Abstract
Louisiana experienced high morbidity and mortality from COVID-19. To assess possible explanatory factors, we conducted a cohort study (ClinSeqSer) of patients hospitalized with COVID-19 in New Orleans during August 2020–September 2021. Following enrollment, we reviewed medical charts, and performed SARS-CoV-2 RT-PCR testing on nasal and saliva specimens. We used multivariable logistic regression to assess associations between patient characteristics and severe illness, defined as ≥ 6 L/min oxygen or intubation. Among 456 patients, median age was 56 years, 277 (60.5%) were Black non-Hispanic, 436 (95.2%) had underlying health conditions, and 358 were unvaccinated (92.0% of 389 verified). Overall, 187 patients (40.1%) had severe illness; 60 (13.1%) died during admission. In multivariable models, severe illness was associated with age ≥ 65 years (OR 2.08, 95% CI 1.22–3.56), hospitalization > 5 days after illness onset (OR 1.49, 95% CI 1.01–2.21), and SARS CoV-2 cycle threshold (Ct) result of < 32 in saliva (OR 4.79, 95% CI 1.22–18.77). Among patients who were predominantly Black non-Hispanic, unvaccinated and with underlying health conditions, approximately 1 in 3 patients had severe COVID-19. Older age and delayed time to admission might have contributed to high case-severity. An association between case-severity and low Ct value in saliva warrants further investigation.
Similar content being viewed by others
Introduction
COVID-19 continues to have a broad clinical spectrum that encompasses a range of acute and longer-term complications1,2. Although overall severity of COVID-19 has become milder since the beginning of the COVID-19 pandemic3, some patients continue to experience severe illness. Louisiana has experienced high morbidity and mortality during the COVID-19 pandemic compared with other regions4, especially among Black non-Hispanic persons, and among those with underlying health conditions5.
Several potential reasons may explain the relatively severe disease in Louisiana. First, impacts of the pandemic have highlighted underlying inequalities6 that may lead to the accumulation of specific comorbidities. Advanced age, male gender and certain comorbidities such as obesity, diabetes mellitus, cardiovascular disease, chronic lung disease and kidney disease have been identified as risk factors for severe outcomes7,8,9. Louisiana has the second highest prevalence of adults diagnosed with diabetes in the United States, at 12.9%, and has the highest rate of newly diagnosed adults with diabetes among states in the continental U.S10. Prevalence of self-reported obesity in Louisiana is 38.6%, ranked 43 of 50 for worst rates of obesity among U.S. states11. Second, inequalities might have been perpetuated by delayed access to care5. Third, severe illness might reflect virologic factors, although this might explain temporal rather than geographic differences in severity12,13,14. Initial variants of SARS-CoV-2 led to more severe illness than since predominance by the Omicron variant13, and lower PCR cycle threshold (Ct) values (which correlate with higher SARS-CoV-2 viral load) are associated with adverse outcomes among patients hospitalized with COVID-1915,16,17,18,19. Fourth, host immunity plays an important role in attenuating severity, whether following infection or vaccination. Populations that were both SARS-CoV-2 naïve and unvaccinated were therefore at particular risk of severe outcomes. Finally, not receiving recommended treatment might have led to more severe illness.
To assess the potential contributions of these factors to severe outcomes among hospitalized patients in Louisiana, we conducted a cohort study among patients hospitalized with COVID-19 during August 2020–September 2021. Following enrollment, we reviewed medical charts, performed SARS-CoV-2 RT-PCR testing on nasal and saliva specimens, and measured SARS-CoV-2 anti-nucleocapsid antibody titers in available serum specimens. We described a range of demographic, clinical and laboratory characteristics among patients hospitalized with COVID-19 during the study period. Using multivariable logistic regression, we then assessed the association between patient characteristics and severe illness, which we defined for analysis purposes as receiving ≥ 6 L/min of supplemental oxygen, intubation or inpatient death.
Results
Participants included
Among 527 patients who were enrolled from August 2020 until September 2021, 456 were included in the analysis; among 458 patients hospitalized with COVID-19-like illness, two patients had died without receiving ≥ 6 L/minute of supplementary oxygen—one patient with atrial fibrillation and hypertension, and another with several comorbidities including chronic myelogenous leukemia with splenectomy and hepatic cirrhosis. We excluded these patients a priori because of other possible causes of death (Fig. 1). Overall, characteristics of the remaining 456 patients who were included in the analysis were similar in age, gender, and ethnicity to those of all inpatients diagnosed with COVID-19 at participating health facilities during the same period, although those enrolled more frequently had Black race (60.5% vs 50.3%) and non-Hispanic ethnicity (96.7% vs. 81.3%; Supplementary Table 1). Among patients enrolled in the study, characteristics of the 456 patients included were generally similar to those of patients who were excluded (Supplementary Table 2).
Demographic and clinical characteristics
Among the 456 hospitalized patients, illness onset was most frequent before Delta predominance (n = 258, 56.6%), the median age was 56 years (range 18–98), 259 (56.8%) were male and 275 (60.3%) were Black non-Hispanic (Table 1). Of 434 patients (95.2%) with at least one underlying health condition, the most prevalent were cardiovascular conditions including hypertension (n = 279, 61.2%), obesity (n = 40.4%, 184) and diabetes mellitus (n = 33.8%, 154); 370 patients (81.1%) had multiple underlying health conditions. Patients were admitted with COVID-19 a median of 5 days (range 2 to 9) after symptom onset. At symptom onset, 388 patients had known vaccination status, of whom 31 (8.0%) had received at least one COVID-19 vaccine dose. Treatments administered included dexamethasone (n = 293, 64.3%), remdesivir (n = 198, 43.4%), anti-spike monoclonal antibody therapy (n = 11, 2.4%), baricitinib (n = 2, 0.4%), tocilizumab (n = 17, 3.7%), and convalescent plasma (n = 3, 0.7%).
Differences in clinical and demographic characteristics by severity
Overall, 187 patients (41.0%) received ≥ 6 L/min oxygen during hospitalization and were classified as having ‘severe illness’; this included 118 (63.1%) patients who were not intubated, and 69 (36.9%) patients who were intubated (Table 2). Among the 187 patients with severe illness, 60 (32.1%) subsequently died during their hospital stay, 39 (20.9%) of whom died during the first 28 days after symptom onset. Patients with severe illness were admitted to hospital for a median 12 days (range 0–88) whereas those classified as non-severe were admitted for a median of 4 days (range 0–48).
Compared with the 269 hospitalized patients who were classified as having ‘non-severe illness’, those with severe illness were more likely to be older, or to have obesity or renal disease, but were less likely to have a history of smoking or substance abuse (Table 1). Patients with severe illness were generally admitted later after symptom onset. Patients who were categorized as having severe illness were also more likely to have received dexamethasone, remdesivir, or other therapy at some point during admission (Table 2). Other characteristics were generally similar by severity (Tables 1 and 2).
In a multivariable model, patients with severe illness were more likely to be older than ≥ 65 years (OR 2.08, 95% confidence interval [CI] 1.22–3.56), admitted > 5 days after illness onset (OR 1.49, 1.01–2.21), and to have received COVID-19 therapy before receiving ≥ 6L/minute oxygen or intubation (OR 3.10, 1.99–4.83) (Table 3).
Cycle threshold (Ct) values
Overall, 361 patients (79.2%) had a Ct value reported from a nasal specimen (including 214 patients with a specimen collected before or without any administration of antiviral medication), and 318 patients (69.7%) had a Ct value reported from a saliva specimen (including 194 with a specimen collected before or without antiviral treatment); 291 (63.8%) had both nasal and saliva specimens. Compared with other patients enrolled, those with available Ct values were more likely to have mild disease, be Black non-Hispanic, and to be admitted before predominance of the Delta SARS-CoV-2 variant (Supplemental Table 3). Among patients with Ct values for specimens collected during the 0–7 days after illness onset, 69/155 (44.5%) of those with a nasal specimen and 63/142 (44.4%) of those with a saliva specimen had an initial Ct value < 32 (Table 4); Ct values tended to be higher when collected later from symptom onset, consistent with lower viral load later in clinical course (Supplementary Fig. 1).
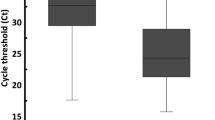
Among patients with a Ct value result before or without any antiviral treatment, severe patients had lower Ct values than non-severe patients in saliva specimens collected during 0–7 days after illness onset (severe, 0–7 days: median (IQR) Ct = 31.5 (26.3–36.7); non-severe, 0–7 days: median (IQR) Ct = 35.1 (28.9–40.0); p = 0.015; Fig. 2). Correspondingly, severe patients were more likely than non-severe patients to have a Ct less than the median value of 32 in a saliva specimen collected 0–7 days post-onset and before or without receipt of antiviral treatment (Table 4). When specimens were collected after antiviral treatment, we observed no association with severity in either specimen type.
Ct values by specimen type, severity and time since onset. Distribution of PCR cycle threshold (Ct) values by time from symptom onset to PCR specimen collection. Panels a and b represent Ct values of nasal and saliva (respective) specimens collected before or without receipt of antiviral treatment (remdesivir, casirivimab/imdevimab, bamlanivimab/etesevimab). Panels c and d represent Ct values of nasal and saliva (respective) specimens collected after receipt of antiviral treatment (includes receipt of remdesivir, casirivimab/imdevimab, bamlanivimab/etesevimab).
In a multivariable model, a Ct value < 32 in a saliva specimen collected during 0–7 days after onset was associated with severe illness if the specimen was collected before or in the absence of treatment (OR 4.79, 95% CI 1.22–18.77) but not if the specimen was collected after treatment (OR 1.96, 95% CI 0.51–7.53). By contrast nasal Ct value was not associated with severe illness (Table 5). These findings were similar for patients with nasal and saliva specimens collected on the same day, for specimens collected before the receipt of any oxygen, and for specimens collected during the 0–14 days after illness onset (Supplementary Table 4).
Anti-nucleocapsid antibody
Anti-nucleocapsid antibody results were available for a convenience sample of 187 out of 456 patients (41.0%), with specimens collected for 125 patients 0–14 days after illness onset, for 25 patients 15–28 days after onset, and for 52 patients outside these time periods. Participants with available results were similar to others included in the analysis (Supplementary Table 5). Anti-nucleocapsid antibodies varied between patients and by severity; low antibody titers were rare more than 14 days after illness onset (Table 4), and, by days 15–28, antibody levels were higher among severe patients [median (IQR) = 60.0 (39.0–83.0)] than non-severe patients [median (IQR) = 25.0 (12.0–54.0); p = 0.024] (Supplementary Fig. 2). Sparse data precluded inclusion of anti-nucleocapsid antibody detection in the multivariable model.
Discussion
In this study we have characterized the demographic, clinical and laboratory aspects of COVID-19 among a population that experienced a disproportionate impact of the pandemic. Among the patients admitted with COVID-19, approximately 1 in 2 received supplemental oxygen, 1 in 3 received high-flow oxygen, and 1 in 10 died during their hospitalization. Our analysis is consistent with other reports of substantial early impact of COVID-19 in Louisiana. Together with evidence from other studies, our findings suggest that several factors may have been important in explaining the high case-severity in this cohort.
We found that patients with COVID-19 requiring high-flow oxygen were more likely to be older, which is consistent with other studies7. Nevertheless, approximately 66% of patients hospitalized with severe COVID-19 were younger than age 65, indicating that other factors were also important. Among all patients included in our analysis, 95% had underlying health conditions, and 80% had multiple comorbidities. Patients most frequently had cardiac disease, obesity, and diabetes mellitus, each of which are risk factors for severe outcomes from COVID-1912. Notably, we found that comorbidity was reflected in hospitalized cases, whether or not they received high-flow supplemental oxygen. Since we conditioned the analysis on hospital admission, comorbidity among non-severe cases might reflect an increased likelihood of admission, leading to potential collider bias20. A lower threshold for admission with underlying health conditions among patients without severe COVID-19 might explain why we did not find overall differences in underlying conditions by illness severity. Our finding that patients with more than two underlying conditions tended to be admitted more rapidly than other patients is suggestive of this. In view of these considerations, the lack of an overall difference in comorbidity by severity does not negate the importance of comorbidities in driving case-severity. Instead, the high prevalence of known risk factors suggests these factors were important drivers of adverse outcomes.
Among patients included in our analysis, 60.5% had Black non-Hispanic race and ethnicity. This proportion is slightly higher than that of inpatients documented to have COVID-19 in the participating hospitals (approximately 50%), and is similar to the proportion reported for New Orleans in the U.S. census (58%)21. Consistent with previous analyses, we did not find a difference in case-severity by race and ethnicity among hospitalized patients5,22. However, Black race was associated with an increased risk of hospitalization with COVID-19 in Louisiana after adjusting for comorbidity and socioeconomic status5, and this elevated risk might reflect an array of other factors, including those related to accessing care6.
Compared with non-severe hospitalized patients, we found that those requiring high-flow supplemental oxygen were more likely to be admitted greater than five days after symptom onset. This suggests that delayed access to healthcare might have contributed to adverse outcomes. In our analysis, patients received high-flow oxygen a median of 8 days after symptom onset, and inpatient deaths occurred a median of 24 days after illness onset. Severe COVID-19 typically progresses over 1–2 weeks23, and patients who were admitted more than five days after illness onset were likely to have more severe illness by the time of presentation. We also found that patients with severe illness were more likely to have received treatment with remdesivir, dexamethasone, or other non-antiviral treatment (baricitinib or convalescent plasma). Since patients who met criteria for severe illness were likely to have been unwell at presentation, this is likely to reflect more treatment for patients presenting with more advanced disease, rather than any effect of treatment on severity; such an interpretation is supported by other evidence from other studies24.
Only 5% of hospitalized patients had completed a primary COVID-19 vaccine series during the period of analysis. This low proportion is likely to reflect both low vaccine coverage early in the pandemic, and an increased risk of COVID-19 if unvaccinated25. Similarly, approximately 20% of the U.S. population were estimated to have had prior infection during the period of analysis26. Since infection-induced immunity confers substantial protection against severe illness, patients admitted with COVID-19 would be expected to have a lower prevalence of prior infection during the period of analysis27. Although we did not have baseline serology results, low antibody titers during 0–7 days is consistent with a low prevalence of prior infection in the cohort.
Our finding that 43% of patients did not have a positive anti-nucleocapsid result within 14 days of illness onset is consistent with other evidence that it can take up to 14 days or longer for new antibodies to develop28. Since a similar proportion of patients with severe and non-severe disease had evidence of seroconversion, we did not find evidence that severe disease reflected inadequate immune responses. However, our modeled estimates were limited by sparse data.
Among patients with available SARS-CoV-2 RT-PCR results, we found that severe COVID-19 was associated with a lower cycle threshold value in saliva specimens that were collected before any antiviral treatment was started. Cycle threshold values reflect the number of RT-PCR amplification cycles needed to detect viral RNA in a specimen, and are inversely related to the level of viral RNA; lower Ct values therefore imply the presence of higher RNA levels. Higher cycle threshold values over time are likely to reflect declining viral load after initial infection29,30. Lower cycle threshold values among patients with severe disease is broadly consistent with evidence of higher viral load in severe illness, after adjusting for other characteristics15,17,19. Detection of SARS-CoV-2 in saliva may reflect involvement of the oral cavity31. Previous studies have found similar detection of SARS-CoV-2 RNA in nasal and saliva specimens early after symptom onset32,33, although with differences in cycle threshold that may reflect differences in specimen collection33,34. Our findings were similar when restricted to patients with paired saliva and nasal specimens on the same day. However, data were sparse for paired specimens, and reasons for an association with saliva but not nasal specimens is unknown. Our findings of an association between case-severity and lower Ct value in saliva are consistent with those of others, who have reported that abundance of SARS-CoV-2 RNA in saliva was significantly higher in patients with risk factors for severe COVID-19, correlated with more severe COVID-19, and was superior to nasopharyngeal viral load as a predictor of mortality35. We did not find an association between lower cycle threshold and severity after treatment that might lower the viral load36,37, possibly because patients with severe illness were also more likely to received such medications, thereby masking differences in viral load.
Before considering implications of our analysis, several strengths and limitations need to be considered, in addition to those listed above. First, although we provided a detailed description of more than 500 patients with severe COVID-19, for some analyses we were limited by sparse data, resulting in wide confidence intervals. Second, our capture of potential confounding factors was incomplete, which might lead to residual or unmeasured confounding in multivariable analyses. Third, although we found a relatively high mortality among hospitalized patients, we may have underestimated deaths that occurred in the community or that did not meet our definition of ‘severe illness’. For example, two patients who died without meeting this definition might have had extrapulmonary manifestations of infection2. Fourth, for analysis purposes we used a relatively low threshold (≥ 6 L/min) to determine severity based on oxygen level, limiting comparability with some other studies that have used 10–15 L/min as a threshold, and with guidelines that define severe illness based on oxygen saturation rather than supplemental flow38,39. Lastly, generalizability of our findings to other populations may be limited. Patients included in the analysis had similar overall demographic characteristics to other patients with COVID-19 in participating hospitals, but might have differed from patients admitted to other hospitals in the New Orleans area. Similarly, although overall patient characteristics were similar by availability of laboratory results, patients with laboratory data might be considered as a convenience sample within the main cohort. Overall, our scope was limited to analysis of patients who were hospitalized before widespread transmission of the Omicron SARS-CoV-2 variant and its subvariants.
Since predominance of the Omicron variant, average case-severity of SARS-CoV-2 infection has become milder3, both because of increased immunity from vaccination and infection40, and because of lower virulence compared with the Delta SARS-CoV-2 variant and ancestral variants13. Nevertheless, severe infections and deaths have continued to occur, both in individuals with and without clear risk factors. In our analysis, substantial comorbidity coupled with late presentation in an unvaccinated population are likely to have contributed to the high case-severity. Our study is relevant both in highlighting a patient population who experienced a disproportionate burden of COVID-19, and in describing severe COVID-19 in this group. Our findings of a correlation between severe illness and low cycle threshold in saliva may support the use of saliva PCR tests as a potential alternative to nasal PCR in the inpatient setting, though more work is needed to explore this association. To prepare for future epidemic and pandemic threats, our findings support broader efforts to address underlying inequalities and strengthen access to healthcare access and resilience of health systems6,41.
Methods
Setting and study participants
We conducted the cohort study at two healthcare systems in New Orleans, Louisiana—Tulane Medical Center and University Medical Center. Participants were eligible for inclusion if they were hospitalized at either site with a positive SARS-CoV-2 clinical nucleic acid amplification test (NAAT) result (from the participating health system or elsewhere), and if verbal informed consent was obtained. This study was reviewed and approved by the Tulane University School of Medicine Institutional Review Board and was performed in accordance with relevant guidelines (see 45 C.F.R. part 46; 21 C.F.R. part 56). Informed consent was obtained from all participants and/or their legal guardians. Among participants enrolled in the overall study, participants were included in the current analysis if they were admitted with COVID-19–associated symptoms and had a recorded date of admission and date of discharge (if survived). Participants were excluded from the analysis if they had enrolled in a clinical trial of medications used to treat COVID-19.
Research specimen collection and testing
For a subset of participants determined by convenience, we collected additional nasal specimens, saliva specimens, or both. Nasal swabs were collected by insertion of collection swab directly into one nare followed by rotation, then repetition in other nare. Saliva was collected by asking subject to spit directly into sample collection tube containing virus transport medium (either 1xPBS with antifungal or OMNIGene oral (OraSure), depending on supply chain availability during pandemic). All samples were placed on wet ice for transport then storage at -80 °C, for later batched aliquoting, virus RNA extraction, and qRT PCR. Nasal swab fluid and saliva were processed for virus RNA using QIAamp Viral RNA Mini Kit (Cat. 52906) according to manufacturer’s instructions. Extracted RNA was tested for SARS-CoV-2 qRT-PCR following CDC protocol (N1, N2, RNase P primers), and including a standard curve (see Supplemental Methods).
For a subset of participants determined by convenience, we collected serum specimens for serologic analyses; this subset was overlapping to those with research respiratory specimens. Samples were aliquoted then stored at − 80 °C until testing for antibody. We performed testing for SARS-CoV-2 anti-nucleocapsid antibody using the reSARS™ CoV-2 (N) IgG enzyme-linked immunosorbent assay (ELISA) test from Zalgen Labs following manufacturer’s recommendations (see Supplemental Methods).
Abstraction of medical information
Using a standardized tool, we abstracted additional information that had been collected as part of routine clinical care, including demographic information (age, sex, race, ethnicity), pre-COVID-19 medical history, pre-COVID-19 medications, SARS CoV-2 vaccination status, COVID-19-like symptoms, oxygen requirements, mortality, and medications administered during inpatient admission.
Variable definitions
For analysis purposes, we considered a participant to have ‘severe’ COVID-19 if they received ≥ 6 L/min of supplemental oxygen, including via high-flow nasal cannula, non-invasive positive-pressure ventilation, or mechanical ventilation; this included patients who subsequently died during hospitalization. We used a cutoff of ≥ 6 L/min, as that is used in the participating hospitals as a threshold for initiation of high-flow nasal oxygen; we considered this level of support or more severe disease to approximate a score of 5–8 using the WHO scale42. We considered participants to have non-severe illness if they were hospitalized with COVID-19 but did not meet the definition of ‘severe’, and were discharged. We considered patients to have died from COVID-19 if death occurred during hospital admission. Patients who died without receipt of ≥ 6 L/min of oxygen were excluded from the analysis, since such patients were considered to have a potential alternative cause of death.
We defined the Delta-predominant period as July 1, 2021 through the end of the study period (September 2021), based on estimated national predominance43. We considered patients to be ‘vaccinated’ if they had received a 2nd mRNA COVID-19 vaccine dose or single dose of the Janssen COVID-19 vaccine at least 14 days before the date of symptom onset. We categorized treatment for COVID-19 as remdesivir, anti-spike monoclonal antibody (e.g., casirivimab/imdevimab, bamlanivimab/etesevimab), dexamethasone, anti-inflammatory monoclonal antibody (baricitinib, tocilizumab) or convalescent plasma; for analysis purposes we considered ‘antiviral medication’ to include remdesivir and anti-spike monoclonal antibodies, based on anticipated effects on viral load36,37. We analyzed laboratory values as continuous and, for simplicity, by whether they were greater or less than the median. Additionally, we limited analysis of cycle threshold (Ct) values to the first available nasal specimen and the first available saliva specimen collected during 0–7 days after symptom onset, since viral load typically declines after this period. We categorized SARS-CoV-2 anti-nucleocapsid antibody assay results by whether the specimen was obtained within 14 days after illness onset, since new seroconversion usually occurs within this period, or whether obtained during 15 to 28 days after onset. Underlying health conditions were categorized based on diagnoses listed in the patient’s medical record (Supplementary Table 1).
Statistical analysis
To assess patient characteristics associated with severe disease, we compared demographic, clinical and laboratory characteristics by severity. In unadjusted analyses, we used the Student’s t-test or Wilcoxon rank sum test to compare continuous variables and the chi squared test to compare categorical variables. We used logistic regression to compare the patient characteristics by severity in a multivariable model, using broader categories to account for sparse data, and limiting treatment to receipt during 0–7 days after illness onset and before receiving ≥ 6 L/min of oxygen. Multivariable models included covariates that were hypothesized to be associated with severe illness (age group, sex, race/ethnicity, comorbidity, vaccination status, days from symptom onset to admission). In addition, we used a hierarchical approach to limit each model to covariates that were considered to be distal to each exposure of interest44. Multivariable assessments of the association between Ct value and severity were stratified by specimen collection before or after receipt of remdesivir or anti-spike monoclonal antibodies that might be expected to suppress viral load. All statistical analyses were performed using SAS Enterprise Guide 9.4 (SAS Institute, Inc., Cary, North Carolina).
Data analysis
The analysis was planned by Ian D. Plumb, Claire Midgely, and Dahlene Fusco with input from other coauthors. Data preparation and analysis was conducted by Dahlene Fusco, Matthew McCullough and Jade James-Gist. Dr. Fusco had full access to all the data in the study and takes responsibility for the integrity of the data.
Ethical approval
This study was reviewed and approved by the Tulane University School of Medicine Institutional Review Board, and was performed in accordance with relevant guidelines (see 45 C.F.R. part 46; 21 C.F.R. part 56). Informed consent was obtained from all participants and/or their legal guardians.
Data availability
Data presented in these analyses available in supplementary material or upon request to corresponding author.
References
Davis, H. E. et al. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21(3), 133–146 (2023).
Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26(7), 1017–1032 (2020).
Koelle, K. et al. The changing epidemiology of SARS-CoV-2. Science 375(6585), 1116–1121 (2022).
CDC COVID 19 mortality final. (2023); Available from: https://www.cdc.gov/nchs/pressroom/sosmap/covid19_mortality_final/COVID19.htm
Price-Haywood, E. G. et al. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 382(26), 2534–2543 (2020).
Bambra, C. Pandemic inequalities: Emerging infectious diseases and health equity. Int. J. Equity Health 21(1), 6 (2022).
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584(7821), 430–436 (2020).
Gao, Y. D. et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 76(2), 428–455 (2021).
Merad, M. et al. The immunology and immunopathology of COVID-19. Science 375(6585), 1122–1127 (2022).
CDC Geographic Distribution of Diabetes in US. (2023); Available from: https://www.cdc.gov/diabetes/library/reports/reportcard/national-state-diabetes-trends.html
CDC Obesity Data Prevalence. (2023); Available from: https://www.cdc.gov/obesity/data/prevalence-maps.html
Russell, C. D., Lone, N. I. & Baillie, J. K. Comorbidities, multimorbidity and COVID-19. Nat. Med. 29(2), 334–343 (2023).
Carabelli, A. M. et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 21(3), 162–177 (2023).
Walsh, K. A. et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 81(3), 357–371 (2020).
Choudhuri, J. et al. SARS-CoV-2 PCR cycle threshold at hospital admission associated with patient mortality. PLOS ONE 15(12), e0244777 (2021).
de la Calle, C. et al. Impact of viral load at admission on the development of respiratory failure in hospitalized patients with SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 40(6), 1209–1216 (2021).
Magleby, R. et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin. Infect. Dis. 73(11), e4197–e4205 (2021).
Rico-Caballero, V. et al. Impact of SARS-CoV-2 viral load and duration of symptoms before hospital admission on the mortality of hospitalized COVID-19 patients. Infection 50(5), 1321–1328 (2022).
Tanner, A. R. et al. SARS-CoV-2 viral load at presentation to hospital is independently associated with the risk of death. J. Infect. 83(4), 458–466 (2021).
Griffith, G. J. et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat. Commun. 11(1), 5749 (2020).
New Orleans US Census Data. (2023); Available from: https://www.census.gov/quickfacts/fact/table/neworleanscitylouisiana/PST045222
Gold, J. A. W. et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb. Mortal Wkly Rep. 69(18), 545–550 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).
Bhimraj, A., et al., Infectious diseases society of america guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19), ciac724 (Clinical Infectious Diseases, 2022).
Scobie, H. M. et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status - 13 U.S. jurisdictions, April 4–July 17, 2021. MMWR Morb. Mortal Wkly. Rep. 70(37), 1284–1290 (2021).
Jones, J. M. et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 326(14), 1400–1409 (2021).
Bobrovitz, N. et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. Lancet Infect. Dis. 23(5), 556–567 (2023).
Orner, E. P. et al. Comparison of SARS-CoV-2 IgM and IgG seroconversion profiles among hospitalized patients in two US cities. Diagn. Microbiol. Infect. Dis. 99(4), 115300 (2021).
Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 28(5), 1031–1041 (2022).
To, K.K.-W. et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 20(5), 565–574 (2020).
Huang, N. et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 27(5), 892–903 (2021).
Congrave-Wilson, Z. et al. Change in saliva RT-PCR sensitivity over the course of SARS-CoV-2 infection. JAMA 326(11), 1065–1067 (2021).
Procop, G. W. et al. A direct comparison of enhanced saliva to nasopharyngeal swab for the detection of SARS-CoV-2 in symptomatic patients. J. Clin. Microbiol. https://doi.org/10.1128/jcm.01946-20 (2020).
Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med. 383(13), 1283–1286 (2020).
Julio, S. et al. Saliva viral load is a dynamic unifying correlate of COVID-19 severity and mortality. medRxiv 2021.01.04.21249236 (2021).
Goldberg, E. et al. A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel. Clin. Microbiol. Infect. 27(6), 917.e1-917.e4 (2021).
Gottlieb, R. L. et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: A randomized clinical trial. JAMA 325(7), 632–644 (2021).
NIH. NIH COVID treatment guidelines. 2024 02/07/2024]; Available from: https://www.covid19treatmentguidelines.nih.gov/
IDSA. IDSA COVID treatment guidelines. 2024 02/07/2024]; Available from: https://doi.org/10.1093/cid/ciac724/6692369.
CDC. COVID data tracker. Centers for disease control and prevention 2020 2020/03/28/; Available from: https://covid.cdc.gov/covid-data-tracker.
Haldane, V. et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 27(6), 964–980 (2021).
Garibaldi, B. T. et al. Patient trajectories among persons hospitalized for COVID-19: A cohort study. Ann. Intern. Med. 174(1), 33–41 (2021).
Taylor, C. A. et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance - COVID-NET, 14 States, January–August 2021. MMWR Morb. Mortal Wkly. Rep. 70(43), 1513–1519 (2021).
Victora, C. G. et al. The role of conceptual frameworks in epidemiological analysis: A hierarchical approach. Int. J. Epidemiol. 26(1), 224–227 (1997).
Acknowledgements
The authors thank Sharon Saydah, PhD MHS, for helpful discussions related to this manuscript. The authors thank all participants and their family members, as well as the hospital communities of Tulane Downtown Medical Center (closed January 13th 2024) and University Medical Center for their support of this work during extremely challenging times. This article is dedicated to the memory of those subjects who died from COVID, in gratitude.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Funding
This work was supported by the Centers for Disease Control and Prevention (CDC) (U01CK000480).
Author information
Authors and Affiliations
Contributions
The analysis was planned by A.D, D.F., I.P., and C.M. with input from other coauthors. Data preparation and analysis was conducted by D.F., M.M., and J.J.G. Data interpretation was conducted by D.F., C.M., and I.P. and manuscript writing was completed by D.F. and I.P. with critical input from all authors. D.F. had full access to all the data in the study and takes responsibility for the integrity of the data. All authors certify that they meet authorship criteria.
Corresponding author
Ethics declarations
Competing interests
DF has served on an Advisory Board for Gilead Sciences and for AXCELLA, and as site PI for clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). AD has served as co site-PI on clinical trials sponsored by Gilead Sciences, Regeneron, MetroBiotech LLC, and the NIH (DMID COVAIL). Other authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Drouin, A., Plumb, I.D., McCullough, M. et al. Clinical and laboratory characteristics of patients hospitalized with severe COVID-19 in New Orleans, August 2020 to September 2021. Sci Rep 14, 6539 (2024). https://doi.org/10.1038/s41598-024-57306-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57306-5