Abstract
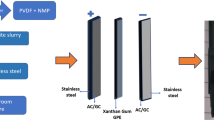
For the first time, electrochemical methods are utilized to study the response of tubulin monomers (extracted from plant source such as Green Peas: Arachis Hypogea) towards charge perturbations in the form of conductivity, conformational changes via self-assembly and adsorption on Au surface. The obtained dimerization and surface adsorption energetics of the tubulins from Cyclic Voltammetry agree well with the literature value of 6.9 and 14.9 kCal/mol for lateral and longitudinal bond formation energy respectively. In addition to the effects of charge perturbations on change in structure, ionic and electronic conductivity of tubulin with increasing load are investigated and found to be 1.25 Sm−1 and 2.89 mSm−1 respectively. The electronic conductivity is 1.93 times higher than the literature value of 1.5 mSm−1, demonstrating the fact that the microtubules (dimer of tubulins, MTs) from plant source can be used as a semiconductor electrode material in energy conversion and storage applications. Thus, motivated by the Monte Carlo simulation and electrochemical results the MTs extracted from plant source are used as cathode material for energy storage device such as Bio-battery and the Galvanostatic Charge/Discharge studies are carried out in coin cell configuration. The configuration of the bio-battery cell is as follows: Al/CB//PP-1M KCl//MTs/SS; where SS and Al are used as current collectors for cathode and anode respectively, Polypropylene (PP) membrane soaked in 1M KCl as electrolyte and Carbon Black (CB) is the anode material. Another configuration of the cell would be replacement of CB by biopolymer such as ethyl cellulose anode (Al/EC/PP-1M KCl/MTs/SS).
Similar content being viewed by others
Introduction
MTs are self-assembled polymeric tubules made of α and β tubulin dimers. MTs are the major constituent of cytoskeleton involved in variety of functions such as cell division, intracellular transport of organelles and prominently neuronal signal transmission1,2,3,4 due to their high ionic conductivity, unique structure and ordered arrangement5. Living cells are known to possess intrinsic electric fields and are sensitive to external electric fields. MTs are influenced by the external electric fields and thereby affect their major role in cell division. As cancer being an uncontrolled cell division6,7,8,9, MTs mechanism of action in treating cancers are also affected by electrical perturbations. It is found in the literature that protein monomers and their polymeric forms differ largely in their conductivity5. Hence proteins in the cells can be regarded as semiconducting in nature10. In the ionic medium inside the cell, counterion condensation effect compensates these unbalanced charges in polymeric forms of protein11. Hence studying the effects of electric field on the monomers and polymer ensembles of tubulin will provide insights in to the role of MTs in signal transmission. MTs are intracellular cytoskeletal cylindrical protein polymer made of tubulin dimer that self-assemble in to MTs under appropriate conditions but also rapidly depolymerize12. These proto filaments form hollow MTs of 15–25 nm diameters and lengths up to few mm, with almost 900 amino acid residues and measures 4 × 5 × 8 nm313. They are involved in many critical cellular functions such as cell division, intracellular transport and signal transduction14. MTs are associated with many proteins to perform various functions. Motor proteins such as kinesin and dynein use MTs as track to carry cargo such as neurotransmitters and deliver them15 (Figs. 1, 2).
MTs are also known to play important role in the functioning of neurons and the brain, hence are said to be responsible for cognition, memory and consciousness16,17. Consciousness is thought to be the emergent phenomena of highly complicated nested complex of neurons within the brain but if we look closer it is indeed due to the quantum effects of the MTs present in the neuronal cells as said in orchestrated OR theory17,18,19. The unit of information processing in brain is the tubulin protein and change in their conformational state20,21. They are known to increase the conductivity of electrolyte solutions by 23%13. This high conductivity can be attributed to the counterion polarization effect where the positive ions such as K+ and Ca2+ adsorb on the highly localized negatively charged surface of MTs and due to the mobility of these ions along the length of the MT, the conductivity increases22,23. They also transmit signals received from the external environment into the cell through the initiation of signal transduction cascade14. Transmission of signal in terms of passage of charged particles such as electron and ions through them is possible because of the nanopores and defects present in their wall24. Cytoskeletal proteins transmit signals in the form of ionic solitons25. Bunch of MTs form bundles in neuronal cells act as bio-electrical transistor to generate electrical signals similar to action potential16. Analogously, here we use electrochemical techniques such as CV as a probe to study the changes in the conformation and conductivity of tubulin protein due to application of charge perturbations. The novelty of this work is that unlike literature10,26,27,28, in which conductivity of MTs were studied using non-electrochemical methods, we use electrochemical methods to study the tubulin monomers instead of MTs and their response to charge perturbations in terms of conductivity and conformational changes through self-assembly and adsorption on Au surface. The free energy of dimerization and surface adsorption were computed by Monte Carlo Simulation and compared with experimental free energy calculated from CV. Coin Cell of configurations Al/CB//1M KCl-PP//MTs/SS and Al/EC//1M KCl-PP//MTs/SS were fabricated and their Galvanostatic Charge/discharge profile and performance is demonstrated.
Methodology
The methodology is to develop general theoretical method to simulate the dynamic behavior of MTs under wide range of conditions similar to experimental conditions like charge perturbations by CV experiments and to validate the theory. From CV, using Randle Sevcik’s Equaion29, the diffusion coefficient of K+ ions in the MT is evaluated. Employing Butler–Volmer equation, the free energy of dynamic instability of MTs is obtained. The exchange current density is written as29
and the heterogeneous rate constant is written via free energy of activation using Arrhenius equation as29
In case of charge perturbation, potential or current (potentiostatic or galvanostatic) is provided as input for MC simulation and the free energy of activation for dynamic instability of MTs in the presence of external perturbation were investigated. The comparison of conductivity (electronic and ionic), between theoretical and experimental procedures can also be made in addition to free energy estimation. The methodology is provided as flow chart in Fig. 3.
Experimental section
As demonstrated elsewhere30, MT is isolated from plant source Arachis Hypogea. The column chromatography eluent is used in KCl electrolyte for electrochemical studies. All experiments were carried out in STP conditions. All the experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, complies with relevant institutional, national, and international guidelines and legislation. All methods were performed in accordance with the relevant guidelines/regulations/legislation. The source of the plant material is as follows:
Nature of Biological resource: Plant.
Common Name: Peanut, Groundnut.
Scientific Name (Genus and Species) : Arachis Hypogea.
Exact part used: Seed.
Source of access (Wild/Culture/Trader): Trader.
Exact place (village, Taluk, District, State) of access of Biological source: Farmers Market, High road, Chengalpattu, Chengalpattu District, Tamil Nadu, India, 603001.
CV studies
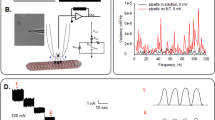
The CV studies were performed for the tubulin fraction obtained from the column chromatography in 0.4 mol/L KCl solution. The electrodes used were (i) Au—working electrode, (ii) Pt wire—counter electrode and (iii) Ag/AgCl—reference electrode employing Zahner-Zennium workstation (Germany). The Area of the electrode exposed to the electrolyte is 0.0005 m2. CV is recorded at a scan rate of 10, 20, 50, 100, 150 and 200 mV/s in the potential window of -1 to 1 V.
Characterization of coin cell Bio-batteries with microtubules as cathode material
The electrochemical characterization of the coin cell of bio-battery fabricated using microtubules as cathode active material coated on SS current collector, carbon black or ethyl cellulose as anode active material coated on Al current collector, PP as membrane soaked in 1 mol/L KCl solution as electrolyte was subjected to Galvanostatic Charge Discharge (GCD) studies employing Zahner Zennium E4 Electrochemical workstation at 0.1 C-rate with the discharge current of 10 mA upto 10,000 cycles.
Theoretical studies
MC code was written using MATLAB software (version 7.5) in which scan rate, distance from the bulk to the electrode surface, chemical potential of α and β tubulin, number of amino acids present in the dimer (N) were provided as input. The random number generator and energy criteria were used to filter out the tubulin which possessed the energy required to move to the next step. The distance from the bulk to electrode surface and number of solvent molecules were fixed and several iterations from 10 to 105 seeds were carried out for convergence. Using the model, energy associated with two configurations α and β tubulin is generated as output. Free energy (ΔG) was calculated as the difference between the energetics of the two configurations of tubulin. This energy difference between the two forms of tubulin is defined as the energy of dimerization of the tubulin monomers. The steps involved in the simulation is provided in Fig. 4. The MC simulation is carried out in STP conditions.
Results and discussion
The energetics obtained from MC simulation and CV experiments for α and β Tubulin were analyzed and compared in the following sections.
CV analysis
The eluent from column is utilized for electrochemical studies in 0.4 mol/L KCl. From Fig. 5, we can infer that at all the scan rates (or load applied), forward scan involved a small peak at 0.3 V triggered by the adsorption of MTs from the solution on Au surface. In the reverse scan, peaks at 0.05 and − 0.3 V were noticed. The former peak could be attributed to the Hydrogen Evolution Reaction (HER)31 whereas the latter one is due to desorption of MTs from the Au surface to the electrolyte solution as can be visualized in Fig. 5. The reversible nature of 0.3 V peak indicates that the adsorbed MT comes back to the electrolyte without any degradation on Au surface. As the applied load increases, the tubulin adsorption process becomes more facile because of the decrease in activation energy (cf. “Free energy of adsorption of MT on Au surface (ΔG) calculated from CV” section). As sulphur has natural affinity towards Au, sulphur containing amino acids such as cysetine and methionine present in tubulin interacts with the Au electrode via Au–S bond32. The first image (I) in Fig. 6 shows the electrochemical interface with the electrolytic solution containing the individual tubulin units forming dimers and diffusing towards the electrode eventually covering its surface with proto-filament chains. The second image (II) of Fig. 6 shows the interaction between the amino acids such as cysteine, methionine, phenylalanine, tyrosine and tryptophan residues of the protein chain with the Au surface.
Free energy of adsorption of MT on Au surface (\(\Delta {\varvec{G}}\) ) calculated from CV
The Free energy of adsorption of MT on Au surface (\(\Delta {\varvec{G}}\) ) can be calculated via Butler-Volmer kinetics. \(\Delta {\varvec{G}}\) is related to ip via the following equations
where ip denotes peak current from CV in Amps, A is the area of electrode in cm2, h represents planks constant (Js), kB depicts Boltzmann constant in JK−1, T is the temperature (298 K), C being the concentration of MTs in mol/cm3 and R represents the gas constant (Jk−1 mol−1). From CV, ip at different load is utilized to evaluate the corresponding \(\Delta \mathbf{G}\) as provided in Table 1. As the load increases, ip increases by 5 times and \(\Delta \mathbf{G}\) decreases by 40 meV (cf. Figure 7). This energy is nearly equivalent to free energy of tubulin immobilization33 (50 meV) on MTs, which indicates the incorporation of tubulin (monomers) in MTs(polymer) with application of load. Thus, it could be inferred that with increased charge perturbation, the feasibility of MT being adsorbed on the Au surface increases. Since there is no degradation seen in CV analysis, \(\Delta \mathbf{G}\) shows very minimal variation with the load applied (40 meV).
Conductivity of MT from CV studies
The charge-transfer resistance (Rct) and electronic conductivity of tubulin dimers were calculated using the following Equations34 and the values are given in Table 1.
It is found that with increase in applied load, ip increases indicating a linear proportionality between the load and the intensity of peak current. From Table 1, it had been observed that the resistivity (ρ) of the dimers decreased linearly with increasing load. The electronic conductivity (σ) showed an exponential increase with increasing load. Hence it is concluded that as the magnitude of the voltage applied increases, the resistance and the free energy of interaction of the dimers decreases, thus they become more reactive to their surroundings by taking up the charged particles around them and conducting the positive ions such as K+ from bulk to the Au surface.
Dynamic stability of MTs on Au surface
Figure 8 shows the evolution of ΔG during the transfer of tubulin monomers to Au surface from the bulk of the solution. Steps (I–V) indicate processes by which the tubulin units diffuse towards the Au surface. As observed, upon experiencing higher load in MΩ, a decreasing trend in the ΔG with applied load indicates facile dimerization of α and β tubulin monomers into MTs. Initially the monomer units coexisted without strong interaction between them (I). As the load applied per second increases, ΔG slowly decreases with the α and β-tubulin interacting to form dimers (II) via electrostatic interaction, H-bond formation and vanderwaals forces35. These dimers adsorb on Au surface due to the interaction between amino acid residues in tubulin such as cysteine, methionine, tyrosine and Au surface. The interaction between Au and tyrosine, tryptophan is through conjugated π- electrons and in the case of cysteine and methionine via hetero sulphur atoms32 as represented in Fig. 8. Many such dimers start covering the electrode (Au) surface (IV) and due to the crowding of dimer units, they associate themselves as self-assembly to form linear polymer filaments (V) and attain stability. As the applied load increases, decrease in ρ (increase in conductivity σ) with simultaneous decrease in ΔG had been noticed. This could be attributed to the self-assembly of monomers forming dimers and the dimers interacting with Au surface. Free monomers in the solution have high energy and when they assemble in to ensembles this energy reduces. In the case of free monomers, the electronic band gap between the conduction and valence band is large and when they start aggregating in an orderly manner, this gap decreases5, making the conductivity (σ) to increase as supported by decrease in resistivity (ρ) in Fig. 8. Conductivity (σ) measurements of several proteins under various conditions indicated a band gap or activation energy of the order of 3 eV10,36,37. Thus, from Fig. 8 which represents the phase transition in MT due to charge perturbations, the maximum limit of ΔG is found to be 1.22 eV and is in satisfactory agreement with the dimerization energy (1.10 eV) of tubulin monomers27,28. The difference in ΔG with respect to the difference in load is nearly 40 meV, such small variation may be due to the conformational change of independent α and β tubulin subunits to α, β-dimer and their incorporation into MT33. Thus Fig. 8, clearly indicates the dimerization of tubulin monomers, its self-assembly into linear proto filaments and adsorbing on Au surface with increase in load applied. It could also be inferred that electronic conductivity (σ) of tubulin monomers (dimerizing and self-assembling) increased upon transfer from bulk to Au/electrolyte interface. The electronic conductivity (σ) evaluated from CV studies is of the order of 0.86 to 3.89 mSm−1 demonstrating the increase in electrical conductivity (σ) by 4.58 times for 20 times increase in the load. This supported the fact that proteins can be semiconducting in nature10, especially MTs and can be of great importance in bioelectronic applications such as biodegradable batteries, pace makers so on and so forth. In addition, Figs. 8 and 9, explicitly depicts the dynamic nature of the MTs formed on Au surface to the self-assembly of tubulin monomers in bulk of the electrolyte upon charge perturbations.
MC simulation analysis
In order to support the experimental data and to get a clear insight about the interfacial reactions, the adsorption of the tubulin proteins on Au surface was modelled using MC simulation.
Assumptions made:
-
Simulation box walls were assumed to be the surface of the electrode (Au)
-
The distance from the center of the box to the walls is maintained constant; dtotal = 690 Å
-
Number of protein units was increased from 1 to 1500; a = 1 to 1500
-
With increasing tubulin units, the concentration per unit area increases.
-
Figure 9 is the analogous representation of electrode surface, Inner Helmholtz Plane (IHP), Outer Helmholtz Plane (OHP) and bulk of the electrolyte, whereas when the protein moves from bulk to the metal surface, its concentration decreases.
-
Figure 9, mimics the electrochemical interface and bulk of the solution with protein units in the center (bulk) and they move from the center to the edge of the box (electrode surface)
-
The iteration continues till the distance between the protein units and the electrode surface (the walls of the box) becomes less than the critical length which is assumed in the present scenario as the Au–S bond distance, d1.
$$while \, d_{total} \ge d_{1}$$ -
The energetics involved in each step movement of the protein units inside the cubic box is calculated using Eqs. (8–15).
-
The difference in the energy associated with the movement of α and β tubulin is calculated to be the energy of dimerization of tubulins on Au surface into MTs (Fig. 10)
-
The tubulin unit dimensions are 4 × 5 × 8 nm3 as represented in Fig. 11.
Adsorption energetics of MT-Au from MC analysis
A model was developed using MATLAB in which the simulation box is a cube, whose side length is considered as 2 × dtotal. This value is fixed as 690 Å. The center of the box is assumed to be the bulk of the electrolyte and walls are the electrode surface. The number of tubulin units and the water molecules are assigned as input. Random number is generated employing the criteria
where F represents Faraday constant (C/mol), µ1 and µ2 denotes the chemical potential (eV) of α and β tubulin units respectively, R being the Gas constant (J/K/mol) and T is the working temperature in K. The tubulin units were allowed to move from the center of the box to the walls, the distance covered by a single tubulin unit during each step (d2) is given by the following equation
where x is the hydrodynamic radius/solvation radius of tubulin monomer in the electrolyte (3 Å), \(\Delta {\varvec{d}}\) is the distance covered in each step, t is the time scale of applied potential (ephiapp), teq is the equilibration or relaxation time between one step and another step, d1 is the Au–S bond distance (2.39 Å)36. The product of solvation radius of each tubulin along with the time fraction will give us the distance that can be covered during single step. Using MC simulation, the energy values assigned are used to filter the tubulin units which has energy value above or equal to a critical energy value employing Arrhenius equation as criteria; (\({e}^{-\left(F\left({\mu }_{2}-{\mu }_{1}\right)/RT\right)})\), those tubulin units which possess this minimum energy will move to next step. This process is repeated for many iteration/seeds to check reproducibility.µ1 and µ2; the chemical potential of α and β tubulin is defined as the energy possessed by them without the externally applied potential and is given by the below equations
where \({E}_{i1}\) and \({E}_{i2}\) are energy associated with the two configurations of tubulin given by the sum of individual α/β energy, distance fraction and miscellaneous energy as given below
Eamt and Ebmt represents the interaction free energy of α-tubulin and β-tubulin with Au surface respectively (Eamt = 0.286 eV, Ebmt = 0.293 eV)32
The miscellaneous energy is the solvation energy of the tubulin units, i.e., H-bonding and is given by the below equation as the sum of distance covered and applied potential over the total box size
The difference between Ei1 and Ei2 is considered as energy of dimerization, Ead
The process is repeated for 103 to 105 seeds to attain convergence and reproducibility (cf. Figure 2).
Variation of Ead with applied load
From Fig. 10, it is observed that with increase in the applied load an exponential decrease in the dimerization energy (Ead) is observed in the system. Initially at lower load, the relaxation time for the tubulin proteins is very high between any applied potential at any instant. Thus the equilibrium between dimerization and de-dimerization process happens at faster rate and hence the higher barrier for dimerization. While as the load increased, high potential pulse is applied per second, dimerization happens instantly without de-dimerizing. Thus, the energy barrier for dimerization diminishes and hence the dimer formation become feasible. In addition, with increasing load, the monomer units are driven more towards the electrode surface, which makes the dimerization and subsequent adsorption process more facile and highly feasible. Figure 10 shows the load dependency of Ead, as the load applied increases, the Ead value decreases, which leads to the conclusion that applied load makes the adsorption of monomer units on the Au surface and dimerization easier which is evident from the exponential drop in the dimerization energy with increasing load. As the voltage applied per second is increased in our model the mobility of monomers towards the Au surface increases and dependency is given by the equation
This equation signifies that in the absence of load (L → 0), the energy of dimerization is 3.0 eV. This is in good agreement with the experimental values of 2.6–3.1 eV found by Eley et al.37 and is approximately equivalent to the forbidden band width between the highest filled and the unfilled band calculated using a simple (Hückel type) LCAO MO method (3.05 eV) in proteins stating the possibility of conduction in proteins like semiconductors38,39,40.
Comparison of ΔG from CV and Ead from MC simulation
For the concentration of 1 mmol/L MTs, experimentally obtained ΔG and the theoretically calculated dimerization energy Ead were compared as shown in Fig. 12. It was found to be linear with a slope of 0.055 and an intercept of 1.10. This signifies that for every 1.0 eV of the dimerization energy there is a 0.055 eV change in the activation energy. The intercept signifies that in the absence of any dimerization process happening in the system (Ead → 0); the average activation energy of tubulin would be 1.10 eV. It is shown in literature41 that the contribution of interfacial hydrogen bonding to the free energy of MT formation from α and β lattices as 462 kJ/mol (4.80 eV). Thus, the activation energy of 1.10 eV being comparatively lesser due to the conditions of the electrochemical setup, solvent, external electric fields and accounts only for the dimerization of α and β tubulin. Upon comparing the theoretical and experimental energetics, it can be inferred that there is exponential variation in the energetics with change in load. As the load increases, the rate of the electron transfer reaction at the Au surface takes place efficiently due to decrease in the energetics as indicated by the exponential decay in Fig. 12. From Fig. 12, it is seen that the experimental value is lower than the theoretical value. The justification behind this higher theoretical value could be attributed to the absence of ascribing parameters for dissociation of tubulin dimers on the Au surface in the MC simulation. It is also observed from Fig. 11 that as the load increases the theoretical and experimental values converge. This convergence demonstrates the diminishing of degradation of the tubulin dimers on the Au surface with increase in the load. The rationale behind this is the fact that, relaxation time of dimers between two impulse of potentials per second is very low at higher load conditions and hence at any instant, the dimers formed on Au surface gets involved in the formation of linear protofilament of MT rather than undergoing dissociation. With increase in the number of tubulin units in the simulation box, exponential decay of the energetics is noticed for all load. The concentration of extracted tubulin in the experiments is 1 mM (a = 1500 units), at that number of units (cf. Fig. 13) the experimental energetics was 1.10 eV but the theoretical values were higher. It is clear from Table 2 that at load of 1.81 to 2.21 MΩ, the energy difference between theoretical and experimental value ranges from 0.69 to 0.38 eV respectively. As can be verified by the literature, the difference in energetics could be attributed to the longitudinal free energy of dissociation of MTs (ca. 0.64 eV)27. This difference in energy varies for different loads and this additional energy in theoretical value is due to the fact that the dimerization energy is higher than the activation energy of tubulin and also the linear protofilament formation of tubulin dimers at higher loads. As the load increases, we observe an exponential decrease in the activation energy and dimerization energy, this is due to the interaction between monomers leading to the formation of aggregates such as dimers and oligomers while being incorporated into MT33, which is clear from the rate of energy drop (40 meV). Initially in their free form when the units exists as monomers the energy values are high. With increase in applied load, this energy drops indicating decrease in activation energy which leads to facile interaction between the monomer units and also facilitates their adsorption on the electrode surface. This decreasing trend in energy is seen both in experimental and theoretical results. This variation in energy is an indication of the process occurring at the interface. Thus, it is clear from Fig. 14, that the monomers are forming dimers, oligomers and MT ensembles and this led to a drop in energy. The dimerization energy calculated from MC simulation (Ead) and the activation energy calculated from CV(∆G) are compared and the difference between them is plotted with increasing load in Fig. 15 and provided in Table 2. It is seen that the difference reduces with applied load indicating a convergence between these two values and this difference can be attributed to the latitudinal (0.3 eV)27 and longitudinal (0.64 eV)27 free energy of dissociation of tubulin-tubulin bonds in MTs.
Ionic conductivity of the electrolytic solution from MC studies
Ionic conduction occurs in MTs due to two reasons (i) one being the highly negatively charged C terminal chain of the tubulin monomer and (ii) second being the pores between the tubulin dimers which has properties like ion channels42,43. The nanopores present in the walls of MTs helps them in passage of ions through the channels to the lumen of the hollow tubes (cf. Fig. 16). The pore size is big enough for K+ ions to pass through but not Ca2+ and Na+. This high negatively charged C terminal chain of the dimer is projected outside on the periphery such that the surface charge density is high and due to this counterions adsorb easily on the surface. The MT assembly consists of two types of lateral arrangement: (i) α-tubulin-α -tubulin, β-tubulin- β-tubulin ie α-tubulin/ β-tubulin in one protofilament lies beside its same counterpart in the neighboring protofilament (ii) α-tubulin-β-tubulin ie α-tubulin in one protofilament lies beside/β-tubulin in the neighboring protofilament and vice versa constituting the A lattice and B lattice respectively44. The ionic conductance of the electrolyte was calculated using the formula
where G-Conductance of the electrolyte in mhos, \({{\Lambda }^{0}}_{{K}^{+}}\)-Molar conductance of K+ in mhos, C—concentration of KCl in the electrolyte in mol/L, A—Area of the cross section of electrodes in cm2, l-distance between the electrodes in cm. The concentration of KCl is varied from 0.1 to 0.8 mol/L and the conductance is calculated for varying box dimensions (in turn concentration of MTs) and plotted as seen in Fig. 15. The molar ionic conductance of K+ ions is 73.5 S cm2/mol/L45 and the calculated value of ionic conductance for 1 mM tubulin at 0.4 mol/L KCl is 5.53 × 10−4 µS (ionic conductivity being 1.1 S m−1). It is seen that there is a sudden drop in conductance when the concentration of MTs reached a critical limit, with further increase in the concentration of MTs, the conductance becomes constant and independent of the concentration. This indicates that the individual tubulin units assemble into a particular conformation, till the critical point and the conductance decreases. After the attainment of critical or saturation point, conductance remains constant with variation in concentration. This behavior is observed for all concentrations of KCl. Analogous results had been reported in the literature, that with increase in the concentration of MT the conductance increases approximately by 0.2 mS up to 60 Hz of frequency of the AC signal and beyond this frequency the conductance starts to decrease and remains constant11 (Supplementary Information 1).
Diffusion coefficient of the electrolyte from CV studies
Using Randle Sevcik’s Eq. 29 for quasi-reversible system the diffusion coefficient was calculated from ip
The mobility of a species in electrolyte, K+ is directly proportional to the diffusion coefficient of that particular species and is given by Einstein–smoluchowski Equations35 as follows
Diffusion coefficient (D) from Randle Sevcik’s equation tells us about the extent of diffusion of analyte (tubulin) and its dependency on applied load. D is linearly proportional to the load in a diffusion limited process. Diffusion coefficient of tubulin in the electrolyte for varying load along with their mobility are given in Fig. 17. From Fig. 17, we infer that with increase in load, diffusion coefficient and mobility follow drastic pattern, increases and decreases with every load application, forming three spikes indicating the increased mobility of monomers in the electrolyte, followed by sudden drop. The reason behind this kind of variation is due to the shift in equilibrium between aggregated monomers (oligomers, dimers) and free monomer forms of tubulin. The aggregated tubulin has less mobility and D value whereas the free forms are able to move unhindered, hence showing a spike in the values of mobility and D, resulting in constant association and dissociation among themselves leading to dynamic instability. The average value of D is obtained as 1.95 × 10–7 cm2/s in agreement with the literature46,47 value of (4.5 ± 0.2) × 10−7 cm2/s for labelled tubulin at nano molar concentration. The calculated average value of mobility for tubulin is 7.73 × 10–6 cm2/Vs. Hence the electrochemical perturbations are clearly making the tubulin units associate and dissociate causing the rise and fall of Diffusion coefficient and mobility values with load.
Galvanostatic charge/discharge profile of non-enzymatic bio-battery coin cell
Theoretical specific capacity of MTs is calculated from the formula.
Theoretical specific capacity = zF/M; where z is the charge, F is Faraday’s constant and M is the molecular weight of the material under consideration. In the present studies, charge of intercalation or deintercalation in the MTs would be one (K+ ions); F = 96,500 Cmol−1, the theoretical specific capacity of MTs were obtained as 89.35 mAh/g. For the charging current is 10 mA and discharging capacity is 89.35 mA, the C-rate obtained is 0.1 C.
The GCD profile of the bio-battery coin cell is represented in Fig. 18a. The cycling of charge/discharge is carried out at 10 mA discharge current. The coin cell is stable up to 10,500 cycles and 175 h at 0.1 C-rate. The specific capacity of Non-enzymatic Bio-battery cell is calculated at every Δt(s) for the discharge current of I (Amps) from the GCD profile for the electroactive mass m (gms) as follows:
Analogously,
Thus, at 0.1C rate of discharge, the initial specific capacity and specific capacitance of 63.2 mAh/g and 173.65 F/g respectively during discharge with Δt = 90 s, I = 1A, m = 0.5 g, ΔV = 0.214 V. The subsequent cycles show slight variation in the capacity and capacitance leading to steady decrement at every cycle and leveled at 36.35 mAh/g and 101.33 F/g after 10,000 cycles. This corresponds to 42.48% capacity retention after 10,000 cycles (cf. Figure 18). This demonstrates that the bio-battery cells fabricated in the present invention possess very long cycling stability upto 10,000 cycles at 0.1 C rate and can be utilized in low power electronic devices and power bank applications. The fabricated full cell is shown in Fig. 19.
The energy density of the Bio-Battery cells is calculated employing the equation
For 1 kg battery, with nominal voltage of 0.214 V and rated battery capacity being 63.2 mAh/g, the gravimetric energy density is obtained as 13.53 Wh/kg attributed to the protein type Microtubules (MTs) cathode and Carbon Black anode with the full cell configuration of Al/CB//PP-1MKCl//MTs/SS. The bio-battery cell of second configuration Al/EC//PP-1MKCl//MTs/SS possessed much less performance with stability upto 500 cycles with lower specific capacity and gravimetric energy density. Hence of all configurations attempted, the one with carbon-based anode such as carbon black, graphite or graphene possessed good performance as biomolecules-based energy storge devices for low power electronic device charging and power banks.
Perspectives and summary
This study will pave way to understand the utility of MTs, synthetic MTs and other similar compounds in bioelectronic applications such as biodegradable batteries, pacemakers so on and so forth. The estimation of electrical conductivity of MTs in liquid phase becomes an interesting study due to the fact that MTs are involved in the electron and proton transport (i) in the cell for phototaxis process, (ii) in centrioles and (iii) in cells coupled with GTP hydrolysis. In addition, as MTs can act as connectors for electric pulse transmission in axons of nerve cells, current study can help in understanding the physical processes associated with consciousness studies. Moreover, as plant MTs are found to handle electromagnetic signals and electrical signals5,16,23, present investigation will be of much utility in the studies of quantum information and signal processing in axons involving MTs. The novelty of the present work lies in the fact of utilization of less computer intensive MC simulation coupled with macro characteristics of solvent to understand and evaluate the free energy and kinetic parameters of MTs. Validating the theory with simple electrochemical techniques to measure electronic conductivity, free energy of activation, electrode kinetics due to charge perturbations so on and so forth. Analogously, from the free energetics of the MT conformation, the conductivity can be estimated as suggested in Butler- Volmer equation. Thus, the process associated with the conformation, energetics and ionic as well as electronic conductivity of MTs can be validated. Thus, from CV studies, the reversible peak at 0.3 V indicated the reversible desorption of MT from Au surface without any degradation. As explained in “Free energy of adsorption of MT on Au surface (ΔG) calculated from CV” section, with increase in the applied load, the reversible tubulin adsorption on Au surface becomes more facile with decrease in the activation energy. The decrement in activation energy with increase of load, is approximately equivalent to free energy of tubulin immobilization33 (50 meV) on MTs. Thus, with increase in the charge perturbation, the feasibility of MTs adsorbed on the Au surface increases with very minimal variation with load applied (40 meV). The charge transfer resistance is also found to decrease with increase in applied load and as a consequence, tubulin become more reactive to the electrolyte medium by associating the charged counter ions around them and conducting the positive ions such as K+ from bulk to the Au surface. The electronic conductivity (σ) ranges from 0.86 to 3.89 mSm−1 (as obtained from CV studies) demonstrating 4.58 times increase in σ for 20 times increase in the load. This supported the fact that proteins can be semiconducting in nature10, especially MTs and can be of great importance in bioelectronic applications such as biodegradable batteries, pace makers so on and so forth. In addition, Fig. 7, explicitly depicts the dynamic nature of the MTs formed on Au surface to the self-assembly of tubulin monomers in bulk of the electrolyte upon charge perturbations. MC simulation showed (cf. Figure 11) the load dependency of Ead. As the load applied increases, the Ead decreased, indicating facile adsorption of monomer units and dimerization of tubulin monomers on the Au surface. From Eq. (16), as L → 0, the energy of dimerization will be 3.0 eV which is in good agreement with the literature reported experimental value of 2.6–3.1 eV by Eley et al.37. Thus the present methodology could predict the experimental value of forbidden band width between the highest filled and the unfilled band calculated in proteins stating the possibility of conduction in proteins like semiconductors38,39,40. The calculated value of ionic conductance for 1 mM tubulin at 0.4 mol/L KCl is 5.53 × 10–4 µS (ionic conductivity being 1.1 S m−1) from MC simulation whereas the electronic conductivity obtained being 2.89 mS m−1 and is 1.93 times higher than the literature. Thus, the current MC simulation in conjunction with CV studies can predict the dynamic stability of MTs and tubulin monomers on Au surface due to external charge perturbations effectively. From the predictions and experimental validation, it is concluded that MTs extracted from plant sources such as Arachis hypogea can act as a semiconductor material on Au surface in KCl environment. Thus, these studies paved way for the future applications of MTs as electrode materials in bioelectronic applications such as biodegradable batteries, pace makers so on and so forth. With the results of theoretical model and the electrochemical experimental validation, two-coin cells of bio-battery fabricated employing MTs as cathode material and CB in cell 1, EC in cell 2 as anode material, PP soaked in 1 mol/L KCl as membrane and electrolyte respectively. At 0.1C rate of discharge, the initial specific capacity and specific capacitance of 63.2 mAh/g and 173.65 F/g respectively during discharge with Δt = 90 s, I = 1A, m = 0.5 g, ΔV = 0.214 V were noticed for cell 1. The subsequent cycles show slight variation in the capacity and capacitance indicating steady decrement at every cycle and leveled at 36.35 mAh/g and 101.33 F/g after 10,000 cycles. This corresponds to 42.48% capacity retention after 10,000 cycles. This demonstrates that the bio-battery cells fabricated in the present investigation possess very long cycling stability upto 10,000 cycles at 0.1 C rate and can be utilized in low power electronic devices and power bank applications (cf. Fig. 18).
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- CV:
-
Cyclic voltammetry
- GCD:
-
Galvanostatic charge discharge
- CB:
-
Carbon black
- MTs:
-
Microtubules
- SS:
-
Stainless steel
- Al:
-
Aluminium
- STP:
-
Standard temperature and pressure
- EC:
-
Ethyl cellulose
- PP:
-
Polypropylene
- MC:
-
Monte carlo
- ED:
-
Energy density
- D:
-
Diffusion co-efficient
- F:
-
Faraday’s constant
- R:
-
Gas constant
- T:
-
Temperature in K
- k:
-
Boltzmann constant in J/K
- h:
-
Planck’s constant in Js
- µ:
-
Mobility
- i :
-
Current density in Acm−2
- G:
-
Conductance of the electrolyte in S
- \({{\Lambda }^{0}}_{{K}^{+}}\) :
-
Molar conductance (mhos) of K+
- C:
-
Concentration of KCl in the electrolyte mol lit−1
- A:
-
Area of the cross section of electrodes in cm2
- l:
-
Distance between the electrodes in cm
- E ad :
-
Dimerization energy in eV
- µ1 and µ2 :
-
The chemical potential of α and β tubulin in eV
- ρ:
-
Resistivity of the dimers in Ohms
- σ:
-
Conductivity of the dimers in Sm−1
- Rct :
-
Charge-transfer resistance in Ohms
- \(\Delta G\) :
-
Free energy of adsorption of MT on Au surface in eV
- ket :
-
Heterogeneous rate constant in ms−1
References
Janke, C. The tubulin code: Daltons components, readout mechanisms, and functions. J. Cell Biol. 206, 461–472 (2014).
Li, H., DeRosier, D. J., Nicholson, W. V., Nogales, E. & Downing, K. H. Microtubule structure at 8 Å resolution. Structure 10, 1317–1328 (2002).
Tuszyński, J. A., Hameroff, S., Satarić, M. V., Trpisova, B. & Nip, M. L. A. Ferroelectric behavior in microtubule dipole lattices: Implications for information processing, signaling and assembly/disassembly. J. Theor. Biol. 174, 371–380 (1995).
Alushin, G. M. et al. High-resolution microtubule structures reveal the structural transitions in αβ-Tubulin upon GTP hydrolysis. Cell 157, 1117–1129 (2014).
Tuszynski, J. A. The bioelectric circuitry of the cell. 2019 Aug 28. In Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018 [Internet] (eds Makarov, S. et al.) (Springer, 2019).
Minoura, I. & Muto, E. Dielectric measurement of individual microtubules using the electroorientation method. Biophys. J. 90(10), 3739–3748 (2006).
Van den Heuvel, M., De Graaff, M., Lemay, S. & Dekker, C. Electrophoresis of individual microtubules in microchannels. Proc. Natl. Acad. Sci. 104(19), 7770–7775 (2007).
Kirson, E. D. et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. 104, 10152–10157 (2007).
Tuszynski, J., Wenger, C., Friesen, D. & Preto, J. An overview of sub-cellular mechanisms involved in the action of TTFields. Int. J. Environ. Res. Public Health 13, 1128 (2016).
Rosenberg, B. Electrical conductivity of proteins. Nature 193(4813), 364–365 (1962).
Santelices, I. B. et al. Response to alternating electric fields of tubulin dimers and microtubule ensembles in electrolytic solutions. Sci. Rep. 7(1), 9594 (2017).
Mitchison, T. & Marc, K. Dynamic instability of microtubule growth. Nature 312(5991), 237 (1984).
Craddock, T. J. A. & Jack, A. T. On the role of the microtubules in cognitive brain functions. NeuroQuantology https://doi.org/10.14704/nq.2007.5.1.116 (2007).
Gundersen, G. G. & Cook, T. A. Microtubules and signal transduction. Curr. Opin. Cell Biol. 11(1), 81–94 (1999).
Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279(5350), 519–526 (1998).
Cantero, M. D. R., Villa Etchegoyen, C., Perez, P. L., Scarinci, N. & Cantiello, H. F. Bundles of brain microtubules generate electrical oscillations. Sci. Rep. 8(1), 11899 (2018).
Barlow, P. W. The natural history of consciousness, and the question of whether plants are conscious, in relation to the Hameroff-Penrose quantum-physical ‘Orch OR’theory of universal consciousness. Commun. Integr. Biol. 8(4), e1041696 (2015).
Stuart, H. Quantum computation in brain microtubules? The Penrose-Hameroff ‘Orch OR’ model of consciousness. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 356(1743), 1869–1896 (1998).
Hameroff, S. & Penrose, R. Orchestrated reduction of quantum coherence in brain microtubules: A model for consciousness. Math. Comput. Simul. 40(3–4), 453–480 (1996).
Craddock, T. J. A., Jack, A. T., Avner, P. & Holly, F. Microtubule ionic conduction and its implications for higher cognitive functions. J. Integr. Neurosci. 9(02), 103–122 (2010).
Sekulić, D. & Satarić, M. V. An improved nanoscale transmission line model of microtubule: The effect of nonlinearity on the propagation of electrical signals. Facta Univ. Ser. Electron. Energetics 28(1), 133–142 (2014).
Frieden, B. R. & Gatenby, R. A. Signal transmission through elements of the cytoskeleton forms an optimized information network in eukaryotic cells. Sci. Rep. 9(1), 6110 (2019).
Dent, E. W. & Baas, P. W. Microtubules in neurons as information carriers. J. Neurochem. 129(2), 235–239 (2014).
Freedman, H. et al. Model of ionic currents through microtubule nanopores and the lumen. Phys. Rev. E 81(5), 051912 (2010).
Satarić, M. V., Sekulić, D. & Živanov, M. Solitonic ionic currents along microtubules. J. Comput. Theor. Nanosci. 7(11), 2281–2290 (2010).
Umnov, M. et al. Experimental evaluation of electrical conductivity of microtubules. J. Mater. Sci. 42(1), 373–378 (2007).
Kononova, O. et al. Tubulin bond energies and microtubule biomechanics determined from nanoindentation in silico. J. Am. Chem. Soc. 136(49), 17036–17045 (2014).
Detrich, H. W. III., Johnson, K. A. & Marchese-Ragona, S. P. Polymerization of Antarctic fish tubulins at low temperatures: Energetic aspects. Biochemistry 28(26), 10085–10093 (1989).
Padmapriya, S. & Harinipriya, S. Hydrogen storage capacity of polypyrrole in alkaline medium: Effect of oxidants and counter anions. J. Market. Res. 8(5), 4435–4447 (2019).
Harinipriya, S., Aarat, K. & Amit, K. M. Physiochemical characterization of tubulin from Arachis hypogaea. Synth. Met. 220, 86–94 (2016).
Sukeri, A. & Bertotti, M. Nanoporous gold surface: An efficient platform for hydrogen evolution reaction at very low overpotential. J. Braz. Chem. Soc. 29(2), 226–231 (2018).
Hoefling, M., Iori, F., Corni, S. & Gottschalk, K. E. Interaction of amino acids with the Au (111) surface: Adsorption free energies from molecular dynamics simulations. Langmuir 26(11), 8347–8351. https://doi.org/10.1021/la904765u (2010).
Mozziconacci, J., Sandblad, L., Wachsmuth, M., Brunner, D. & Karsenti, E. Tubulin dimers oligomerize before their incorporation into microtubules. PLoS ONE 3(11), e3821 (2008).
Bard, A. & Falkner, L. R. Electrochemical Methods 103 (Wiley, 1990).
Sept, D., Baker, N. A. & McCammon, J. A. The physical basis of microtubule structure and stability. Protein Sci. 12(10), 2257–2261 (2003).
Bürgi, T. Properties of the gold–sulphur interface: From self-assembled monolayers to clusters. Nanoscale 7(38), 15553–15567 (2015).
Cardew, M. H. & Eley, D. D. The semiconductivity of organic substances. Part 3.—Haemoglobin and some amino acids. Discuss. Faraday Soc. 27, 115–128 (1959).
Ladik, J. Energy band structure of proteins. Nature 202, 1208–1209. https://doi.org/10.1038/2021208a0 (1964).
Rosenberg, B. & Postow, E. Semiconduction in proteins and lipids—Its possible biological import. Ann. N. Y. Acad. Sci. 158(1), 161–190 (1969).
Evans, M. G. & Gergely, J. A discussion of the possibility of bands of energy levels in proteins electronic interaction in non-bonded systems. Biochem. Biophys. Acta. 3, 188–197 (1949).
Ayoub, A. T., Craddock, T. J., Klobukowski, M. & Tuszynski, J. Analysis of the strength of interfacial hydrogen bonds between tubulin dimers using quantum theory of atoms in molecules. Biophys. J. 107(3), 740–750 (2014).
Shen, C. & Guo, W. Ion permeability of a microtubule in neuron environment. J. Phys. Chem. Lett. 9(8), 2009–2014 (2018).
Craddock, T. J., Tuszynski, J. A., Priel, A. & Freedman, H. Microtubule ionic conduction and its implications for higher cognitive functions. J. Integr. Neurosci. 9(02), 103–122 (2010).
McIntosh, J. R., Morphew, M. K., Grissom, P. M., Gilbert, S. P. & Hoenger, A. Lattice structure of cytoplasmic microtubules in a cultured Mammalian cell. J. Mol. Biol. 394(2), 177–182 (2009).
Benson, G. C. & Gordon, A. R. A reinvestigation of the conductance of aqueous solutions of potassium chloride, sodium chloride, and potassium bromide at temperatures from 15 to 45 °C. J. Chem. Phys. 13(11), 473–474 (1945).
Krouglova, T., Vercammen, J. & Engelborghs, Y. Correct diffusion coefficients of proteins in fluorescence correlation spectroscopy. Application to tubulin oligomers induced by Mg2+ and Paclitaxel. Biophys. J. 87(4), 2635–2646 (2004).
Stracke, R., Böhm, K. J., Wollweber, L., Tuszynski, J. A. & Unger, E. Analysis of the migration behaviour of single microtubules in electric fields. Biochem. Biophys. Res. Commun. 293(1), 602–609 (2002).
Acknowledgements
The authors acknowledge the internal funding by Ram Charan Company Pvt Ltd – Entity1, Chennai, Tamil Nadu, India. The authors also acknowledge the HPCC Facility of SRMIST, Kattankulathur, Chennai, Tamil Nadu, India.
Author information
Authors and Affiliations
Contributions
Mrs. P.L.—modelling and carrying out experiments Mr. K.A.P.—idea generation and discussion Dr. S.H.—idea generation, theory and experiments design, data analysis and manuscript writing Dr. V.S.—Theortical studies, data analysis, discussions and manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palicha, K.A., Loganathan, P., Sudha, V. et al. Monte Carlo simulation and experimental validation of plant microtubules cathode in biodegradable battery. Sci Rep 13, 10393 (2023). https://doi.org/10.1038/s41598-023-36902-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-36902-x