Abstract
Both body mass index (BMI) and family history of cancer are established risk factors for female breast cancer. However, few studies explored the potential interaction between both factors. We assessed the association of BMI and its interaction with family cancer history on the risk of female breast cancer in Shanghai, China. Based on a population-based prospective cohort study started from 2008 to 2012 with 15,055 Chinese female participants in Minhang district, Shanghai. Cox regression models were used to estimate the association of BMI and its interaction with a family history of cancer on breast cancer risk. The additive interaction was evaluated by the relative excess risk due to interaction (RERI) and the attributable proportion due to interaction (AP), and the multiplicative interaction was assessed by the product term (BMI* family history of cancer) in the Cox regression model. Compared with BMI of < 24 kg/m2 and no family history of cancer, women with BMI of ≥ 24 kg/m2 and a family history of cancer had a higher risk for breast cancer with HR 2.06 (95% CI 1.39, 3.06). There was an additive interaction between BMI and family history of cancer on breast cancer incidence, with the RERI being 0.29 (95% CI 0.08, 0.51) and the AP being 0.37 (95% CI 0.08, 0.66). The coexistence of obesity and cancer family history may exacerbate breast cancer incidence risk, highlighting the importance of weight management in women with a family history of cancer.
Similar content being viewed by others
Introduction
Breast cancer is the most commonly diagnosed cancer worldwide, with cases expected to reach 4.4 million by 20701,2 Among women, breast cancer accounts for approximately 25.8% of all cancer cases and 15.6% of cancer deaths based on GloboCan 20201,3. Recent socioeconomic developments and lifestyle changes in some Asian countries, including China, have led to an increase in breast cancer incidence rate4. Breast cancer is now the most common cancer among Chinese women, with cases in China accounting for 12.2% of newly diagnosed cases worldwide5,6,7. As a result of the high incidence of breast cancer, not only women will be burdened with this disease, but also families and society will bear the high expense of medical treatment8.
Among women, obesity and a family history of cancer are both established risk factors for breast cancer9,10,11. Women with a high body mass index (BMI) have a much higher risk of breast cancer than those with a normal BMI12. A study showed that with each 5 kg/m2 increase in BMI, the risk of breast cancer increased by 31%13. The effect of family history of cancer as an unmodifiable risk factor for breast cancer has also been explored by many studies, with the population-attributable risk proportion of family history of cancer being 8.7%14,15. Although the relationship between obesity and family cancer history on breast cancer has been well established, the interaction between BMI and family cancer history on breast cancer remains unclear. A good understanding of the interaction is vital to identify target groups for weight management advice and effectively reduce the risk of breast cancer.
In this prospective population-based cohort study, we investigated the possible nonlinear association between BMI and the incidence of breast cancer in women. On this basis, we further explored the interaction of BMI and cancer family history on the incidence of breast cancer and quantified the increased risk of breast cancer caused by a higher BMI in women with a family history of cancer. The study is essential to clarify whether there is an additional benefit of interventions for obesity among female individuals with a family history of cancer.
Materials and methods
Study population
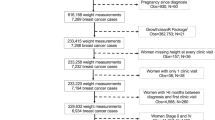
This population-based prospective cohort study was based on a community colorectal cancer screening program implemented in an urban–rural integration town named Qibao, located in Minhang District, south-eastern Shanghai, China16. 15,101 female participants were initially enrolled in the study from 2008 to 2012. We excluded 46 participants with follow-up durations of less than 3 months (N = 9), missing demographic information (N = 30), and missing information on exposures and other covariates (N = 7). Ultimately, 15,055 participants were included in this study. All participants were followed up until the date of cancer diagnosis, death, or loss to follow-up, or June 30, 2021.
Data collection
During the baseline recruitment period, data were collected through face-to-face interviews by trained interviewers using a standard questionnaire that included demographic and lifestyle factors such as sex (male, female), birthday, education level (primary, secondary, tertiary), marital status (married/remarried, divorced/separated/widowed/unmarried), 3-month income level (< 2000¥, 2000–4000¥, ≥ 4000¥), smoking status (yes, no), drinking status (yes, no), frequency of eating preserved/fried and smoked/high-fat foods per week (at least once, not even once) and intake of fruits and vegetables per day (< 300 g, ≥ 300 g). Data on the diagnosis of type 2 diabetes (yes, no) were identified through linkage to the Diabetes Standardized Management Program (DSMP) based on the local electronic health record management system (EHR)17.
According to the standard protocol, height and weight were measured without shoes and light clothing, and BMI was calculated as weight (kg) divided by the square of height(m). The BMI cut-off for Chinese individuals proposed by the China Obesity Task Force was used to classify participants into four groups: underweight, < 18.5 kg/m2; normal weight, 18.5–23.9 kg/m2; overweight, 24–27.9 kg/m2; and obesity, ≥ 28 kg/m218. Family history of cancer was divided into family history of cancer (including parents, siblings, children, second-degree relatives, and collateral relatives with cancer) and no family history of cancer according to baseline self-report data.
Definition of outcome
The outcome of interest in this study was the incidence of breast cancer cases (Classification of Diseases and Related Problems, 10th Revision, Clinical Modification, C50) during the study period. Until June 30, 2021, newly diagnosed breast cancer patients were identified using a record linkage system with the Shanghai Cancer Registry and Shanghai Vital Statistics through the Chinese Resident Identity Card number19. Person-years of follow-up were calculated from the date that participants were first investigated to the date on which breast cancer was diagnosed, or death date, or June 30, 2021, whichever occurred first.
Statistical analyses
Participants were grouped by the category of baseline BMI, and baseline characteristics were presented as the median (interquartile range) for continuous variables and as the frequency (%) for categorical variables. The χ2 test for categorical variables and the Kruskal‒Wallis test for continuous variables were used to compare demographic characteristics and lifestyle factors. We presented the cumulative incidence risk of breast cancer across baseline BMI classifications.
We presented the crude incidence rate(CIR) of breast cancer across baseline BMI classifications and family history of cancer. The CIR calculation formula is as follows:
After adjusting for covariates, Cox proportional hazard models were used to estimate the association of BMI and the joint effect between BMI and family history of cancer on breast cancer risk. The proportional hazard assumption for BMI and family history of cancer were evaluated using log–log survival plots20,21. The potential curvilinear relationship of BMI with breast cancer risk was assessed by restricted cubic splines (RCS) using the 5th, 50th, and 95th percentiles as fixed knots. The BMI equal to 24 kg/m2 was chosen as the reference group. A p value for nonlinearity < 0.05 suggested a nonlinear association between BMI and breast cancer.
The interactions between BMI and family history of cancer on breast cancer were measured using additive and multiplicative scales. The coefficient of the product term (BMI* family history of cancer) in the Cox regression model assesses the multiplicative interaction. Three indicators of additive interaction were used to evaluate the interaction between BMI and a family history of cancer: (1) the relative excess risk due to interaction (RERI), (2) the attributable proportion due to interaction (AP), and (3) the synergy index (SI), defined as follows22:
Here HR11 represented the hazard ratio of women with BMI ≥ 24 and with family history of cancer, HR10 for women with BMI ≥ 24 and no family history of cancer, and HR01 for women with BMI < 24 and with family history of cancer. There was an additive interaction if RERI and AP were equal to 0 or S was unequal to 1. SAS version 9.4 software (SAS Institute, Cary, NC, USA) was used to analyze the data. All statistical analyses were two-sided, and a result for which P < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The study was approved by the Institutional Ethical Approval Committee of the Center for Disease Prevention and Control of Minhang district, Shanghai, China. The Institutional Review Board of the Center for Disease Prevention and Control in Minhang District, Shanghai, waived the requirement for informed consent from participants involved in the study, as the data analyzed in the study were compiled from anonymized data from electronic medical records. All methods were carried out by the relevant guidelines and regulations.
Results
Basic demographic characteristics
A total of 15,055 women were included in the analyses. Table 1 shows the demographic characteristics and lifestyle factors by baseline BMI categories. Among all participants, the median age was 55.87 years, and 34.0% were over 60 years old. Significant differences were found for age(P < 0.001), marriage status(P < 0.001), educational levels(P < 0.001), 3-month income levels(P < 0.001), diabetes status(P < 0.001), frequency of eating preserved food per week(P = 0.025) and frequency of eating high-fat food per week(P < 0.001) across BMI categories.
Breast cancer incidence and hazard ratio by BMI and family history of cancer
As shown in Table 2, after 174,281.91 person-years of follow-up, 196 participants were diagnosed with breast cancer, giving rise to a CIR of 110.74 (95% CI 96.19, 127.52)/100,000 person-years. The incidence risk of breast cancer in the obese group was highest, with a CIR of 166.32 (95% CI 126.73, 218.26)/100,000 person-years, which was 2.09 (95% CI 1.42, 3.07) times the incidence risk of the normal weight group after adjusting for other variables. The breast cancer incidence risk of participants with cancer family history was 1.63 (95% CI 1.22, 2.49) times the incidence risk of participants without family history of cancer after adjusting for other variables, with CIR of 163.08 (95% CI 130.81, 203.32)/100,000 person-years. Figure 1 shows cumulative incidence risk of breast cancer estimates based on baseline BMI categories after accounting for competing risks (Gray chi-square = 8.55, P = 0.036).
As shown in Fig. 2, BMI was positively associated with breast cancer (Poverall = 0.001) in a linear pattern (Pnonlinearity = 0.165). Statistically speaking, there was no correlation between BMI and breast cancer when BMI was less than 24 kg/m2. However, when BMI was greater than 24 kg/m2, breast cancer incidence risk rose as BMI increased. As shown in Fig. 3, BMI was significantly related to the risk of breast cancer for women with a family history of cancer (Poverall = 0.004) in a linear pattern (Pnonlinearity = 0.097). Similarly, after BMI was greater than 24 kg/m2, BMI was positively correlated with the incidence of breast cancer. However, for women without a family history of cancer, BMI was not statistically associated with breast cancer risk (Poverall = 0.116).
HR (95% CIs) between BMI (kg/m2) and breast cancer, allowing non-linear effects (adjusted for age; marriage status; education; personal monthly income; smoking; drinking; daily fruit and vegetable intake; frequency of eating preserved food per week; frequency of eating fried and smoked food per week; frequency of eating high-fat food per week; diabetes; family history of cancer). The reference BMI (with HR fixed as 1.0) was 24 kg/m2.
HR (95% CIs) between BMI (kg/m2) and breast cancer, stratified by family history of cancer, allowing non-linear effects (adjusted for age; marriage status; education; personal monthly income; smoking; drinking;daily fruit and vegetable intake; frequency of eating preserved food per week; frequency of eating fried and smoked food per week; frequency of eating high-fat food per week; diabetes). The reference BMI (with HR fixed as 1.0) was 24 kg/m2.
Interaction and Joint effect between BMI and family history of cancer
Table 3 shows the joint association between BMI and family history of cancer. The statistical association between family history of cancer and breast cancer incidence only existed among women with BMI ≥ 24, but not among women with BMI < 24.Compared with BMI of < 24 kg/m2 and no family history of cancer, women with BMI of ≥ 24 kg/m2 and a family history of cancer had a higher risk of breast cancer, with a CIR of 186.54 (95% CI 138.25, 251.53) and the adjusted hazards ratio was 2.06 (95% CI 1.39, 3.06). We found a positive additive interaction between BMI and family history of cancer, with the RERI being 0.29 (95% CI 0.08, 0.51) and the AP being 0.37 (95% CI 0.08, 0.66). The multiplicative interactions were not statistically significant (HR:1.25; 95% CI 0.71, 1.65).
Discussion
In this population-based cohort study, we found that obesity and cancer family history were associated with a higher risk of breast cancer in females. Among 15,055 participants, when BMI ≥ 24, the risk of breast cancer increased as BMI increased. The same trend can be observed in women with family history of cancer, but not in women without family history of cancer, indicating that the association between BMI and breast cancer is affected by whether there is family history of cancer. Moreover, there was an interaction between overweight/obesity and cancer family history. When both risk factors were presented, the risk of breast cancer incidence was 2.06-fold that of neither risk factor (95% CI 1.39, 3.06). Although multiplicative interaction was not significant, a positive additive interaction between BMI and family history of cancer on breast cancer incidence was observed [RERI:0.29 (HR 0.08–0.51)], implying that BMI ≥ 24 and family history of cancer together may have amplified association on the incidence breast cancer compared to the sum of their individual associations.
The results of our study show that women who are overweight or obese have a significantly increased risk of breast cancer, which is consistent with the findings of other studies7,23,24.In postmenopausal women, a positive association between BMI and breast cancer risk has been observed in several studies25,26. Our findings are consistent with previous research results. Some studies have also shown that the incidence of breast cancer in women with family history is much higher than that in women without family history27,28, and family history of cancer other than family history of breast cancer is also related to the incidence of breast cancer29. However, there has been no report about the interaction and joint effect between BMI and cancer family on the risk of breast cancer in previous studies.
Our study revealed the additive interaction between BMI and cancer family history on the incidence of breast cancer. Although multiplicative interaction was not significant, we found a positive association between BMI and breast cancer incidence among women with a family history of cancer. The association between BMI and breast cancer incidence may be modified by a family history of cancer. Additive interaction measures the absolute change of risk, while multiplicative interaction measures the relative risk change. Additive interaction has more public health significance and is more related to biological interaction30,31
Based on the findings of our study, more efforts should be invested in promoting keeping fit as a way to reduce the risk of breast cancer among women with cancer family history. Therefore, reasonable weight control can not only reduce the risk of overweight and obesity on breast cancer but can also reduce the combined risk of BMI and cancer family history on breast cancer.
The mechanisms and causal pathways of obesity affecting the onset of breast cancer are complex. For example, increased estrogens, insulin resistance, mammary fat inflammation, increased aromatase expression, and elevated leptin levels are all thought to play a role in the pathogenesis of obesity-associated breast cancer32,33,34,35,36,37. The specific mechanism of the interaction between BMI and a family history of cancer has not yet been reported. However, there was evidence of an interaction between breast density and genetic risk measures, with low breast density more likely to carry high-risk breast cancer susceptibility mutations and an inverse association between breast density and BMI38,39. It may be one of the mechanisms by which BMI interacts with a family history of cancer in breast cancer incidence.
Overweight and obesity are modifiable risk factors, while family history of cancer is not. Nevertheless, the prevalence of obesity is on the rise in China as a result of the development of Chinese regional society and a change in people's lifestyles40. China has thus far been the country with the highest number of obese people, resulting in enormous health, economic, and social risks, making obesity prevention and control a very serious public health problem41,42. Overweight and obesity, in addition to being associated with breast cancer on their own, also interacted with a family history of cancer to increase breast cancer risk, according to our study. Therefore, interventions and treatments for overweight and obesity in women with a family history of cancer have high public health benefits.
This study analyzed the association between BMI and female breast cancer and combined it with restricted cubic splines to assess the potential nonlinear association between the two. Further analysis of the interaction between BMI and cancer family history on breast cancer risk provided new insights into the causal pathways of breast cancer incidence. Several limitations should also be noted. First, cancer family history was collected through face-to-face interviews, which may have induced some self-report and recall biases. Second, the variables of height and weight were only measured once at baseline, but the human body weight may change over the follow-up duration. In future studies, the variables should be measured multiple times to determine the relationship between body changes and breast cancer risk in women. Third, information about the use of hormones, menopausal age, and physical exercise of the women that may affect the conclusions of this study was not collected. In follow-up studies, we need to collect for further research.
Conclusions
Our study suggested that obesity and family history of cancer together may have an amplified association on the risk of breast cancer compared to their independent association, highlighting the importance of keeping fit in women with family history of cancer, which suggested that obesity status should be given greater attention in high-risk groups with a family history of cancer and that obesity interventions in women with a family history of cancer may be able to provide additional health care benefits.
Data availability
The datasets generated and analyzed in the study are not publicly available but are available from the corresponding authors at reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Soerjomataram, I. & Bray, F. Planning for tomorrow: Global cancer incidence and the role of prevention 2020–2070. Nat. Rev. Clin. Oncol. 18(10), 663–672 (2021).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72(6), 524–541 (2022).
Lei, S. et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (Lond.) 41(11), 1183–1194 (2021).
Fan, L. et al. Breast cancer in China. Lancet Oncol. 15(7), e279–e289 (2014).
Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 66(2), 115–132 (2016).
Xu, H. L. et al. Body mass index and cancer risk among Chinese patients with type 2 diabetes mellitus. BMC Cancer 18(1), 795 (2018).
Meng, Q. et al. Trends in access to health services and financial protection in China between 2003 and 2011: A cross-sectional study. Lancet 379(9818), 805–814 (2012).
Picon-ruiz, M. et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67(5), 378–397 (2017).
Organization WH. Obesity and overweight. Fact sheet number 311. World Health Organisation, 2014: 1–5.
Frank, C. et al. The population impact of familial cancer, a major cause of cancer. Int. J. Cancer 134(8), 1899–1906 (2014).
Benn, M. et al. High body mass index and cancer risk-a Mendelian randomisation study. Eur. J. Epidemiol. 31(9), 879–892 (2016).
Renehan, A. G. et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 371(9612), 569–578 (2008).
Zheng, G. et al. Family history of breast cancer as a second primary malignancy in relatives: A nationwide cohort study. BMC Cancer 21(1), 1210 (2021).
Engmann, N. J. et al. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 3(9), 1228–1236 (2017).
Li, J. et al. Environmental tobacco smoke and cancer risk, a prospective cohort study in a Chinese population. Environ. Res. 191, 110015 (2020).
Xu, H. et al. Body mass index and the risk of mortality among Chinese adults with Type 2 diabetes. Diabet. Med. 35(11), 1562–1570 (2018).
He, W. et al. Lower BMI cutoffs to define overweight and obesity in China. Obesity (Silver Spring) 23(3), 684–691 (2015).
Xiang, Y. B., Jin, F. & Gao, Y. T. Cancer survival in Shanghai, China, 1992–1995. IARC Sci. Publ. 162, 55–68 (2011).
Hess, K. R. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat. Med. 14(15), 1707–1723 (1995).
George, B., Seals, S. & Aban, I. Survival analysis and regression models. J. Nucl. Cardiol. 21(4), 686–694 (2014).
Li, R. & Chambless, L. Test for additive interaction in proportional hazards models. Ann. Epidemiol. 17(3), 227–236 (2007).
Klintman, M. et al. Postmenopausal overweight and breast cancer risk; results from the KARMA cohort. Breast Cancer Res. Treat. 196(1), 185–196 (2022).
Park, J. W. et al. Obesity and breast cancer risk for pre- and postmenopausal women among over 6 million Korean women. Breast Cancer Res. Treat. 185(2), 495–506 (2021).
Recalde, M. et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: A prospective cohort study of 3.5 million adults in Spain. BMC Med. 19(1), 10 (2021).
Chlebowski, R. T. et al. Weight loss and breast cancer incidence in postmenopausal women. Cancer 125(2), 205–212 (2019).
Colditz, G. A. et al. Family history and risk of breast cancer: Nurses’ health study. Breast Cancer Res. Treat. 133(3), 1097–1104 (2012).
Liu, L. et al. Correlation between family history and characteristics of breast cancer. Sci. Rep. 11(1), 6360 (2021).
Bethea, T. N. et al. Family history of cancer in relation to breast cancer subtypes in African American women. Cancer Epidemiol. Biomark. Prev. 25(2), 366–373 (2016).
Blot, W. J. & Day, N. E. Synergism and interaction: Are they equivalent?. Am. J. Epidemiol. 110(1), 99–100 (1979).
Rothman, K. J., Greenland, S. & Walker, A. M. Concepts of interaction. Am. J. Epidemiol. 112(4), 467–470 (1980).
Coleman, W. B. Obesity and the breast cancer methylome. Curr. Opin. Pharmacol. 31, 104–113 (2016).
Grossmann, M. E. et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev 29(4), 641–653 (2010).
Li, N. et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the global burden of disease study 2017. J. Hematol. Oncol. 12(1), 140 (2019).
Xu, Y. et al. The FTO/miR-181b-3p/ARL5B signaling pathway regulates cell migration and invasion in breast cancer. Cancer Commun. (Lond.) 40(10), 484–500 (2020).
Iyengar, N. M. et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev. Res. (Phila) 8(5), 349–358 (2015).
Mullooly, M. et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 19(1), 8 (2017).
Li, J. et al. Breast cancer genetic risk profile is differentially associated with interval and screen-detected breast cancers. Ann. Oncol. 27(6), 1181 (2016).
Nguyen, T. L. et al. Interval breast cancer risk associations with breast density, family history and breast tissue aging. Int. J. Cancer 147(2), 375–382 (2020).
Wu, Y. et al. The impact of urbanization on the community food environment in China. Asia Pac. J. Clin. Nutr. 26(3), 504–513 (2017).
Wang, Y., Wang, L. & Qu, W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur. J. Clin. Nutr. 71(1), 149–150 (2017).
Wang, H. & Zhai, F. Programme and policy options for preventing obesity in China. Obes. Rev. 14(Suppl 2 (0 2)), 134–140 (2013).
Acknowledgements
We acknowledge all the participants in our study and all the staff of the colorectal cancer screening program. All those involved in preparing this manuscript contributed significantly to its completion.
Funding
This study was supported by the Nature Science Foundation of Minhangdistrict, Shanghai, China (2022MHZ024).
Author information
Authors and Affiliations
Contributions
H.X., K.G., and J.C. designed and conceived the research. Y.P., M.W., and J.C. analyzed and interpreted the data. JL and JC drafted the article. H.X., G.Q., Z.Z., and K.G. contributed to the guidance and supervision of the manuscript. All the authors have reviewed this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, J., Li, J., Zhang, Z. et al. Interaction between body mass index and family history of cancer on the risk of female breast cancer. Sci Rep 14, 4927 (2024). https://doi.org/10.1038/s41598-024-54762-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54762-x