone during the stress induce a depressive-like state in mice. Behav. Brain Res. 347, 17–25 (2018).
Picciotto, M. R., Higley, M. J. & Mineur, Y. S. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129 (2012).
Wang, H. L. & Morales, M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 29, 340–358 (2009).
Weitemier, A., Tsivkovskaia, N. & Ryabinin, A. Urocortin 1 distribution in mouse brain is strain-dependent. Neuroscience 132, 729–740 (2005).
Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36, 165–186 (1983).
Honda, T. & Semba, K. An ultrastructural study of cholinergic and non-cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei in the rat. Neuroscience 68, 837–853 (1995).
Cadet, J. L. Epigenetics of stress, addiction, and resilience: therapeutic implications. Mol. Neurobiol. 53, 545–560 (2016).
Ryabinin, A. E. et al. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol 46, 349–357 (2012).
Volkow, N. D., Koob, G. F. & McLellan, A. T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 374, 363–371 (2016).
Chang, F.-C. & Opp, M. R. Corticotropin-releasing hormone (CRH) as a regulator of waking. Neurosci. Biobehav. Rev. 25, 445–453 (2001).
Deussing, J. M. & Chen, A. The corticotropin-releasing factor family: physiology of the stress response. Physiol. Rev. 98, 2225–2286 (2018).
Romero-Leguizamón, C. R. & Kohlmeier, K. A. Stress-related endogenous neuropeptides induce neuronal excitation in the Laterodorsal Tegmentum. Eur. Neuropsychopharmacol. 38, 86–97 (2020).
Dautan, D. et al. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat. Neurosci. 19, 1025–1033 (2016).
Omelchenko, N. & Sesack, S. R. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J. Comp. Neurol. 483, 217–235 (2005).
Omelchenko, N. & Sesack, S. R. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J. Comp. Neurol. 494, 863–875 (2006).
Forster, G. L. & Blaha, C. D. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur. J. Neurosci. 12, 3596–3604 (2000).
Forster, G. L., Yeomans, J. S., Takeuchi, J. & Blaha, C. D. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J. Neurosci. 22, RC190 (2002).
Nelson, C., Wetter, J., Milovanovic, M. & Wolf, M. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience 146, 41–49 (2007).
Forster, G., Falcon, A., Miller, A., Heruc, G. & Blaha, C. Effects of laterodorsal tegmentum excitotoxic lesions on behavioral and dopamine responses evoked by morphine and d-amphetamine. Neuroscience 114, 817–823 (2002).
Laviolette, S. R., Priebe, R. P. & Yeomans, J. S. Role of the laterodorsal tegmental nucleus in scopolamine-and amphetamine-induced locomotion and stereotypy. Pharmacol. Biochem. Behav. 65, 163–174 (2000).
Razavinasab, M., Sheibani, V., Kohlmeier, K. A., Nazeri, M. & Shabani, M. Hyperexcitability of VTA dopaminergic neurons in male offspring exposed to physical or psychological prenatal stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 101, 109923 (2020).
Dagytė, G., Den Boer, J. A. & Trentani, A. The cholinergic system and depression. Behav. Brain Res. 221, 574–582 (2011).
Williams, M. J. & Adinoff, B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology 33, 1779–1797 (2008).
Kanwal, A. et al. Anti-amnesic activity of Vitex negundo in scopolamine induced amnesia in rats. Pharmacol. Pharm. 1, 1 (2010).
Blokland, A. Acetylcholine: A neurotransmitter for learning and memory?. Brain Res. Rev. 21, 285–300 (1995).
Drachman, D. A. & Leavitt, J. Human memory and the cholinergic system: A relationship to aging?. Arch. Neurol. 30, 113–121 (1974).
Hagan, J. & Morris, R. The cholinergic hypothesis of memory: a review of animal experiments. in Handbook of Psychopharmacology 237–323 (1988).
Unal, C. T., Golowasch, J. P. & Zaborszky, L. Adult mouse basal forebrain harbors two distinct cholinergic populations defined by their electrophysiology. Front. Behav. Neurosci. 6, 21 (2012).
Berger, M. A., Barros, V. G., Sarchi, M. I., Tarazi, F. I. & Antonelli, M. C. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem. Res. 27, 1525–1533 (2002).
Coimbra, B. et al. Impairments in laterodorsal tegmentum to VTA projections underlie glucocorticoid-triggered reward deficits. Elife 6, e25843 (2017).
Kippin, T. E., Szumlinski, K. K., Kapasova, Z., Rezner, B. & See, R. E. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology 33, 769–782 (2008).
Deminière, J. M. et al. Increased locomotor response to novelty and propensity to intravenous amphetamine self-administration in adult offspring of stressed mothers. Brain Res. 586, 135–139 (1992).
Yang, J. et al. Enriched environment treatment counteracts enhanced addictive and depressive-like behavior induced by prenatal chronic stress. Brain Res. 1125, 132–137 (2006).
Ungless, M. A., Argilli, E. & Bonci, A. Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci. Biobehav. Rev. 35, 151–156 (2010).
Shaham, Y. & Hope, B. T. The role of neuroadaptations in relapse to drug seeking. Nat. Neurosci. 8, 1437–1439 (2005).
Polter, A. M. & Kauer, J. A. Stress and VTA synapses: implications for addiction and depression. Eur. J. Neurosci. 39, 1179–1188 (2014).
Smith, R. J. & Aston-Jones, G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct. Funct. 213, 43–61 (2008).
Laplante, D. P. et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr. Res. 56, 400–410 (2004).
Soliani, F. C. et al. Unpredictable chronic prenatal stress and manifestation of generalized anxiety and panic in rat’s offspring. Progr. Neuro-Psychopharmacol. Biol. Psychiatry 85, 89–97 (2018).
Nazeri, M. et al. Psychological or physical prenatal stress differentially affects cognition behaviors. Physiol. Behav. 142, 155–160 (2015).
Aghaei, I., Arjmand, S., Yousefzadeh Chabok, S., Tondar, M. & Shabani, M. Nitric oxide pathway presumably does not contribute to antianxiety and memory retrieval effects of losartan. Behav. Pharmacol. 28, 420–427 (2017).
Iturra-Mena, A., Arriagada-Solimano, M., Luttecke-Anders, A. & Dagnino-Subiabre, A. Effects of prenatal stress on anxiety-and depressive-like behaviours are sex-specific in prepubertal rats. J. Neuroendocrinol. 30, e12609 (2018).
GhotbiRavandi, S., Shabani, M., Bakhshaei, S., Nazeri, M. & Nozari, M. Effects of psychological or physical prenatal stress on attention and locomotion in juvenile rats. Int. J. Neurosci. 131, 233–238 (2021).
Shabani, M. & Naderi, R. Phytohormone abscisic acid elicits positive effects on harmaline-induced cognitive and motor disturbances in a rat model of essential tremor. Brain Behav. 12(5), e2564 (2022).
Naderi, R., Esmaeili-Mahani, S. & Abbasnejad, M. Phosphatidylinositol-3-kinase and protein kinase C are involved in the pro-cognitive and anti-anxiety effects of phytohormone abscisic acid in rats. Biomed. Pharmacother. 96, 112–119 (2017).
Naderi, R., Esmaeili-Mahani, S. & Abbasnejad, M. Extracellular calcium influx through L-type calcium channels, intracellular calcium currents and extracellular signal-regulated kinase signaling are involved in the abscisic acid-induced precognitive and anti-anxiety effects. Biomed. Pharmacother. 109, 582–588 (2019).
Kobrin, K. L. et al. Acquisition of morphine conditioned place preference increases the dendritic complexity of nucleus accumbens core neurons. Addict. Biol. 21, 1086–1096 (2016).
Kohlmeier, K. A. & Polli, F. S. Plasticity in the brainstem: prenatal and postnatal experience can alter laterodorsal tegmental (LDT) structure and function. Front. Synapt. Neurosci. 12, 3 (2020).
Takano, S. et al. Electrophysiological effects of ghrelin on laterodorsal tegmental neurons in rats: an in vitro study. Peptides 30, 1901–1908. https://doi.org/10.1016/j.peptides.2009.07.014 (2009).
Razavinasab, M., Moazzami, K. & Shabani, M. Maternal mobile phone exposure alters intrinsic electrophysiological properties of CA1 pyramidal neurons in rat offspring. Toxicol. Ind. Health 32, 968–979. https://doi.org/10.1177/0748233714525497 (2016).
Acknowledgements
Funding for this study was provided by Iran National Science Foundation INSF (Grant#: 4,005,231) and Kerman University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors have conceived and designed the concept and road map of the study, searched the literature, contributed to acquisition of animal data, and drafted the manuscript. M.R. critically reviewed the manuscript for its content, originality, usage of English language, and accuracy of interpreted data. All authors have made substantive contribution and attest to approving the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shabani, M., Ilaghi, M., Naderi, R. et al. The hyperexcitability of laterodorsal tegmentum cholinergic neurons accompanies adverse behavioral and cognitive outcomes of prenatal stress. Sci Rep 13, 6011 (2023). https://doi.org/10.1038/s41598-023-33016-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-33016-2
This article is cited by
-
Quercetin nanoemulsion ameliorates tremor and neuroinflammatory dysregulation: behavioral and molecular insights in a mouse model of essential tremor
Behavioral and Brain Functions (2025)
-
Effects of subanesthetic dose of ketamine on motor and cognitive outcomes of harmaline-induced essential tremor model: a focus on Lingo-1 and inflammatory pathways
BMC Neuroscience (2025)
-
Co-administration of low-dose-Naltrexone and Carbamazepine remarkedly ameliorate allodynia and cognitive deficit in a rat model of trigeminal neuralgia
Scientific Reports (2025)
-
Unveiling the therapeutic potential of octreotide in treating morphine dependence
Scientific Reports (2025)
-
Maternal prenatal stress induces sex-dependent changes in tRNA fragment families and cholinergic pathways in newborns
Molecular Psychiatry (2025)