Abstract
Methylene blue (MB) dye is considered a well-known dye in many industries and the low concentration of MB is considered very polluted for all environment if it discharged without any treatment. For that reason, many researchers used advanced technologies for removing MB such as the electrochemical methods that considered very simple and give rapid response. Considering these aspects, a novel quartz crystal microbalance nanosensors based on different concentrations of PVC@SiO2 were designed for real-time adsorption of MB dye in the aqueous streams at different pHs and different temperatures. The characterization results of PVC@SiO2 showed that the PVC@SiO2 have synthesized in spherical shape. The performance of the designed QCM-Based PVC@SiO2 nanosensors were examined by the QCM technique. The sensitivity of designed nanosensors was evaluated at constant concentration of MB (10 mg/L) at different pHs (2, 7 and 11) and temperatures (20 °C, 25 °C, and 30 °C). From the experimental, the best concentration of PVC@SiO2 was 3% for adsorbed 9.99 mg of cationic methylene blue at pH 11 and temperature 20 °C in only 5.6 min.
Similar content being viewed by others
Introduction
Plastics play a vital role in our life as many objects which are using daily are made of different kinds of plastics such as packaging, films, covers, bags and containers, to construction, electrical and electronic applications of plastics1.
Polyvinyl chloride (PVC) is among the most abundant plastics worldwide, it can be used in different fields of industry including architecture, electronic, chemical engineering, pharmaceutical packaging2,3, transportation, while mechanical and thermal properties of PVC can be modified by incorporating some materials during PVC production4. Its eminent properties and good performance with low cost, great process ability, synthetic resistance and low combustibility were improved5.
Moreover, PVC is considered a part of plastic industry, furthermore, PVC can combine with fillers as thermal stabilizer and plasticizer, before preparing and utilizing the ideals of toughness, acid, alkali resistance and grating resistance6. Due to PVC little thermal stability, some materials should be added during its production to improve its thermal stability, and this was due to the unique structure and remarkable mechanical, optical, thermal and electrical properties of the used materials. By the time, PVC nanocomposites had attracted great interest because the researches have been improved the properties of PVC with loading a very low amount of nanoparticles, compared to the conventional composite materials6,7,8.
Nanoparticles additives can improve performance of polymers due to their small size, large specific area, quantum confinement effects and a strong interfacial interaction9,10. PVC/graphite nano-composites can be used for attenuation and electromagnetic interference (EMI) shielding11. PVC/CaCO3 nanocomposite can be used for flexibility and strength due to its novel properties12. These studies showed that PVC properties can be modified by adding nanoparticles and used more widely in the environment13. On the other side, silica nanoparticles are widely studied for many applications such as photonic crystals, chemical sensors, biosensors, nano-fillers for advanced composite materials, markers for bio imaging, substrate for quantum dots, and catalysts14,15,16. However, many wonderful reports illustrated silica uses in production of modern polymer composites, the mechanical properties and process ability of PVC filled with nano-SiO2 particles are widely studied17.
It is remarkable that polymer gives rise to new types of nanocomposite polymer electrolytes filling PVC polymers with nano-sized silica gives new types of nanocomposite polymer electrolytes. Due to optical properties of silica nanoparticles including transparency and refractive index, they are used in the research field18. From all the above, it is clear that, addition of nano silica to PVC matrix improved the optical and dielectric properties of the nanocomposite films19,20.
Population growth and industrial development have increasing pollution of the environment and needs for more energy and electricity21. This pollution affecting mostly water bodies and this leads to make water unsuitable for consumption22. One of the most common reasons of water pollution is dyes which are used in many industries, such as textiles, food, and cosmetics, and are also used for medical purposes, including as antiseptic agents23 or present naturally in some types of wastewater such as leachate24. Dyes are not only considered one of the leading water pollutants but also, they have every chance of affecting the surrounding environment in several ways25. Dyes pollution considered very dangerous as they have a lasting effect on exposed animals, depending on the concentration and duration of exposure and this increase bacterial growth for photosynthesis dependent bacteria and this affecting negatively on aquatic plants as bacteria will interfere with the light26. Dyes are used in the food industry to improve the appearance, smell, taste, color, texture, calorie content and shelf life of food. Most dyes are considered mutagenic and carcinogenic due to the presence of aromatic rings in their structures and could be present in natural and hazardous wastewater like leachate27,28. The presence of these dyes in food and their appearance in surface and groundwater are considered unsafe for human well-being29. A variety of health problems, including allergic reactions, skin irritation, gastrointestinal problems, and overactivity in children can be occurring at high doses30,31. Due to all these harmful effects of dyes on the health, very high regulations controlling the presence of dyes in foods are considered, and scientists are always working on developing simple and inexpensive methods for detecting and extracting dyes to lowering their contamination in the wastewater as much as they can. Detecting and identifying the content of organic dyes in food products is very hard, and, therefore, various methods are used for this purpose, including UV spectrophotometry, high-performance liquid chromatography, spectrofluorometry, electrochemical voltammetry, and mass spectrometry32,33. For example, methylene blue (MB) is an aromatic heterocyclic basic dye34 having a molecular weight of 319.85 g mol−134,35. It is highly water-soluble, and thus forms a stable solution with water at room temperature36,37,38. Water contamination by methylene blue (MB) is a threat to human health and aquatic biota due to its toxicity, persistence, and non-biodegradability39. From all the above, the performance of PVC@SiO2 nanocomposite was investigated by measuring the removal efficiency of the methylene blue dye.
Identification of hazardous gas species and concentrations of hazardous pollutants is crucial because, in addition to polluting our living environment, toxic compounds also pose harm to human health once they reach a specific level40,41,42,43,44. To this purpose, a variety of gas and hazardous pollutants sensors, such as the quartz crystal microbalance (QCM)41,42, have been developed for effective environment monitoring and respiratory analysis. The Quartz Crystal Microbalance (QCM) is a physical nano-gram-sensitive device44 which is suited as a transducer element for electrochemical sensors because it is considered as rapid, easy to be used, highly stable and portable. QCM-based sensors can be used in detecting several analytes in very different matrix environment45. The QCM-based sensors can accurately detect the trace mass changes in the nano-gram range that absorbed onto the electrode of the Quartz Crystal surface in both air or liquid44, but using tiny molecules in making these devices make it difficult to design QCM sensors to be used continuously. So, polymeric materials can modify the surface characteristics of the QCM and hence, increase the range of QCM sensors’ applications46,47.
In light of this, PVC@SiO2 nanocomposite for QCM applications was created. atomic force microscopy) AFM), X-ray diffraction (XRD), dynamic light scattering (DLS), Brunauer–Emmett–Teller (BET) surface area, zeta-potential measurements, scanning and transmission electron microscopes (SEM and TEM), were used to characterize the nanocomposite. The QCM technology for detection of methylene blue in the aqueous solution was used to test the activity of the PVC/SiO2 nancomposite related to this technology. According to the findings, the PVC/SiO2 nanocomposite effectively could detect the methylene blue in the aqueous solution.
Materials and methods
Synthesis of PVC@SiO2 nanocomposite
Synthesis has been done by two steps; the first one synthesis of nano filler (silica nanoparticles) by sol–gel technique as used before in some literature such as Refs.17,19,48 and the second step was the synthesis of nanocomposite by hybrid nano silica into PVC polymer. In any case, silica nanoparticles synthesis was performed by following the gel formation method (hydrolysis of sodium silicate in acidic media using sulfuric acid). In typical synthesis, 1g of sodium silicate was dissolved in 145 ml of doubled deionized water then sulfuric acid was added drop by drop until pH becomes 2.0 and clear gel was obtained. Gel was dried under temperature of 600 °C for 2 h and finally gently milling in mortar to get fine powder of nano silica. PVC@SiO2 nanostructure was synthesized by dissolving 5g of PVC in 100 DHF with stirring for 5 h at 60 °C until a transparent solution was watched. The calculated weight of the prepared SiO2 nanoparticles (0.05, 0.1, 0.15 and 0.2g) was added to the solution under ultrasonic stirrer at room temperature (RT) for 30 min to prevent the agglomeration of the nanoparticles. Then, the synthesized 1%, 2%, 3% and 4% PVC@SiO2 nanocomposites were cast in glass dish of 10 cm diameters and air dried for 24 h17,48. During the synthesis of the nanocomposites, the conditions were well adjusted, and the silica nanoparticles were well distributed to prevent the agglomerates as shown in the following results.
Instrumentation
X-ray diffraction (EQUINOX 1000, Thermo Scientific CO., Lafayette, CO, USA) was used to determine the composition and phase of PVC@SiO2 nanocomposites. Cu Kα radiation with a current of 31 mA and an applied voltage of 33 kV was used. The 2θ angles ranged between 0° to 85°, and the scan speed was adjusted to 0.1°/min. In addition, the surface charge and particle size of PVC@SiO2 were determined using the zeta seizer instrument (NanoSight NS500, Malvern Panalytical, Malvern, UK). The prepared samples were further examined with a TEM instrument (JEOL, JEM-2100 high-resolution, Peabody, MA, USA) to determine the morphology of PVC@SiO2. The PVC@SiO2 sample was sonicated for 20 min using an ultrasonic probe sonicator (UP400S, Hielscher, Oderstraße, Teltow, Germany) at a frequency of 55 kHz, an amplitude of 55%, and a cycle of 0.55 before TEM analysis. The dispersed mixture was then deposited in drops with a diameter of five to ten microns across a copper grid that had been coated with carbon before being subjected to TEM analysis. AFM was carried out by AFM instrument model of (5600Ls manufacture by Agilent technology, USA). The surface area and pore size of a sample were determined using BET Surface Area Analyzer manufacture by (Quanta chrome model of NOVA touch 2LX) as reported before49. The QCM measurements have been done using a QCM system (QCM, Q-senses, Biolin Scientific, Linthicum Heights, MD, USA) and these measurements have been done following some researches50.
Application of QCM
QCM base-method depends on piezoelectric properties of quartz mineral chips in which quartz development electric charge when subjected to stress load by nanogram50. Detection of MB have been done by using PVC@SiO2 nanostructure adsorber for MB in aqueous solution which can be determined by QCM where some information about the period enough to absorb MB from solution in different temperatures and pH parameters using constant weight of MB and different concentrations of PVC@SiO2 nanostructure was gutted. The weight of MB adsorbed was monitored by studying the behavior of adsorbed MB on surface of PVC@SiO2 nanostructure with time. Twenty four QCM chips were prepared in three steps. The first step involved cleaning of QCM chips by cleaning solution consisting of 5:1:1 v/v/v solution of aqueous ammonia, H2O2, and double-distilled water) for 15 min. The second step is the precipitation of thin film of PVC@SiO2 nanostructure on QCM chip surface using EMITECH K450X sputter coater. In this step 10 mg of 1%, 2%, 3% and 4% of PVC@SiO2 nanostructure was dissolved in 20 ml DHF and coated on the surface of QCM chip by sputter coater. Finally, the QCM chips were air dried for 24 h in vacuum system. The third step is the insertion of QCM@PVC@SiO2 in QCM device and injecting the background doubled deionized water as electrolyte solution to blank baseline. After a stable base line, the device was now ready for the evaluation of the adsorption of MB on surface of QCM@ PVC@SiO2 at different temperatures and pHs46. Each data measurement was performed by injecting 10 mg of MB dissolved in 50 ml doubled deionized distilled water onto the surface of QCM@ PVC@SiO2 either at various temperatures (20 °C, 25 °C and 30 °C) or pH values (2, 7 and 11). The MB solution was then injected continuously until the frequency signal stabilized which indicating that the equilibrium of the binding interaction between the QCM@PVC@SiO2 and the MB had been reached.
Consent to participate
All of the authors consented to participate in the drafting of this manuscript.
Results and discussion
XRD for PVC@SiO2 nanostructure
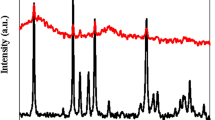
X-ray diffraction (XRD) was used to characterize the sensitized nano-composite of PVC@SiO2 with different concentrations namely 1%, 2%, 3% and 4%. The films of PVC were loaded with the mentioned concentrations of silica oxide nanoparticles. The formed wide peak from 15 to 35∘ in all cases confirmed the formation of PVC according to many previous literature studies51. Furthermore, the formed nanocomposite intimated a wide and shallow peak, which confirmed the formation of an amorphous structure of the nanocomposites. This showed that the addition of silica nanoparticles causes a decrease in the degree of crystallinity and a simultaneous increase in the amorphicity of the composite (Fig. 1)48,51. The intensities in Fig. 1, of the nanocomposite are quite similar due to the synthesis conditions which confirmed that the homogeneity and the arrangement of the silica crystal within PVC polymer for all concentrations48.
AFM for PVC@SiO2 nanocomposite
To obtain a more in-depth insight into the shape, size, and crystalline nature of the PVC@SiO2 nanocomposite, AFM was employed. The 3D and 2D surface morphology images of PVC@SiO2 nanocomposite are consistent with what will be shown by TEM analysis. Figure 2 from -A to K- showed the AFM of the prepared nano-composite with the different concentrations. Overall, the nano-scale of the different concentrations are being cleared which confirmed that the preparation was as expected. However, the increasing of the SiO2 nanoparticles from 1 to 4% leads to changing in the morphology and decreasing the surface topography of the prepared nanocomposite. As a general observation, adherent and continuous silica-based films could be obtained by sol–gel on PVC substrate and moreover, increasing the concentrations to 4% lead to form the PVC@SiO2 nanocomposite without voids and cracks as shown in Fig. 2G. Furthermore, AFM showed that the spectral range was from 90 to 0 nm. A major problem in all nanocomposites is tendency of nanoparticles to create agglomerates at the surface of polymer as a result of their high surface area and high concentration as had been shown in a literature52.
SEM and TEM for PVC@SiO2 nanocomposie
Microstructure studies were carried out in order to detect voids or agglomerates that could be formed through processing steps. SEM images for PVC@SiO2 nanocomposies are shown in Fig. 3. It is depicted that nano silica are homogenously dispersed up to 4%. It could be observed that the smooth shape has been formed up to 3% however, the shape of the nanocomposite becomes rougher after increasing the amount of the nano-silica to 4%. This could be due to the incorporation of nano-silica particles to the PVC resulted from the various interactions between nano-silica particles and PVC molecular chains. This probably induces aggregates and agglomerates on the surface of PVC nanocomposites. As a result of this phenomenon, the surface roughness of prepared samples is increased which is in good agreement with the other sophisticated analyses. This phenomenon that the nanocomposite becomes rougher after increasing the amount of nano-silica has been observed with good agreement with other literatures53.
TEM microscopy has been done in this study to provide detailed information about the morphology and structural characteristics of PVC@SiO2 nanocomposite, allowing researchers to better understand the properties and behavior of such composite materials regarding the dispersion of the nano-silica particles within the PVC matrix. Figure 4 showed that the 1% concentration of PVC@SiO2 nanocomposite had size of 35 nm, however, the size of PVC@SiO2 nanocomposite concentrations of 2%, 3% and 4% was ranged from 50 to 60 nm which confirmed the preparation of the nanocomposite in good manner.
Furthermore, Fig. 4 showed that the nano-silica particles were well-dispersed as individual and uniformly distributed particles with a consistent size and spherical-shape for the concentrations of 1%, 2% and 3%. Regarding the last concentration (4%), it can be shown that in some cases, an interaction could be occurred between nano silica and PVC and resulted in the formation of an aggregation and clustering of nano silica particles, and this can affect the properties and performance of the composite materials during their application on the treatment54.
BET surface area of PVC@SiO2 nanocomposite
Table 1 and Fig. 5 showed BET analysis of the surface area, pore size and pore volume of PVC@SiO2 nanocomposites (1–4%). The BET surface area for 1% PVC@SiO2 nanocomposite was 458.4 m2/g and the pore volume was 0.91 cc/g. The more loading of the nano-silica, a decrease in surface area to 277.1 m2/g was observed due to the filling of pores of the PVC with the nano-silica structure and this was in accordance with Thamilselvi and Radha study55. Silica nanoparticle loaded PVC showed further decrease in surface area to 276.9 m2/g and 208.9 m2/g for increasing the loaded nano-silica to 3% and 4%. The pore volume decreased from 0.91 cc/g to 0.57 cc/g, 0.56 cc/g, and 0.38 cc/g for PVC@SiO2 nanocomposite concentrations of 1%, 2%, 3%, and 4%, respectively. These results confirmed that the silica nanoparticle was loaded on PVC surfaces correctly and with a good manner. Also, the porous solid materials are classified by IUPAC into: (i) microporous materials with pore sizes up to 2.0 nm, (ii) mesoporous materials with pore sizes intermediate between 2.0 and 50.0 nm, (iii) macroporous materials with pore sizes exceeding 50.0 nm56. So, this confirmed that PVC@SiO2 nanocomposites were mesoporous materials.
Zeta potential and DLS studies for PVC@SiO2 nanocomposites
From Fig. 6, it can be shown that the particle size distribution, as was found by DLS analysis, showed an average particle size of 56 nm for 1%, 48 nm for 2%, 51 nm for 3% and 31 nm for 4% of PVC@SiO2 nanocomposites which confirmed that the prepared nanocomposites fell in the required range before being used in this study. Figure 7 showed the zeta potential that can help as a measure of the stability of a colloidal system and this analysis could be used for evaluation of the particles within a colloid of the prepared nanocomposite57. In this study, the prepared PVC@SiO2 by different concentrations was a colloid. The ZP values were approximately − 32, − 30, − 21 and − 37 mV for the prepared PVC@SiO2 of 1%, 2%, 3% and 4%, respectively. Zeta potentials of − 30 mV value at which colloids formed nanocomposites are commonly considered to be stabilized electrostatically and this has been confirmed by the authors of Refs.58,59.
MB adsorption using QCM-based PVC@SiO2 nanosensors at different pH and different temperatures
The sensor based on quartz crystal microbalance (QCM) is an essential and promising sensing device for the real-time detection of dyes in aqueous solution. A QCM resonator can sensitively and precisely monitor the change in quartz resonance frequency caused by the mass adsorbed on the piezoelectric quartz crystal. The QCM-based sensor has been thoroughly investigated for detecting the trace mass changes in the nanogram range that are absorbed onto the electrode surface of the quartz crystal in air or in liquid, and it is exceedingly sensitive. For the very sensitive and precise detection of heavy metal ions in aqueous media, several QCM sensors containing layers of tiny molecules have been developed60,61. Due to the limitations of tiny molecules in manufacturing tools, the design of QCM sensors for use in continuous fluids is still difficult60,61.
QCM technique was used for adsorption of MB cationic dye using a constant concentration of MB to illustrate the role of change in QCM@PVC@SiO2 concentration while changing pH value and changing temperature values as will be discussed in the following section. The QCM frequency nanogram curve of 1%, 2%, 3% and 4% PVC@SiO2 nanocomposite was shown in Fig. 8. This figure illustrated the increase in the amount of MB adsorbed by increasing pH value from 2.0 to 7.0 and 11.0. This increase in the adsorption value can be accounted for the increase in the charge density in surface of QCM@PVC@SiO2 which increased the interaction between cationic MB dye and PVC@SiO2 nanocomposite. However, according to QCM data all samples succeeded in absorbing 10 mg of MB dye at all pH values but the time of removal of MB dye from solution was different as shown in Fig. 8. All PVC@SiO2 dosages (1%, 2%, 3% and 4%) showed super speed absorption and removal for MB dye from solution at the time from 5 to 7 min whereas the previous work utilized 100mg/L of MB showed adsorption of 7.25 to 6.67 mg of MB in 90 min using nanotube and nanosheet of titania62. However, frequency change (Δf) of (1%, 2%, 3% and 4%) PVC@SiO2 curves for different pH illustrated the best mass of MB adsorbed on QCM@PVC@SiO2 surface at pH 11, 2 and 7, respectively. The experimental results illustrated decrease in the time and rate of adsorbed cationic MB dye on the surface of QCM@PVC@SiO2 by increasing temperature due to weakness of electrostatic interaction by increasing temperature. Frequency change (Δf) of (1%, 2%, 3% and 4%) PVC@SiO2 curves for different temperatures illustrated the best mass of MB adsorbed on QCM@PVC@SiO2 surface at temperatures 20 °C, 25 °C and 30 °C, respectively. However, from our experimental data, the best concentration of PVC@SiO2 nanocomposite was 3% for adsorption of 9.99 mg of cationic methylene blue dye at pH 11 for temperature 20 °C in only 5.6 min and a summary chart can conclude our findings as shown in Fig. 9. The reason for that the best concentration was 3% of PVC@SiO2 may be revealed to that after increasing the amount of SiO2 to 4% on the PVC surface, a formation of an aggregation and clustering of nano-silica particles has been happen, and this can affect the properties and performance of the composite materials. The selectivity of the PVC@SiO2 sensor towards methylene orange (MO) dye was evaluated by QCM measurements as shown in Fig. 10. PVC@SiO2 with 3% concentration was coated onto QCM electrodes by drop casting method. Solutions of MO of different concentrations of (0.1 ppm, 0.5 ppm and 1 ppm) were flowed over the sensor surface at a flow rate of one mL/min for 7 min for every one with recording of Δf. With 0.1 ppm MO, a small Δf of − 6 Hz was observed indicating MO binding to the sensor surface. When the concentration was increased to 0.5 ppm, a higher frequency change of − 7 Hz occurred due to more deposition of MO. At 1 ppm MO, the frequency further dropped to − 10 Hz as more binding sites were occupied. After each MO injection, buffer was flowed which resulted in an increase in frequency back to baseline. This suggests the bound MO was removed from the sensor surface demonstrating the reversible deposition-leaching cycle. In contrast, a control experiment was performed with methylene blue (MB) dye under identical conditions. While MB also showed concentration dependent frequency decreased, the frequency did not return to baseline on buffer injection. This indicates irreversible binding of MB without leaching. The QCM results confirm the selective detection of MO by the PVC@SiO2 sensor with showing a reversible MO binding that was dependent on concentration. The selectivity can be attributed to the hydrogen bonding and electrostatic interactions between MO and the SiO2 and PVC components.
Mechanism of QCM sensing
The PVC@SiO2 nanocomposite film demonstrated greater sensitivity may be due to the presence of PVC and SiO2 NPs within the hydrogel matrix. By incorporating PVC and SiO2 NPs into the polymer matrix, the sensing properties of the PVC@SiO2 nanocomposite were improved63. Therefore, the combination of PVC and SiO2 NPs can complement each other and effectively produce nanocomposites film with excellent sensing performance owing to the large specific surface area and many alkenyl, chloride and oxygen-containing functional groups of the PVC@SiO2 hybrid system63. As a result, it serves as a good sensor platform characterized by high sensitivity due to its ability to enhance electron transfer when immobilized into QCM electrodes. It can be concluded that physisorption is the primary process that drives MB dye adsorption by PVC@SiO2 NPs are the most significant contributor to this process. When in solution, PVC atoms tend to become protonated and acquire a negative charge, which enables them to attract positively charged of MB dye molecules through electrostatic forces. While the SiO2 NPs assisted by increasing the loading area and the surface area for PVC@SiO2 NPs, which also resulted in the removal of some MB molecules through intercalation. The loading area and the surface area could reach at the maximum to adsorb the maximum amount of the methylene blue depending on the crystallinity and the homogeneity of the materials and after the maximum point, adding any amount of SiO2 on the PVC will lead to different shape and change in the crystallinity and could lead to decreasing the adsorption capacity for the material. In this study, the optimum concentrations of the SiO2 on the PVC was 3% and after this percent, the efficiency of the materials in the adsorption capacity will decrease as happen in some modifications that used for adsorption process and confirmed that after increasing the concentrations of the nanoparticles, the adsorption process could be decreased64,65.
Conclusion
In this work, novel QCM-based PVC@SiO2 nanosensors with different concentrations have been developed for adsorption of MB dye from the water streams. X-ray diffraction (XRD) was used to characterize the sensitized PVC@SiO2 nanocomposite with different concentrations namely 1%, 2%, 3% and 4%. The films of PVC were loaded with the mentioned concentrations of silica oxide nanoparticles. The formed wide peak from 15 to 35∘ in all cases confirmed the formation of PVC. The concentration of PVC/nano silica concentrations of 1% was in the size of 35 nm and the size of PVC/nano silica concentrations of 2%; 3% and 4% was ranged from 50 to 60 nm which confirmed the preparation of the nanocomposite was in the good manner. The sensitivity of designed nanosensors was evaluated at constant concentration of MB (10 mg/L) at different pHs and temperatures (20 °C, 25 °C, and 30 °C). From our experimental, the best concentration of PVC@SiO2 nanocomposite was 3% for adsorption of 9.99 mg of cationic methylene blue dye at pH 11 for temperature 20 °C in only 5.6 min.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gibb, B. C. Plastics are forever. Nat. Chem. 11, 394–395 (2019).
Skelly, P. W., Li, L. & Braslau, R. Internal plasticization of PVC. Polym. Rev. 62, 485–528 (2022).
Bassani, F., Rodrigues, C., Marques, P. & Freire, F. Life cycle assessment of pharmaceutical packaging. Int. J. Life Cycle Assess. 27, 978–992 (2022).
Li, D. & Liu, P. Trends and prospects for thermal stabilizers in polyvinyl chloride. J. Vinyl Addit. Technol. 28, 669–688 (2022).
Elashmawi, I. S., Alatawi, N. S. & Elsayed, N. H. Preparation and characterization of polymer nanocomposites based on PVDF/PVC doped with graphene nanoparticles. Results Phys. 7, 636–640 (2017).
Rabiee, H., Shahabadi, S. M. S., Mokhtare, A., Rabiei, H. & Alvandifar, N. Enhancement in permeation and antifouling properties of PVC ultrafiltration membranes with addition of hydrophilic surfactant additives: Tween-20 and Tween-80. J. Environ. Chem. Eng. 4, 4050–4061 (2016).
Ari, G. A. & Aydin, I. Rheological and fusion behaviors of PVC micro-and nano-composites evaluated from torque rheometer data. J. Vinyl Addit. Technol. 16, 223–228 (2010).
Sterky, K., Jacobsen, H., Jakubowicz, I., Yarahmadi, N. & Hjertberg, T. Influence of processing technique on morphology and mechanical properties of PVC nanocomposites. Eur. Polym. J. 46, 1203–1209 (2010).
Kadham, A. J., Hassan, D., Mohammad, N. & Ah-yasari, A. H. Fabrication of (polymer blend-magnesium oxide) nanoparticle and studying their optical properties for optoelectronic applications. Bull. Electr. Eng. Inform. 7, 28–34 (2018).
Hassan, G. K., Al-Shemy, M., Adel, A. & Al-Sayed, A. Boosting brackish water treatment via integration of mesoporous γ-Al2O3 NPs with thin-flm nanofltration membranes. Sci. Rep. 12, 1966 (2022).
Malak, S. A., Kazma, S. & El Achkar, C. The mechanical and blast resistance properties of polyvinyl chloride/calcium carbonate (PVC/CaCO3) nanocomposites. Mater. Today Proc. 65, 895–900 (2022).
Thabet, A. & Ebnalwaled, A. A. Improvement of surface energy properties of PVC nanocomposites for enhancing electrical applications. Measurement 110, 78–83 (2017).
Gao, W., Rigout, M. & Owens, H. Facile control of silica nanoparticles using a novel solvent varying method for the fabrication of artificial opal photonic crystals. J. Nanoparticle Res. 18, 1–10 (2016).
Islam, S., Bidin, N., Riaz, S. & Naseem, S. Self-assembled hierarchical phenolphthalein encapsulated silica nanoparticles: Structural, optical and sensing response. Sensors Actuators A Phys. 266, 111–121 (2017).
Alhadhrami, A. et al. Behavior of silica nanoparticles synthesized from rice husk ash by the sol–gel method as a photocatalytic and antibacterial agent. Materials (Basel) 15, 8211 (2022).
Akbarian, M., Gholinejad, M., Mohammadi-Samani, S. & Farjadian, F. Theranostic mesoporous silica nanoparticles made of multi-nuclear gold or carbon quantum dots particles serving as pH responsive drug delivery system. Microporous Mesoporous Mater. 329, 111512 (2022).
Gharehbash, N. & Shakeri, A. Modification of the surface of silica nanoparticles; studying its structure and thermal properties in order to strengthen it in preparing nanocomposites. J. Am. Sci. 9, 602–606 (2013).
Abdel-Baset, T., Elzayat, M. & Mahrous, S. Characterization and optical and dielectric properties of polyvinyl chloride/silica nanocomposites films. Int. J. Polym. Sci. 2016, 1–13 (2016).
Numan, A. et al. Rationally engineered nanosensors: A novel strategy for the detection of heavy metal ions in the environment. J. Hazard. Mater. 409, 124493 (2021).
Malik, L. A., Bashir, A., Qureashi, A. & Pandith, A. H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 17, 1495–1521 (2019).
Al-Hazmi, H. E. et al. An advanced synergy of partial denitrification-anammox for optimizing nitrogen removal from wastewater: A review. Bioresour. Technol. 381, 129168 (2023).
Tawfik, A. et al. Electron donor addition for stimulating the microbial degradation of 1,4 dioxane by sequential batch membrane bioreactor: A techno-economic approach. Chemosphere 306, 135580 (2022).
Hassan, M. M. & Carr, C. M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 265, 129087 (2021).
Hassan, G. K., Gad-Allah, T. A., Badawy, M. I. & El-Gohary, F. A. Remediation of ammonia-stripped sanitary landfill leachate by integrated heterogeneous Fenton process and aerobic biological methods. Int. J. Environ. Anal. Chem. 1, 1–14 (2021).
Shabir, M. et al. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 112, 1–19 (2022).
Slokar, Y. M. & Le Marechal, A. M. Methods of decoloration of textile wastewaters. Dye. Pigment 37, 335–356 (1998).
Uddin, M. J., Ampiaw, R. E. & Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 284, 131314 (2021).
Hassan, G. K. & El-Gohary, F. A. Evaluation of partial nitritation/anammox process for reduction of pollutants from sanitary landfll leachate. Water Air Soil Pollut. 232, 134 (2021).
Jawad, A. H., Abdulhameed, A. S. & Mastuli, M. S. Acid-factionalized biomass material for methylene blue dye removal: A comprehensive adsorption and mechanism study. J. Taibah Univ. Sci. 14, 305–313 (2020).
Cusioli, L. F., Quesada, H. B., Baptista, A. T. A., Gomes, R. G. & Bergamasco, R. Soybean hulls as a low-cost biosorbent for removal of methylene blue contaminant. Environ. Prog. Sustain. Energy 39, e13328 (2020).
Lebron, Y. A. R., Moreira, V. R. & de Souza Santos, L. V. Biosorption of methylene blue and eriochrome black T onto the brown macroalgae Fucus vesiculosus: Equilibrium, kinetics, thermodynamics and optimization. Environ. Technol. 42, 279–297 (2021).
Mokhtari, P., Ghaedi, M., Dashtian, K., Rahimi, M. R. & Purkait, M. K. Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: Kinetic and isotherm investigation. J. Mol. Liq. 219, 299–305 (2016).
Saleh, M. M. S., Hashem, E. Y. & Al-Salahi, N. O. A. Oxidation and complexation-based spectrophotometric methods for sensitive determination of tartrazine E102 in some commercial food samples. Comput. Chem. 4, 51 (2016).
Sahu, S., Pahi, S., Sahu, J. K., Sahu, U. K. & Patel, R. K. Kendu (Diospyros melanoxylon Roxb) fruit peel activated carbon—An efficient bioadsorbent for methylene blue dye: Equilibrium, kinetic, and thermodynamic study. Environ. Sci. Pollut. Res. 27, 22579–22592 (2020).
Amode, J. O., Santos, J. H., Md. Alam, Z., Mirza, A. H. & Mei, C. C. Adsorption of methylene blue from aqueous solution using untreated and treated (Metroxylon spp.) waste adsorbent: Equilibrium and kinetics studies. Int. J. Ind. Chem. 7, 333–345 (2016).
Makeswari, M. & Saraswathi, P. Photo catalytic degradation of methylene blue and methyl orange from aqueous solution using solar light onto chitosan bi-metal oxide composite. SN Appl. Sci. 2, 336 (2020).
Sabar, S. et al. Preparation of sulfonated chitosan for enhanced adsorption of methylene blue from aqueous solution. React. Funct. Polym. 151, 104584 (2020).
Wei, X. et al. Different adsorption-degradation behavior of methylene blue and Congo red in nanoceria/H2O2 system under alkaline conditions. Sci. Rep. 9, 4964 (2019).
Trokourey, A., N’Goran, K. M. & Blonde, L. D. Removal of methylene blue from industrial effluents using corncob activated carbon. Int. Res. J. Pure Appl. Chem. https://doi.org/10.9734/irjpac/2022/v23i5789 (2022).
Chen, C. et al. Ni-Co-P hollow nanobricks enabled humidity sensor for respiratory analysis and human-machine interfacing. Sens. Actuators B Chem. 370, 132441 (2022).
Su, Y. et al. High-performance piezoelectric composites via β phase programming. Nat. Commun. 13, 4867 (2022).
Su, Y. et al. Maxwell displacement current induced wireless self-powered gas sensor array. Mater. Today Phys. 30, 100951 (2023).
Pan, H. et al. Biodegradable cotton fiber-based piezoresistive textiles for wearable biomonitoring. Biosens. Bioelectron. 222, 114999 (2023).
Bragazzi, N. L. et al. Quartz-crystal microbalance (QCM) for public health: An overview of its applications. Adv. Protein Chem. Struct. Biol. 101, 149–211 (2015).
Emir Diltemiz, S., Keçili, R., Ersöz, A. & Say, R. Molecular imprinting technology in quartz crystal microbalance (QCM) sensors. Sensors 17, 454 (2017).
Al-Gethami, W., Alhashmialameer, D., Al-Qasmi, N., Ismail, S. H. & Sadek, A. H. Design of a novel nanosensors based on green synthesized CoFe2O4/Ca-alginate nanocomposite-coated QCM for rapid detection of Pb(II) ions. Nanomaterials 12, 3620 (2022).
Hassan, G. K. et al. Multi-functional of TiO2@Ag core–shell nanostructure to prevent hydrogen sulfide formation during anaerobic digestion of sewage sludge with boosting of bio-CH4 production. Fuel 333, 126608 (2023).
Saied, M. A. & Ward, A. A. Physical, dielectric and biodegradation studies of PVC/silica nanocomposites based on traditional and environmentally friendly plasticizers. Adv. Nat. Sci. Nanosci. Nanotechnol. 11(035003), 1–11 (2020).
Mersal, M., Zedan, A. F., Mohamed, G. G. & Hassan, G. K. Fabrication of nitrogen doped TiO2/Fe2O3 nanostructures for photocatalytic oxidation of methanol based wastewater. Sci. Rep. 13, 4431 (2023).
Johannsmann, D., Langhoff, A. & Leppin, C. Studying soft interfaces with shear waves: Principles and applications of the quartz crystal microbalance (QCM). Sensors 21, 3490 (2021).
Lobo, H. & Bonilla, J.V. Handbook of plastics analysis. (2003).
Kango, S. et al. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 38, 1232–1261 (2013).
Gashti, M. P., Allahyary, H., Nasraei, P. & Gashti, M. P. SiO2-kaolinite affecting the surface properties of ternary poly (vinyl chloride)/silica/kaolinite nanocomposites. Fibers Polym. 14, 1870–1876 (2013).
Hung, D. P. et al. Effect of silica nanoparticles on properties of coatings based on acrylic emulsion resin. Vietnam J. Sci. Technol. 56, 117–125 (2018).
Thamilselvi, V. & Radha, K. V. Silver nanoparticle loaded silica adsorbent for wastewater treatment. Korean J. Chem. Eng. 34, 1801–1812 (2017).
ALOthman, Z. A. A review: Fundamental aspects of silicate mesoporous materials. Materials (Basel) 5, 2874–2902 (2012).
Hassan, G. K. et al. Harnessing Cu@Fe3O4 core shell nanostructure for biogas production from sewage sludge: Experimental study and microbial community shift. Renew. Energy 188, 1059–1071 (2022).
Xu, P., Wang, H., Tong, R., Du, Q. & Zhong, W. Preparation and morphology of SiO 2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym. Sci. 284, 755–762 (2006).
Garcia, A. L. Nano-Silica Production at Low Temperatures From the Dissolution of Olivine: Synthesis, Tailoring and Modelling (Eindhoven University of Technology, 2014).
Cao, Z. et al. Detection of heavy metal ions in aqueous solution by P (MBTVBC-co-VIM)-coated QCM sensor. Sensors Actuators B Chem. 157, 34–41 (2011).
Thies, J. W., Kuhn, P., Thürmann, B., Dübel, S. & Dietzel, A. Microfluidic quartz-crystal-microbalance (QCM) sensors with specialized immunoassays for extended measurement range and improved reusability. Microelectron. Eng. 179, 25–30 (2017).
Zaki, A. H., Rashad, S., Lee, M. J. & Shehata, N. Unexpected behavior during methylene blue adsorption over H-titanate nanotubes and nanosheets. Mater. Adv. 3, 8977–8988 (2022).
Mallakpoura, S. & Nazaria, H. Y. Ultrasonic-assisted fabrication and characterization of PVC-SiO2 nanocomposites having bovine serum albumin as a bio coupling agent. Ultrason. Sonochem. 39, 686–697 (2017).
Lukhele, E. J., Khutlane, J. T., Báthori, N. B. & Malgas-Enus, R. Reduction and removal of methylene blue from aqueous solutions via recyclable magnetic gold nanomaterials. Surf. Interfaces 31, 101970 (2022).
Dang, A. et al. Graphene oxide mediated carbon foam/CNTs composites for highly efficient adsorption of methylene blue and mechanism insight. Ceram. Int. 49, 36970–36978 (2023).
Acknowledgements
This work was carried out with financial assistance from Chemistry and Physics Departments, Faculty of Science—Cairo University.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
S.S.A.: Conceptualization, Designing- Outline, figures etc., Writing- Original draft. S.H.I.: Methodology, writing. A.A.E. and M.H.: Supervision, Conceptualization, writing, reviewing, and editing. G.G.M.: Supervision, Conceptualization, writing, Reviewing, and Editing. G.K.H.: Writing original draft, Reviewing and editing. All of the authors consented to publish this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, S.S., Hassan, G.K., Ismail, S.H. et al. Exploration of PVC@SiO2 nanostructure for adsorption of methylene blue via using quartz crystal microbalance technology. Sci Rep 13, 19621 (2023). https://doi.org/10.1038/s41598-023-46807-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-023-46807-4
This article is cited by
-
Synthesis of a Novel Multifunctional Nano Silica-based Polymer Composite for Efficient Removal of Dye From Wastewater
Silicon (2025)
-
Evaluation of PVC@Silver Nanocomposite as Sensor for Low Limit Detection of Cadmium Ion By Surface Plasmon Resonance Based Method
Journal of Inorganic and Organometallic Polymers and Materials (2024)