Abstract
Tomato pomace (TP), an antioxidant-rich byproduct, may be suitable for noble applications. The regulation of ROS generation and the anti-inflammatory response can help to prevent ulceration. The purpose of this study was to examine TP for antioxidants, in silico anti-inflammatory properties, and its potential to protect against ulceration and erosion triggered by indomethacin. Tomato pomace extract (TPE) was encapsulated either alone or with probiotics to maximize its potential effect. These microcapsules were investigated in indomethacin-treated rats. TPE demonstrated antioxidant activity as well as high levels of carotenoids (15 mg/g extract) and polyphenols. Because of their binding affinity as well as hydrophobic and hydrogen bond interactions with the active sites of TNF-α and IL-1β inflammatory cytokines, ellagic acid and rutin may be implicated in the anti-inflammatory effect of TPE, according to the docking study. TPE microcapsules, either alone or in combination with probiotics, demonstrated a protective effect against enterocolitis by reducing oxidative stress and inflammation, as evidenced by the decrease in stomach and intestinal MDA, NO, IL-1β, IL-6, and TNF-α levels and the increase in CAT, SOD, and GSH activities. The produced microcapsules are suggested to be promising candidates for protection against gastric ulcers and erosion.
Similar content being viewed by others
Introduction
Tomato pomace (TP) is a by-product rich in nutrients and bioactive components such as phenolic compounds, carotenoids (lycopene, carotenes, and lutein), sugars, and fibers. Therefore, it may be suitable for noble applications. The advantages of valorizing TP include reducing food waste, minimizing environmental disruption, and generating products with added value for food and pharmaceutical applications. The bioactive components of TP provide health benefits due to several biological effects such as antioxidant, anti-hypertensive, anti-proliferative, and anti-inflammatory1. Polyphenols, the most widespread phytochemicals, have been demonstrated to have apoptotic, immunomodulatory, anti-inflammatory, and antioxidant activities. In addition to locally reducing oxidative stress, their actions can be directed at cellular targets by changing the expression of genes linked to inflammation, such as NF-B, Nrf-2, Jak/STAT, and MAPKs, inhibiting the production of downstream cytokines (such as IL-8, IL-1β, and TNF-α), and enhancing the body’s antioxidant enzymes (SOD and GPx). Furthermore, as prebiotics, they may be implicated in probiotic viability improvement, healthy microbiota maintenance, short-chain fatty acid production, gut permeability enhancement, and tight junction stability improvement2.
Probiotics are living bacteria that, when given in appropriate amounts, alter the gut microbiota. Because probiotics help to restore the mucosa and promote anti-inflammatory effects, they considerably reduce intestinal inflammation when used as a treatment, eliminate discomfort and edema, and overall improve the quality of life. It’s also important to point out that probiotics are significantly less expensive and have less potential side effects than other forms of gastrointestinal inflammation treatment3. Combining probiotic strains with phenol-rich extract as antioxidants may be beneficial in extending their viability and providing health benefits to the host4.
Several studies have demonstrated that increased reactive oxygen species (ROS), decreased cell proliferation, and increased inflammation can all lead to stomach injury. It is critical to regulate ROS generation and the anti-inflammatory response to prevent stomach ulcers5. Free radicals of reactive oxygen (and nitrogen) are also involved in the pathophysiology of inflammatory bowel disease (IBD), which includes Crohn’s disease (CD), which affects all portions of the digestive system, and ulcerative colitis (UC), which affects the large intestine. Protective agents and alternative treatments for gastrointestinal inflammation may be beneficial in preventing the side effects of the conventional treatments (antibiotics, corticosteroids, immunosuppressants, and inhibitors of tumor necrosis factor), which include hemopathies, diarrhea, vomiting, and thrombocytopenia6.
Tomato pomace may be potentially useful for the treatment and prevention of gastrointestinal inflammation7 due to its bioactive components, notably carotenoids and phenolic compounds, which have powerful antioxidant and oxygen-quenching activities. Nutritional regimens supplemented with bioactive substances may help to create an environment ideal for the growth of beneficial bacteria, treat intestinal dysbiosis, prevent recurrence, and promote full recovery from gastrointestinal inflammation. However, in the absence of concurrent probiotic therapy, these nutritional regimens may be ineffective8. To sustain the desired advantages, probiotics and bioactive compounds need to be protected. Microencapsulation has been considered a successful strategy in the food industry for the development of functional foods. It improves the handling and utilization of the bioactive compounds, controls the release of the bioactive compounds on demand and to specific targets, and increases the bio-availability of the bioactive compounds1.
This study was designed to achieve several goals. The first was an evaluation of the characteristics and in silico anti-inflammatory effect of tomato pomace extract. The second was producing microcapsules from tomato pomace extract, either alone or in combination with probiotics. The third was to assess the protective effect of the produced microcapsules against indomethacin-induced enterocolitis in a rat model.
Materials and methods
Materials
Tomato fruits (Solanum lycopersicum) were purchased from an outlet selling Egyptian Ministry of Agriculture products in Giza Governorate, Egypt. In this study, tomatoes grown in Egyptian Ministry of Agriculture farms and harvested in October 2022 were used to be identical in cultivation. Probiotics, Lactobacillus acidophilus (LA-5) and Bifidobacterium bifidum (Bb-12), were obtained from Chr. Hansen`s Laboratory, Copenhagen, Denmark. Sodium alginate (medium viscosity) was purchased from Loba Chemie, Pvt Ltd—Mumbai, India. 2,2-Dipheny l-1-picryl hydrazyl (DPPH) and Trolox were purchased from Sigma-Aldrich Chemical Co., (St. Louis, USA). All of the solvents, chemicals, and culture media used in this study are of analytical grade.
Methods
Preparation of tomato pomace extract (TPE)
Tomato fruits were washed, cut, and squeezed out with a manual tomato juicer. The pomace (mix of seeds and peels) was freeze-dried at − 47 °C and 0.100 mbar for 48 h, fine-grained, sieved, and then kept at 4 °C for further use. According to Solaberrieta et al.9, ultrasound-assisted extraction (UAE) was used to prepare the tomato pomace extract. In a 150 ml beaker, freeze-dried tomato pomace (2.0 g) was mixed with 100 ml of ethanol. The ultrasonic probe was submerged in the beaker, and it was chilled in an ice bath. The extraction employed ethanol: water (60% v/v), a 10-min extraction time, and a 70% amplitude as optimal conditions for obtaining the highest yield, according to the results of Solaberrieta et al.9. Tomato pomace extract was centrifuged for 10 min at 5300 rpm. The extraction solvent was used to wash the solid residue twice and then discarded. Subsequently, ethanol was evaporated under decreased pressure (around 75 torr, 99 mbar at 55 °C), and water was removed using a freeze dryer (Crest Alpha 1–4 LSC plus Germany) at − 47 °C and 0.100 mbar for 48 h. The extraction yield was measured by using the following equation:
where TPE is the weight of extract obtained after freeze-drying, and TP is the weight of dried tomato pomace.
Estimation of total phenolic content of TPE
The total phenolic content of TPE was measured using the Folin-Ciocalteu test, as modified by Toor and Savage10. An aliquot (0.5 ml) of the extract was mixed with 2.5 ml of Folin-Ciocalteu reagent that had been previously diluted in distilled water (1:10, v/v). Aqueous sodium carbonate (2.0 ml of 7.5 wt%) was added. The mixture was then vortexed, and the absorbance was measured at 765 nm after 30 min of incubation at 45 °C in the dark. Gallic acid in ethanol: water (60% v/v) was employed as a quantitative reference standard. The results were given in milligrams of gallic acid equivalents (GAE) per gram of dried TP. The extract was estimated in triplicate.
Estimation of antioxidant Activity of TPE
TPE’s DPPH scavenging activity was assessed using the method of Szabo et al.11. TPE (0.4 ml) was mixed with 2.1 ml of newly prepared DPPH solution (10−4 mol L−1 in ethanol). The mixture was vortexed and incubated in the dark at room temperature for 120 min. The absorbance was measured at 517 nm in comparison to a pure ethanol blank. Trolox in ethanol: water (60% v/v) was employed as a quantitative reference standard. The results are given in milligrams of Trolox equivalents (TE) per gram of dried TP. The extract was estimated in triplicate.
Estimation of total carotenoid content of TPE
Total carotenoid concentration was spectrophotometry measured using the method of Jamaleddine et al.12. TPE (0.1 g) was ultrasonicated for 1 min in 50 ml ethanol. Total carotenoid content was calculated by measuring the absorbance at 446 nm and was represented as milligrams of β-carotene equivalent per gram of dried TP. The extract was estimated in triplicate.
High-performance liquid chromatography (HPLC) fingerprinting of TPE
An Agilent 1260 series was used for the HPLC analysis. Eclipse C18 column (4.6 mm × 250 mm i.d., 5 μm) was used for the separation. Water (A) and 0.05% trifluoroacetic acid in acetonitrile (B) were the components of the mobile phase, which had a flow rate of 0.9 ml/min. The linear gradient was sequentially programmed into the mobile phase as follows: 0 min (82% A); 0–5 min (80% A); 5–8 min (60% A); 8–12 min (60% A); 12–15 min (82% A); 15–16 min (82% A) and 16–20 (82% A). At 280 nm, the multi-wavelength detector was monitored. The freeze-dried TPE (100 mg) was re-dissolved in 1 ml HPLC grade methanol and filtrate through a 0.2 μm filter sterilized membrane before the injection. The sample solutions (5 μl) were injected into the HPLC device, the column was kept at a constant temperature (40 °C), then the retention times of the identified compounds of interest were measured. The extract was estimated in triplicate. The concentration of the sample is determined by comparing the peak area of the sample with that of the standard relative to the standard’s concentration. 18 standard (gallic acid, chlorogenic acid, catechin, methyl gallate, coffeic acid, syringic acid, pyrocatechol, rutin, coumaric acid, vanillin, ferulic acid, naringenin, daidzein, querectin, cinnamic acid, apigenin, kaempferol and hesperetin) were used.
Assessment of TPE effect on the probiotic strain’s viability
The probiotic bacteria (Lactobacillus acidophilus, Bifidobacterium bifidum or their mixture) were enumerated using the pour plate technique and De-Man Rogoza and Sharp (MRS) agar media (Oxoid) according to De Man et al.13 in the presence of TPE (2%). The plates were incubated anaerobically at 37 °C for 48 h. The microbiological results were expressed as log colony-forming units (CFU).
In silico predictions of TPE anti-inflammatory through docking assay
The binding affinity score of TPE bioactive molecules namely, ellagic acid and rutin on the active sites of the inflammatory cytokines namely, human IL-1β (PDB: 1HIB) and tumor necrosis factor (TNF-alpha) (PDB: 2AZ5)14 was determined using PyRx tool. 3D chemical structures of ellagic acid and rutin (SMILE code) were obtained from the PubChem database “https://pubchem.ncbi.nlm.nih.gov/” and drawn using the Chem Draw tool. The target proteins and ligands were loaded into the PyRx software and then converted to PDBQT15, the GRID parameters were maximized, then the docking assay was performed16. Complex of protein and ligand was visualized using PyMOl and Discovery Studio.
Preparation of the probiotics pellet
The probiotic bacteria were activated individually using De-Man Rogoza and Sharp (MRS) broth and incubated for 24 h at 37 °C anaerobically to obtain high biomass. The cell pellets were obtained by centrifugation at 5000 rpm, for 15 min at 4 °C. The obtained cell pellets were washed by a sterile saline solution (0.9% w/v of NaCl) and stored at 4 °C for the encapsulation procedure.
Microencapsulation technique of probiotics and TPE
The sodium alginate (3% w/v) was dissolved in distilled water and stirred continuously until the fully dissolved. The sodium alginate solution was sterilized in an autoclave for 15 min at 121 °C. The TPE (15% on a dry basis) was swirled magnetically for 5 min. At 16,000 rpm, the mixture was homogenized for 2 min. To produce the co-encapsulated TPE with probiotics, Probiotic pellets at a concentration of around 8 log CFU/ml were added to the mixture of sodium alginate solution and TPE at a concentration of 25%. The mixture was then agitated using a magnetic stirrer at room temperature for 20 min to combine the cells thoroughly with the mixture. The extrusion technique17 was used to produce either the microcapsules of TPE or the co-encapsulated TPE with probiotics. With moderate stirring for 30 min, either the sodium alginate and TPE mixture or the sodium alginate, TPE, and probiotic strains mixture was separately extruded into the hardening solution (CaCl2, 0.2 M). Filtration was used to collect the produced microcapsules, which were subsequently cleaned using a sterile saline solution. The microcapsules were then placed in the refrigerator until use.
Encapsulation efficiency (EE)
According to Toprakç et al.17, the encapsulation efficiency (EE) for either the microcapsules of TPE or the co-encapsulated TPE with probiotics was determined. EE was expressed by the following equation:
where TPC is the total phenolic content and SPC is the surface phenolic content. Simply, 100 mg of the microcapsules were dissolved in 3 ml of a 50:8:42 (v/v/v) mixture of ethanol, acetic acid, and water. The mixture was exposed to an ultrasonic bath at ambient conditions for 1 min after mixing with a vortex, and TPC was then measured colorimetrically. SPC was assessed colorimetrically after dissolving of 100 mg of the microcapsules in a 3:1 ethanol-to-methanol solution by being placed in an ultrasonic bath for 5 min at room temperature.
Scanning electron microscope (SEM) of TPE with probiotics microcapsules
The morphological shape of TPE and TPE with probiotic microcapsules were the same. Therefore, SEM was examined only for the co-encapsulated TPE with probiotics. To remove moisture content before the scanning, the microcapsules were dried in a lab oven at 40 °C for 2 h. After coating the microcapsules using Quorum Q 150 ES (United Kingdom) for 60 s with a gold layer of around 20 nm, a high-resolution scanning electron microscope model (TESCAN VEGA 3 with field emission gun, Czech Republic) was utilized to examine the morphological structure of these microcapsules.
The animal experiment
Animals
Adult male albino rats of Wistar strain weighing 173.5 ± 6.10 g as Mean ± SD were used in this study and obtained from the Animal Care Unit of the National Research Centre, Cairo, Egypt. Animals were kept individually in stainless steel metabolic cages at temperature (23 ± 1 °C), relative humidity (55 ± 1%), and 12/12 h light/dark cycles. A maintenance standard diet was prepared according to Reeves et al.18 to contain 12% protein, 10% corn oil, 10% sucrose, 58.5% starch, 5% fiber, 3.5% AIN-93 salt mixture, and 1% AIN-93 vitamin mixture. Food and water were provided ad libitum.
Preparation of the jelly candy
Jelly candy was chosen as a suitable delivery vehicle for the soft-produced microcapsules. According to Halim et al.19, gelatin was dissolved in water to prepare the jelly candy. After heating to a boil and cooling to 40 °C, the microcapsules of TPE, or the microcapsules of TPE with probiotics, of the required concentrations, were added to the jelly solution, then the mixture was poured into mould and cooled for an hour in the refrigerator.
Grouping and treatments
A total of thirty rats were acclimatized to laboratory conditions for 1 week before the starting time of the experiment, then divided into five groups (n = 6) with equal average weight as follows:
-
Control normal group (NC): Untreated rats.
-
Indomethacin group (INDO): Rats were subcutaneously injected with 10 mg/kg20 of indomethacin for 2 sequential days (16th and 17th).
-
Jelly candy group (JC): Rats daily fed 10 g jelly candy (from the 1st to the 18th day) and injected with 10 mg/kg of indomethacin for 2 sequential days (16th and 17th).
-
Jelly candy with the microcapsules of TPE group (JC + TPE): Rats daily fed 10 g jelly candy containing 0.8 g of TPE microcapsules (from the 1st to the 18th day) and injected with 10 mg/kg of indomethacin for 2 sequential days (16th and 17th).
-
Jelly candy with the microcapsules of TPE and probiotics group (JC + TPE + PC): Rats daily fed 10 g jelly candy containing 1 g of co-encapsulated TPE and probiotics (from the 1st to the 18th day) and injected with 10 mg/kg of indomethacin for 2 sequential days (16th and 17th).
TPE microcapsules (0.8 g) and the co-encapsulated TPE with probiotics (1 g) were produced from the extrusion of an equal volume of sodium alginate solution. The co-encapsulated TPE and probiotics (1 g) contained 108 CFU of probiotics. All over the experiment period, the animals fed on the aforementioned maintenance-balanced diet. The animal’s body weight was recorded at the baseline and the end of the experiment. Food intake was recorded daily. Body weight gain or loss was calculated. On the 18th day, blood was collected and the animals were scarified by cervical dislocation and dissected to remove the stomach, small intestine, and colon, which were then gently washed with saline. Both the colon and small intestine were measured for length. Through the microscopical examination, the scoring pattern method21 was applied to a section of rat stomach tissues and scored as follows:
No. visible change | 0 |
|---|---|
Hyperemia at sites | 1 |
Lesions with a diameter of l mm or less | 2 |
Lesions with a diameter of 2 mm or less than 5 | 5 |
Lesions with a diameter of more than 2 mm | 6 |
Number 5–10 | 7 |
Number > 10 | 8 |
Freshly excised stomach and small intestine from each group were washed with saline, and preserved in a 10% formaldehyde solution for histopathological examination. The paraffin-embedded blocks were sectioned at 5-micron thickness and stained with Hematoxylin and Eosin22 for histopathological examination by a light microscope (Olympus BX50, Japan). The histopathological alterations of the stomach and intestine were graded23 as follows:
No. changes | 0 |
|---|---|
Mild changes | + |
Moderate changes | + + |
Severe changes | + + + |
Estimation of the oxidative and inflammatory markers in the intestine and stomach homogenates
Small portions of the stomach and intestine were individually used to prepare homogenates (10% w/v) in a cold homogenization buffer (100 mM potassium phosphate buffer, pH 7.4). The homogenates were centrifuged for 10 min at 4 °C, and supernatants were used for conducting the needed biochemical assays. According to Sedlak and Lindsay24, Nishikimi et al.25, Montgomery and Dymock26, Aebi27, and Ohkawa et al.28, the collected supernatants were tested for reduced glutathione oxidase (GSH), superoxide dismutase (SOD), nitric oxide (NO), catalase (CAT), and malondialdehyde (MDA), respectively using UVPC spectrophotometer (Jasco V-730, serial No. A 112,361,798, Japan). Sandwich ELISA detection kits (SinoGeneclon Biotech Co., Ltd.) were used to measure interleukin-6 (IL-6), tumor necrosis factor (TNF-α), and interleukin-1β (IL-1β).
Estimation of serum liver and kidney biochemical indicators
According to Rheinhold and Seligron29, Bessey et al.30, Zimmerman and Weinstein31, and Reitman and Frankel32, respectively, the total protein, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine transaminase (ALT) in each rat’s serum were estimated using a UVPC spectrophotometer (Jasco V-730, serial No. A 112,361,798, Japan). Larsen33, Fawcett and Scott34, and Doumas et al.35 methods were used to assess the amounts of creatinine, urea, and albumin, respectively using a UVPC spectrophotometer. The albumin/globulin (A/G) and globulin ratios were computed.
Statistical analysis
Using SPSS version 21(SPSS Inc., Chicago, Illinois, USA), statistical analysis was conducted. The results were presented as mean ± standard error (SE) and one-way analysis of variance (ANOVA) was used to conduct a statistical analysis of the results. To examine the statistical variances between groups, the Duncan test was performed. P ≤ 0.05 was used to determine the difference’s statistical significance.
Ethics approval and consent to participate
The study was given approval by the National Research Center’s Medical Research Ethics Committee (MREC) with Ethical Approval Certificate No 1496072023. and all procedures were carried out in accordance with the ethical standards, all methods are reported in accordance with ARRIVE guidelines. Experimental research and field studies on plants including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Results and discussion
Characteristics of TPE
Yield, total phenolic content, radical scavenging activity and carotenoid content of TPE
Tomato pomace is one of the most valuable agro-industry by-products. This may be attributed to its enrichment of bioactive compounds such as polyphenols, carotenoids, pectin, dietary fibers, and fatty acids35. In this study, ultrasound-assisted extraction (UAE) was used in the preparation of TPE because it has been demonstrated to be an efficient method for obtaining bioactive molecules with high antioxidant activity from tomato seeds and peels. Additionally, it has the potential to be scaled up for the commercialization of tomato industrial wastes9. TPE recorded yield, total phenolic content, radical scavenging activity and carotenoids at 16.5 ± 0.04%, 2.27 ± 0.2 mg GAE, 4.7 ± 0.1 mg TE, and 184.7 ± 2.08 mg β-carotene equivalent per gram of dried TP, respectively (see Supplementary Table S1 online). The measured amounts are larger than those found in previous studies on tomato pomace from other varieties10,36. The obtained results are in accordance with the previous report37 on Egyptian tomato waste (skin and seeds) where tomato pomace was extracted by UAE with different solvents. El-Malah et al.37 found that aqueous and ethanol extracts of TP recorded the highest yield, antioxidant activity, and total phenolic content among the other solvent extracts. Additionally, it was found that the ethanol extract had a higher concentration of carotenoids than the aqueous extract37.
HPLC phenolic profile of TPE
In this study, 16 phenolic compounds were identified in TPE by the HPLC. According to the findings (Table 1 and Fig. 1), the most abundant phenolic compounds in TPE were ellagic acid, rutin, chlorogenic acid, and gallic acid. El-Malah et al.37 reported that rutin and rutin derivatives were the identified polyphenols in the pomace of the same Egyptian tomato variety. In different tomato varieties, chlorogenic acid, rutin, and naringenin were the main identified phenolic compounds in the extract of tomato seeds20 whereas Grassino et al38 found that chlorogenic acid and its derivatives were the major identified compounds in the extract of tomato skin.
Effect of TPE on the probiotic’s viability
With the TPE, the counts of Lactobacillus acidophilus and Bifidobacterium bifidum or their mixture (see Supplementary Table S2 and Fig. S1A–C, respectively online) increased from 22 × 105, 28 × 105, and 33 × 105 CFU, respectively (the initial counts) to 18 × 108, 25 × 108, and 45 × 108 CFU, respectively. These findings indicate to the promotion effect of TPE on the viability of L. acidophilus and B. bifidum or their mixture. The beneficial effect of TPE on the viability of probiotic bacteria may be attributed to its antioxidant activity and polyphenol content (as noted in the obtained results) that act as prebiotics. The beneficial effects of polyphenols on the viability of probiotic bacteria were demonstrated in several studies as confirmed by Ibrahim et al.39 who found that polyphenols of pomegranate peel improved the viability of probiotic bacteria. According to Garca-Alonso et al.40, there was a positive correlation between polyphenols intake and Lactobacillus bacteria while the polyphenols intake negatively correlated to the presence of Enterobacteriaceae. Garca-Alonso et al.40 also declared that the regulation of bacterial populations and SCFA concentrations may be positively affected by polyphenol ingestion.
Docking assay
Inhibitors of tumor necrosis factor are among the conventional treatments of gastrointestinal inflammation6. The inhibitors of tumor necrosis factor bond to its active sites, preventing receptor binding and thereby stopping the activation of downstream signaling complexes that induce inflammation and other signaling pathways41. Therefore, human TNF-α and IL-1β inflammatory cytokines were chosen as the target proteins. Enrichment of TPE in ellagic acid and rutin stimulated us to carry out the docking assay between them and the target proteins, however further studies on the other compounds are required. According to the findings (Table 2), rutin interacted with the active sites of TNF-α with hydrophobic interactions at the TYR 59, HIS 15, VAL 123 and LEU 55, LEU 57, and LEU 157 residues. Ellagic acid interacted with the active sites of TNF-α with hydrophobic interaction at the TYR 59 residue. Rutin interacted with the active sites of IL-1β with 3 conventional hydrogen bonds and hydrophobic interaction at the LYS 77 residue. Ellagic acid interacted with the active sites of IL-1β with 2 conventional hydrogen bonds and hydrophobic interaction at the PHE 133 residue. Zia et al.41 investigated the TNF-α inhibition activity of several compounds in silico and suggested that the docked compounds with strong hydrophobic interactions and hydrogen bond contacts with TNF-α inhibited the inflammation.
Characteristics of the produced microcapsules
Encapsulation efficiency (EE)
Despite of the potential health benefits of the natural phenolic compounds and antioxidants, the instability of these compounds’ physical and chemical properties represent a challenge when they are used in industrial processes. These limitations are overcome by microencapsulation42. The encapsulation techniques promote the viability and the effective delivery of living cells to the intended location. Furthermore, the encapsulation techniques protect living cells from the challenging physiological conditions of the stomach43. In this context, co-extrusion encapsulation of TPE and probiotics was used in this study. Encapsulation efficiency of TPE and TPE with probiotics were at 88.2 ± 0.8 and 90.2 ± 1.04%, respectively. According to Al-Hindi and Abd El Ghani44, probiotic bacterial counts increased upon adding polyphenols from pomegranate peel to fermented milk beverages. Using an aqueous delivery system, Shinde et al.45 showed that co-extrusion encapsulation of probiotic L. acidophilus and apple skin polyphenol extract recorded microencapsulation efficacy more than 96%. In the study by Al-Moghazy et al.46, co-extrusion encapsulation of B. lactis probiotic with pomegranate peel extract recorded a microencapsulation efficiency of more than 89.65%.
Morphological structure by SEM
The homogeneous shape of TPE and probiotic encapsulated beads is observed from the photograph image (Fig. 2A). The beads’ particles recorded about 1.5 mm in diameter. The spherical surface of the TPE and probiotic encapsulated beads could be seen in the SEM images (Fig. 2B,C). In the SEM image, the shrinkage on the surface of the beads’ particles might be attributed to the drying process.
The protective effect of the produced microcapsules against indomethacin-induced enterocolitis
Effect of the produced microcapsules on the growth performance
Non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and indomethacin are commonly used to treat pain, fever, and inflammation. However, administration of NSAIDs, due to its widespread use and ease of accessibility, causes gastrointestinal tract (GIT) ulcers. Indomethacin promotes the development of ulcers by suppressing prostaglandin production and increasing the production of free oxygen radicals, which in turn stimulate various of transcription factors resulting in the differential expression of some genes involved in inflammatory pathways47. According to Shanmugam et al.20, the treatment of indomethacin (10 mg/kg) was fatal and caused perforating tiny intestinal ulcers in rats through 2–4 days. In the current study, rats were injected with indomethacin for 2 sequential days (16th and 17th) to induce GIT ulceration. The protective effect of microencapsulated TPE either alone or with probiotics against GIT ulceration was evaluated. L. acidophilus and B. bifidum probiotic bacteria were selected in this study as they are the most common probiotics reported to have beneficial health effects. Additionally, they have been used safely in several food products48. In accordance with Shanmugam et al.20, upon the treatment with indomethacin, the body weight gain (see Supplementary Table S3 online) of the INDO group (34.67 g) significantly was less than the NC group (40.50 g). Also, the length of both the small intestine and colon of the INDO group (102.5 and 16.17 cm, respectively) significantly was higher than the NC group (95.42 and 13.83 cm, respectively). In contrast, the body weight gain was higher in rats with either TPE or the co-encapsulated TPE with probiotics than the INDO group. While, the length of both the small intestine and colon was less in rats treated with either TPE or the co-encapsulated TPE with probiotic than the INDO group as strong evidence for the reduction of inflammation condition. The results revealed that the jelly candy was not involved in any improvement in the studied parameters or improvement in inflammation, but it was only a way to deliver the produced microcapsules.
Effect of the produced microcapsules on oxidative and inflammatory markers in the stomach and intestine homogenates
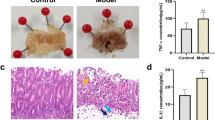
The pathophysiology of GIT ulceration and the excessive generation of ROS are both known to be significantly influenced by oxidative stress and an imbalance between oxidants and antioxidants49. Figures 3 and 4 show the findings of the oxidative and inflammatory markers of the stomach and intestine. In accordance with the results reported by Shanmugam et al.20 and Danisman et al.5, indomethacin treatment significantly altered the levels of inflammatory markers in the stomach and intestine, including IL-6 (76.33 ± 0.95 and 58.93 ± 0.93 pg/g tissue, respectively), IL-1β (35.84 ± 0.16 and 34.17 ± 0.31 pg/g tissue, respectively) and TNF-α (34.65 ± 0.46 and 20.72 ± 0.37 pg/g tissue, respectively) when compared to the normal control group. The levels of stomach and intestine oxidative markers including SOD (2.62 ± 0.07 and 2.73 ± 0.07 U/g tissue, respectively), CAT (239.83 ± 2.98 and 224.67 ± 1.14 μmol/g tissue, respectively), GSH (17.17 ± 10.94 and 16.32 ± 0.6 mg/g tissue, respectively), NO (4.27 ± 0.07 4.35 ± 0.07 nmol/g tissue, respectively) and MDA (37.5 ± 0.76 and 34.78 ± 0.57 μmol/g tissue, respectively) were drastically altered in the INDO group comparing to the normal control group. On the other hand, rats treated with TPE or the co-encapsulated TPE with probiotics exhibited lower levels of the stomach and intestine inflammatory markers as well as MDA and NO than the INDO group. Furthermore, rats treated with TPE or the co-encapsulated TPE with probiotics showed significantly higher activities of the antioxidant enzymes SOD, CAT and GSH than the INDO group. Comparable to the results of the INDO group, rats treated with the co-encapsulated TPE with probiotics exhibited significant improvement than TPE microcapsules. These effects might be attributed to the combined effect of probiotics and the bioactive compounds of TPE. According to the findings (see Supplementary Table S1 online and Table 1), carotenoids, polyphenols, and flavonoids are among the major bioactive compounds of TPE. Carotenoids (β-carotene, lutein, and lycopene) have antioxidant and anti-inflammatory properties, making them potentially beneficial phytochemicals for gut health50. Polyphenols can transfer electrons to the free radicals and suppress them, preventing cell damage. Additionally, polyphenols activate the antioxidant enzymes and reduce oxidative stress and inflammation51. The anti-inflammatory effect of flavonoid derivatives (EGCG, rutin, apigenin, naringenin) and phenolic acids was demonstrated by several reports52. Probiotics have become more well-liked in recent years as a result of their ability to treat diseases caused by inflammation. Probiotics are implicated in the enhancement of intestinal permeability, regulation of immune function, and reduction of pro-inflammatory cytokines53. Additionally, it has been demonstrated that probiotic bacteria and/or bacterial metabolites affect the host by changing the amounts of both endogenous and exogenous ROS resulting in oxidative stress reduction54.
Effect of the produced microcapsules on stomach inflammatory and oxidative markers. (A): TNF-α, (B): IL-1β, (C): IL-6, (D): MDA, (E): GSH, (F): NO, (G): SOD, (H): CAT. NC: normal control group, INDO: indomethacin group, JC: jelly candy group, JC + TPE: rats treated with jelly containing the microcapsules of tomato pomace extract, JC + TPE + PC: rats treated with jelly containing the microcapsules of tomato pomace extract with probiotics. Data are mean values ± SE (n = 6). A significant difference at P ≤ 0.05 can be identified from a different superscript letter in on the bars.
Effect of the produced microcapsules on intestinal inflammatory and oxidative markers. (A): TNF-α, (B): IL-1β, (C): IL-6, (D): MDA, (E): GSH, (F): NO, (G): SOD, (H): CAT. NC: normal control group, INDO: indomethacin group, JC: jelly candy group, JC + TPE: rats treated with jelly containing the microcapsules of tomato pomace extract, JC + TPE + PC: rats treated with jelly containing the microcapsules of tomato pomace extract with probiotics. Data are mean values ± SE (n = 6). A significant difference at P ≤ 0.05 can be identified from a different superscript letter on the bars.
Effect of the produced microcapsules on liver and kidney biochemical indicators
Supplementary Table S4 online includes information on the results of liver and kidney functions for the different groups. In line with Mahmoud et al.55 and Stephanie et al.56 but in contrast to the findings of Shanmugam et al.20, the levels of serum ALP (129.33 U/L), AST (43 U/L), ALT (33.17 U/L) and LDH (282.17 U/L) increased in the INDO group. However, no significant change among the groups was recorded for urea, creatinine, albumin, and total protein. Rats treated with TPE and the co-encapsulated TPE with probiotics recorded liver and kidney functions that were closely comparable to those of the control group. High serum activity of AST, ALT, and ALP enzymes, may be useful quantitative markers for assessing GIT injury. These enzymes are normally not present in serum; but, when tissue damage occurs, some of these enzymes may leak into the serum57. Similarly, the combination of probiotics and TPE bioactive substances may play a role in the significant effect on liver and kidney functions. Previously, L. acidophilus and B. bifidum administration in conjunction with aflatoxin maintained liver and kidney functions close to the normal rats58. Phenolic and flavonoid compounds, including ellagic acid and rutin, demonstrated protective effects against liver injury and preserved liver functions59,60.
Effect of the produced microcapsules on macroscopic changes and ulceration score
The macroscopic examination revealed that indomethacin had a more severe impact on the stomach than the small intestine, where it manifested as chronic intestinal inflammation. Therefore, the stomach ulcer score was determined. The calculated ulcer score (see Supplementary Table S5 online) was significantly less in TPE and the co-encapsulated TPE with probiotic groups compared with the INDO group. Moreover, the protection % of the co-encapsulated TPE with probiotics (85.91%) was significantly higher than the TPE microcapsules (71.94%). These results verified the protective effect of TPE and the co-encapsulated TPE with probiotic against gastrointestinal tract (GIT) ulceration. The decrease in ulcer score and the increase in the protection % of TPE may be attributed to the phenolic compounds as confirmed by20. Furthermore, the combination action of TPE and probiotics may be responsible for the decrease in lesion scores in rats treated with the co-encapsulated TPE and probiotics.
Effect of the produced microcapsules on morphological and histopathological alterations of stomach and intestine
All the recorded lesions in the stomach and intestine were scored as shown in Supplementary Table S6 online. The control animals’ stomach tissues had typical gross morphology (Fig. 5A). The gastric mucosa of the indomethacin-treated rats displayed obvious ulceration and bleeding foci (Fig. 5B). The macroscopic examination of the stomach from the jelly candy group displayed obvious ulceration and bleeding foci (Fig. 5C). The macroscopic examination of the stomach from the TPE microcapsules group showed mild erosion in the gastric mucosa (Fig. 5D). The macroscopic examination of the stomach from the the co-encapsulated TPE with probiotics group showed normal gastric mucosa (Fig. 5E).
Photographs (× 4) and light micrographs of stomachs from the studied groups (stained with H&E X100). (A) normal control, (B) indomethacin group, (C) jelly candy group, (D) TPE microcapsules group, E: TPE and probiotics microcapsules group. (a) Normal control group (0), (b) indomethacin group with multiple mucosal erosions (arrows) and submucosal edema together with blood vessel congestion (+ + +), (c) jelly candy group with multiple mucosal erosions (arrows) and submucosal edema without blood vessel congestion (+ +), (d) TPE microcapsules group with normal mucosa and mild submucosal edema (star) ( +), (e) TPE and probiotic microcapsules group with intact mucosa and normal submucosa (0).
Histopathologically, the stomach from the normal control group showed normal mucosa with normal intact epithelium and normal submucosa without edema nor blood vessel congestion (Fig. 5a). The stomach from the indomethacin group revealed multiple mucosal erosions and submucosal edema together with blood vessels congestion (Fig. 5b). Jelly candy group showed as multiple mucosal erosions, as the indomethacin group, together with submucosal edema without blood vessel congestion (Fig. 5c). In TPE microcapsules group, the stomach mucosa was as normal intact as recorded in control group, while the submucosa showed mild edema (Fig. 5d), while the stomach from the co-encapsulated TPE with probiotics group recorded no histological alterations than the control normal group (Fig. 5e).
The control animals’ intestinal tissues had typical gross morphology (Fig. 6A). The indomethacin-treated animals’ intestines displayed chronic inflammation (Fig. 6B). The intestine from the jelly candy group displayed chronic inflammation also (Fig. 6C). The macroscopic examination of the intestine from the TPE microcapsules group showed a normal intestine (Fig. 6D). The macroscopic examination of the intestine from the co-encapsulated TPE with probiotics group showed a normal intestine (Fig. 6E).
Photographs (× 4) and light micrographs of intestines from the studied groups (stained with H&E X100). (A) normal control, (B) indomethacin group, (C) jelly candy group, (D) TPE microcapsules group, E: TPE and probiotics microcapsules group. (a) Normal control group (0), (b). indomethacin group with multiple mucosal erosions (arrows) and submucosal edema (+ + +), (c) jelly candy group with few mucosal erosions (arrows), there is no submucosal edema (+ +), (d) TPE microcapsules group with mild mucosal epithelium sloughing and normal submucosa ( +), (e) TPE and probiotic microcapsules group with intact mucosa and normal submucosa (0).
Histopathologically, the intestine from the normal control group showed normal mucosa with normal intact epithelium, intestinal glands, and normal submucosa without edema nor blood vessel congestion (Fig. 6a). The intestine from the indomethacin group revealed multiple mucosal erosions and submucosal edema (Fig. 6b). The jelly candy group showed fewer mucosal erosions than the indomethacin group, there was no submucosal edema (Fig. 6c). In the TPE group, the intestinal mucosa revealed mild epithelial sloughing, there was no edema in the submucosa (Fig. 6d), while the intestine from the co-encapsulated TPE with probiotics group recorded no histological alterations than the control normal group (Fig. 6e).
Conclusions
The obtained results demonstrated the antioxidant activity of tomato pomace extract, which may be strongly related to its phenolic component and carotenoid content. The presence of TPE in the growth media increased the viability of Lactobacillus acidophilus and Bifidobacterium bifidum probiotic bacteria. According to the docking results, the extract’s highest concentrations of phenolic compounds, ellagic acid, and rutin, were demonstrated to bind to the active sites of TNF-α and IL-1β cytokines, suggesting that these compounds may be responsible for the extract’s anti-inflammatory actions. When probiotic bacteria were co-encapsulated with TPE, high encapsulation efficiency was recorded. The reduction in oxidative stress and inflammation was demonstrated by the reduction in stomach and intestine MDA, NO, IL-1β, IL-6, and TNF-α levels and the increase in CAT, SOD, and GSH activities in rats treated with TPE microcapsules and the co-encapsulated TPE with probiotics. The produced microcapsules are thought to be promising candidates for protecting against indomethacin-induced erosion and stomach ulcers.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- TP:
-
Tomato pomace
- TPE:
-
Tomato pomace extract
- HPLC:
-
High-performance liquid chromatography
- SEM:
-
Scanning electron microscope
- EE:
-
Encapsulation efficiency
- JC:
-
Jelly candy
- IBD:
-
Inflammatory bowel disease
- IL-1β:
-
Interleukin 1 beta
- TNF-α:
-
Tumor necrosis factor alpha
- IL-6:
-
Interleukin 6
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- LDH:
-
Lactate dehydrogenase
- GIT:
-
Gasrointestinal tract
- CAT:
-
Catalase
- GSH:
-
Reduced glutathione
- NO:
-
Nitric oxide
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
References
Corrêa-Filho, L. C., Lourenço, S. C., Duarte, D. F., Moldão-Martins, M. & Alves, V. D. Microencapsulation of tomato (Solanum lycopersicum L.) pomace ethanolic extract by spray drying: Optimization of process conditions. Appl. Sci. 9(3), 612. https://doi.org/10.3390/app9030612 (2019).
Kaulmann, A. & Bohn, T. Bioactivity of polyphenols: preventive and adjuvant strategies toward reducing inflammatory bowel diseases—promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016, 9346470. https://doi.org/10.1155/2016/9346470 (2016).
Vakadaris, G. et al. The role of probiotics in inducing and maintaining remission in Crohn’s disease and ulcerative colitis: A systematic review of the literature. Biomedicines 11(2), 494. https://doi.org/10.3390/biomedicines11020494 (2023).
Wang, Z. B. et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2019, 3862971. https://doi.org/10.1155/2019/3862971 (2019).
Danisman, B. et al. Tsatsakis and A. Taghizadehghalehjoughi, carnosic acid ameliorates indomethacin-induced gastric ulceration in rats by alleviating oxidative stress and inflammation. Biomedicines 11(3), 829. https://doi.org/10.3390/biomedicines11030829 (2023).
Cai, Z., Wang, S. & Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 8, 765474. https://doi.org/10.3389/fmed.2021.765474 (2021).
Reifen, R., Nur, T., Matas, Z. & Halpern, Z. Lycopene supplementation attenuates the inflammatory status of colitis in a rat model. Int. J. Vitamin Nutr. Res. 71(6), 347–351. https://doi.org/10.1024/0300-9831.71.6.347 (2001).
Liso, M. et al. A bronze-tomato enriched diet affects the intestinal microbiome under homeostatic and inflammatory conditions. Nutrients 10(12), 2018. https://doi.org/10.3390/nu10121862 (1862).
Solaberrieta, I., Mellinas, C., Jiménez, A. & Garrigós, M. C. Recovery of antioxidants from tomato seed industrial wastes by microwave-assisted and ultrasound-assisted extraction. Foods 11(19), 3068. https://doi.org/10.3390/foods11193068 (2022).
Toor, R. K. & Savage, G. P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 38(5), 487–494. https://doi.org/10.1016/j.foodres.2004.10.016 (2005).
Szabo, K., Diaconeasa, Z., Cătoi, A. F. & Vodnar, D. C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 8(8), 292. https://doi.org/10.3390/antiox8080292 (2019).
Jamaleddine, A. et al. In vitro bioactivities of extracts from tomato pomace. Front. Biosci. 27(9), 259. https://doi.org/10.31083/j.fbl2709259 (2022).
De Man, J. C., Rogosa, M. & Sharpe, M. E. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 23(1), 130–135. https://doi.org/10.1111/j.1365-2672.1960.tb00188.x (1960).
Pasala, P. K. et al. Molecular docking and in vivo immunomodulatory activity of Albizia procera bark on doxorubicin induced immunosuppressive rats. J. King Saud Univ.-Sci. 34(3), 101828. https://doi.org/10.1016/j.jksus.2022.101828 (2022).
Othman, I. M. et al. Toward a treatment of antibacterial and antifungal infections: Design, synthesis and in vitro activity of novel arylhydrazothiazolylsulfonamides analogues and their insight of DFT, docking and molecular dynamic simulations. J. Mol. Struct. 1243, 130862. https://doi.org/10.1016/j.molstruc.2021.130862 (2021).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461. https://doi.org/10.1002/jcc.21334 (2010).
Toprakçı, İ, Torun, M. & Şahin, S. Development of an encapsulation method for trapping the active materials from sour cherry biowaste in alginate microcapsules. Foods. 12(1), 130. https://doi.org/10.3390/foods12010130 (2022).
Reeves, P. G., Nielsen, F. H. & Fahey, G. C. AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition Ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 123(11), 1939–1951. https://doi.org/10.1093/jn/123.11.1939 (1993).
Halim, Y., Nugroho, R. D. T. & Handayani, R. Application of guava leaves extract on jelly candy to inhibit Streptococcus mutans. J. Appl. Biol. Biotechnol. 9, 104–111. https://doi.org/10.7324/jabb.2021.9414 (2021).
Shanmugam, S. et al. Protective effects of flavonoid composition rich P. subpeltata Ortega. on indomethacin induced experimental ulcerative colitis in rat models of inflammatory bowel diseases. J. Ethnopharmacol. 248, 112350. https://doi.org/10.1016/j.jep.2019.112350 (2020).
Morris, G. P. et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 96(3), 795–803. https://doi.org/10.1016/0016-5085(89)90904-9 (1989).
Bancroft, D., Stevens, A. & Tumer, R. Theory and Practice of Histologic Technique 4th edn. (Churchill Livingstone, 2012).
Arsad, S. S., Esa, N. M. & Hamza, H. Histopathologic changes in liver and kidneys tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) Schott extract. J. Cytol. Histol. S4, 001. https://doi.org/10.4172/2157-7099.s4-001 (2014).
Sedlak, J. & Lindsay, R. H. Estimation of total, protein-bound and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analyt. Biochem. 25, 192–205. https://doi.org/10.1016/0003-2697(68)90092-4 (1968).
Nishikimi, M., Rao, N. A. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(2), 849–854. https://doi.org/10.1016/s0006-291x(72)80218-3 (1972).
Montgomery, H. A. C. D. J. & Dymock, J. F. Determination of nitrite in water. Analyst 87, 374. https://doi.org/10.1039/an9628700374 (1961).
Aebi, H. Catalase in vitro. Methods Enzymol. 105, 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3 (1984).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. https://doi.org/10.1016/0003-2697(79)90738-3 (1979).
Rheinhold, J. & Seligron, D. Total Protein, Albumin and Globulin in Standard Methods of Clinical Chemistry (Academic Press, Inc., 1953).
Bessey, O. A., Lowry, O. H. & Brock, M. J. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 164(1), 321–329. https://doi.org/10.1016/s0021-9258(18)43072-4 (1946).
Zimmerman, H. J. & Weinstein, H. G. Lactic dehydrogenase activity in human serum. J. Lab. Clin. Med. 48, 607–616 (1956).
Reitman, S. & Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 28(1), 56–63. https://doi.org/10.1093/ajcp/28.1.56 (1957).
Larsen, K. Creatinine assay by a reaction-kinetic principle. Clin. Chim. Acta. 41, 209–217. https://doi.org/10.1016/0009-8981(72)90513-x (1972).
Fawcett, J. K. & Scott, J. E. A rapid and precise method for the determination of urea. J. Clin. Pathol. 13(2), 156–159. https://doi.org/10.1136/jcp.13.2.156 (1960).
Doumas, B. T., Watson, W. A. & Biggs, H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 31(1), 87–96. https://doi.org/10.1016/0009-8981(71)90365-2 (1971).
Szabo, K., Dulf, F. V., Diaconeasa, Z. & Vodnar, D. C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. Lwt 116, 108558. https://doi.org/10.1016/j.lwt.2019.108558 (2019).
El-Malah, M. H., Hassanein, M. M. M., Areif, M. H. & Al-Amrousi, E. F. Utilization of Egyptian tomato waste as a potential source of natural antioxidants using solvents, microwave and ultrasound extraction methods. Am. J. Food Technol. 10(1), 14–25. https://doi.org/10.3923/ajft.2015.14.25 (2015).
Ninčević Grassino, A. et al. Valorisation of tomato peel waste as a sustainable source for pectin, polyphenols and fatty acids recovery using sequential extraction. Waste Biomass Valoriz. 11(9), 4593–4611. https://doi.org/10.1007/s12649-019-00814-7 (2019).
Ibrahim, A., Awad, S. & El-Sayed, M. Impact of pomegranate peel as prebiotic in bio-yoghurt. Br. Food J. 122(9), 2911–2926. https://doi.org/10.1108/bfj-04-2019-0296 (2020).
García-Alonso, F. J. et al. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD). Food Funct. 8(10), 3542–3552. https://doi.org/10.1039/c7fo00393e (2017).
Zia, K. et al. Identification of potential TNF-α inhibitors: From in silico to in vitro studies. Sci. Rep. 10(1), 20974. https://doi.org/10.1038/s41598-020-77750-3 (2020).
Shaygannia, S., Eshaghi, M. R., Fazel, M. & Hashemiravan, M. The effect of microencapsulation of phenolic compounds from lemon waste by persian and basil seed gums on the chemical and microbiological properties of Mayonnaise. Prev. Nutr. Food Sci. 26(1), 82–91. https://doi.org/10.3746/pnf.2021.26.1.82 (2021).
Singh, S. et al. Natural sources and encapsulating materials for probiotics delivery systems: Recent applications and challenges in functional food development. Front. Nutr. 9, 971784. https://doi.org/10.3389/fnut.2022.971784 (2022).
Al-Hindi, R. R. & Abd El Ghani, S. Production of functional fermented milk beverages supplemented with pomegranate peel extract and probiotic lactic acid bacteria. J. Food Qual. 2020, 4710273. https://doi.org/10.1155/2020/4710273 (2020).
Shinde, T., Sun-Waterhouse, D. & Brooks, J. Co-extrusion encapsulation of probiotic Lactobacillus acidophilus alone or together with apple skin polyphenols: An aqueous and value-added delivery system using alginate. Food Bioproc. Tech. 7(6), 1581–1596. https://doi.org/10.1007/s11947-013-1129-1 (2014).
Al-Moghazy, M., El-Sayed, H. S. & Abo-Elwafa, G. A. Co-encapsulation of probiotic bacteria, fish oil and pomegranate peel extract for enhanced white soft cheese. Food Biosci. 50, 102083. https://doi.org/10.1016/j.fbio.2022.102083 (2022).
Khalaf, H. M., Ahmed, S. M., Welson, N. N. & Abdelzaher, W. Y. Rivastigmine ameliorates indomethacin experimentally induced gastric mucosal injury via activating α7nAChR with inhibiting oxidative stress and apoptosis. J. Biochem. Mol. Toxicol. 36(10), e23147. https://doi.org/10.1002/jbt.23147 (2022).
Hassani, M., Sharifi, A., Mohammadi Sani, A. & Hassani, B. Growth and survival of Lactobacillus Acidophilus and Bifidobacterium Bifidum in probiotic yogurts enriched by barberry extract. J. Food Saf. 36(4), 503–507. https://doi.org/10.1111/jfs.12269 (2016).
Almeer, R. S., Mahmoud, S. M., Amin, H. K. & Abdel Moneim, A. E. Ziziphus spinachristi fruit extract suppresses oxidative stress and p38 MAPK expression in ulcerative colitis in rats via induction of Nrf2 and HO-1 expression. Food Chem. Toxicol. 115, 49–62. https://doi.org/10.1016/j.fct.2018.03.002 (2018).
Silva Meneguelli, T. et al. Effect of carotenoids on gut health and inflammatory status: A systematic review of in vivo animal studies. Crit. Rev. Food Sci. Nutr. https://doi.org/10.1080/10408398.2023.2234025 (2023).
Liu, W. et al. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 193, 106812. https://doi.org/10.1016/j.phrs.2023.106812 (2023).
Rakotondrabe, T. F., Fan, M. X., Muema, F. W. & Guo, M. Q. Modulating inflammation-mediated diseases via natural phenolic compounds loaded in nanocarrier systems. Pharmaceutics 15(2), 699. https://doi.org/10.3390/pharmaceutics15020699 (2023).
Cristofori, F. et al. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 12, 578386. https://doi.org/10.3389/fimmu.2021.578386 (2021).
Zheng, Y. et al. Probiotics supplementation attenuates inflammation and oxidative stress induced by chronic sleep restriction. Nutrients 15(6), 1518. https://doi.org/10.3390/nu15061518 (2023).
Mahmoud, S. H. et al. Effect of indomethacin (Non steroidal anti-infilammatory drug) on intestinal permeability and liver functions. Int. J. Med. 114, hcab114. https://doi.org/10.1093/qjmed/hcab114.005 (2021).
Stephanie, T. A., David, O. O. & Farombi, E. O. Thymol Reduces Hepatorenal Oxidative Stress, Inflammation and Caspase-3# xd; Activation in Rats Exposed to Indomethacin. Egypt. J. Basic Appl. Sci. 9(1), 136–150. https://doi.org/10.1080/2314808x.2022.2050114 (2022).
Appidi, J. R., Yakubu, M. T., Grierson, D. S. & Afolayan, A. J. Toxicological evaluation of aqueous extracts of Hermannia incana Cav. leaves in male Wistar rats. Afr. J. Biotechnol. 8, 2016–2020. https://doi.org/10.1358/mf.2010.32.1.1464613 (2009).
Badr, A. N. et al. Protective impact of encapsulated probiotics and Ziziphus spinachristi against liver oxidative stress and DNA damage induced by aflatoxin M1. J. Appl. Pharmaceut. Sci. 12, 082–094. https://doi.org/10.7324/japs.2022.120809 (2022).
Rahmani, S., Naraki, K., Roohbakhsh, A., Hayes, A. W. & Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 11(1), 39–56. https://doi.org/10.1002/fsn3.3041 (2023).
Zhao, L. et al. Protective effects of ellagic acid against alcoholic liver disease in mice. Front. Nutr. 8, 744520. https://doi.org/10.3389/fnut.2021.744520 (2021).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
K.F. and R.S.M. performed the animal study, wrote the main manuscript text, and prepared tables and figures. A.M.M. performed the microbiological analysis, wrote the main manuscript text, and prepared Figure S1. S.S.A. performed the histopathology examination, wrote the main manuscript text, and prepared the histopathological figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fouda, K., Mabrouk, A.M., Abdelgayed, S.S. et al. Protective effect of tomato pomace extract encapsulated in combination with probiotics against indomethacin induced enterocolitis. Sci Rep 14, 2275 (2024). https://doi.org/10.1038/s41598-024-52642-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-52642-y