Abstract
Repurposing of FDA-approved drugs is a quick and cost-effective alternative to de novo drug development. Here, we identify genes involved in bortezomib sensitivity, predict cancer types that may benefit from treatment with bortezomib, and evaluate the mechanism-of-action of bortezomib in breast cancer (BT-474 and ZR-75–30), melanoma (A-375), and glioblastoma (A-172) cells in vitro. Cancer cell lines derived from cancers of the blood, kidney, nervous system, and skin were found to be significantly more sensitive to bortezomib than other organ systems. The in vitro studies confirmed that although bortezomib effectively inhibited the β5 catalytic site in all four cell lines, cell cycle arrest was only induced in G2/M phase and apoptosis in A-375 and A-172 after 24h. The genomic and transcriptomic analyses identified 33 genes (e.g. ALDH18A1, ATAD2) associated with bortezomib resistance. Taken together, we identified biomarkers predictive of bortezomib sensitivity and cancer types that might benefit from treatment with bortezomib.
Similar content being viewed by others
Introduction
The 26S proteasome is a large barrel-shaped protein complex, consisting of up to three subunits (one 20S and one or two 19S subunits) and three catalytic sites (β1 [caspase-like], β2 [trypsin-like], and β5 [chymotrypsin-like]), that is tasked with degrading up to 90% of temporary and misfolded, ubiquitinated proteins in the cell1,2,3,4. Proteolysis is necessary to prevent protein accumulation, provide the cell with new building blocks (amino acids) for new proteins, regulate certain cellular processes (e.g. cell cycle, immune response and apoptosis), and control protein quality5,6. Although abnormally high proteasome activity is found in neoplastic tissue, cancer stem cells or cancer cells with stem cell characteristics have been associated with decreased proteasome activity7,8. Elevated proteasome activity is caused by several factors, including mutations resulting in an accumulation of misfolded proteins in the cell9. Inhibition of proteasome catalytic activity can in turn lead to accumulation of proteins, thereby resulting in the disruption of key cellular processes, a reduced amount of free amino acids available for protein synthesis, cell cycle arrest, and ultimately cell death10,11.
Bortezomib (VELCADE®, formerly PS-341) was the first proteasome inhibitor to pass clinical trials and be approved by US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as first-line treatment for patients diagnosed with multiple myeloma12. Bortezomib and proteasome inhibitors in general primarily bind to the β5 catalytic site and secondarily to the β1 and β2 sites at higher concentrations9,13. A number of proteasome inhibitors have been developed from various chemical classes (e.g. boronate, epoxyketone), binding kinetics (reversible or irreversible binding), and routes of administration (intravenous, subcutaneous, and oral)14. Bortezomib is a dipeptide boronic acid analog with a half-life of 100 min that reversibly inhibits the chymotrypsin-like activity of the β5 catalytic site15. As bortezomib-associated side effects and treatment resistance have become an issue, several second-generation proteasome inhibitors such as carfilzomib, delanzomib, and epoxomicin were developed16,17.
Drug development is a time consuming and costly process with high risk of failure and unknown drug side effects. In contrast, drug repurposing (also called drug repositioning) is a relatively quick, cost effective, and low-risk process that can potentially identify new indications for FDA/EMA-approved drugs with previously known side effects (patient safety)18. Compared to drug repurposing, the conventional drug development process takes about 5–7 years longer from target discovery to clinical implementation18,19. A very common way of drug repurposing is to identify “positive” side effects of drug treatment that emerge during or shortly after clinical trials that could be used in the treatment of other diseases. Tamoxifen and aspirin are noteworthy examples of drug repurposing. Tamoxifen was originally intended to be used as a contraceptive that failed in clinical trials. Later studies revealed Tamoxifen to have antitumor effects; the drug is currently first-line treatment for hormone-positive breast cancer20,21. Besides its use as a painkiller, long-term daily aspirin use has been linked to reduced risk of colorectal cancer22. Here, we identify bortezomib as a multifaceted drug that significantly decreases cell viability in cancer cell lines derived from hematological malignancies, kidney cancer, as well as cancers of the nervous system. We also pinpoint important genomic and transcriptomic profiles associated with bortezomib sensitivity. It is therefore important to predict specific cancer types where patients could benefit from treatment with bortezomib in order to obtain prolonged survival and lower risk of recurrence.
Results
Cancers of the blood, kidney, nervous system and skin were most sensitive to bortezomib
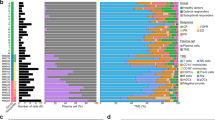
To assess cancer cell line sensitivity to bortezomib (proteasome inhibitor), we used two datasets (Sanger and Massachusetts General Hospital) from the Genomics of Drug Sensitivity in Cancer (GDSC) database that together contained drug sensitivity data (IC50 and AUC) for 860 cancer cell lines originating from 13 organ systems (30 cancer types and 11 unclassified groups, Fig. 1a and Supplementary Table 1). In total, 49/860 (5.7%) cancer cell lines were classified as bortezomib-sensitive and 38 (4.4%) as bortezomib-insensitive (Fig. 1b,c). These data demonstrated that hematologic malignancies (ALL, CLL, DLBC, LAML, LCML, MM, and Unclassified) and cancers of the nervous system (GBM, LGG, MB, and NB), kidney (KIRC and Unclassified), and skin (SKCM and Unclassified) were shown to be sensitive to bortezomib, while cancer of the lung (LUAD, LUSC, MESO, SCLC, and Unclassified), urogenital (BLCA, CESC, OV, PRAD, UCEC, and Unclassified), and digestive organ systems (COADREAD, LIHC, STAD, and Unclassified) were bortezomib-insensitive (Fig. 1d,e). However, an analysis of the distribution of organ systems in the whole cohort versus the bortezomib-insensitive or bortezomib-sensitive groups identified lung cancer to be significantly (P = 4.6E-08) overrepresented in the bortezomib-insensitive group and blood (P = 0.0007), kidney (P = 0.0002), nervous system (P = 0.0080) and skin (P = 0.0004) to be significantly overrepresented in the bortezomib-sensitive group. Although bortezomib-sensitivity was heterogeneous within all organ systems, cell lines derived from e.g. lung cancer and hematologic malignancies were present in both bortezomib-sensitivity groups.
Drug response data from Genomics Drug Sensitivity in Cancer (GDSC) were compiled from the GDSC1 (n = 791) and GDSC2 (n = 756) datasets, and merged into a single dataset containing 860 cancer cell lines (duplicates found in both datasets were excluded). (a) Pie charts depicting the number (and percentage) of cell lines associated with each organ system. Venn diagram showing the number of cancer cell lines classified as (b) bortezomib-sensitive (n = 84) and (c) bortezomib-insensitive (n = 80) based on the logarithmic half-maximal inhibitory concentration (LNIC50: < -6 for sensitivity and > -3 for insensitivity) and/or area under the curve (AUC: < 0.6 for sensitivity and > 0.9 for insensitivity) values. Boxplots depicting sensitivity to bortezomib according to (d) LNIC50 and (e) AUC, with the red horizontal lines illustrating thresholds for bortezomib-sensitivity (LNIC50 < -6 and AUC < 0.6) and insensitivity (LNIC50 > -3 and AUC > 0.9). Using both LNIC50 and AUC values, 49 bortezomib-sensitive and 38 bortezomib-insensitive cell lines were identified for use in subsequent analyses.
To examine the mechanism-of-action for bortezomib (induction of cell cycle arrest, apoptosis, proteasome inhibition) and validate bortezomib sensitivity in human cancer cells, three cell lines from the bortezomib-sensitive group (A-172, A-375, and RPMI-8226) and two from the bortezomib-insensitive group (BT-474 and ZR-75–30) were treated with bortezomib for 24h and 72h. As expected, A-172, A-375, and RPMI-8226 cells had lower cell viability (according to IC50 and AUC values) after treatment than BT-474 and ZR-75–30 cells (Fig. 2a,b). Massive cell death for the A-172 and A-375 cell lines was also observed at 50 nM (Fig. 2c). At 1000 nM bortezomib, about 80% inhibition of the proteasome β5 catalytic site was shown in all cell lines (Fig. 2d). At a concentration of 50 nM, cell cycle arrest in G2/M phase was observed in A-172 and A-375 cells but could not be determined in the other cell lines (Fig. 3a,b). Evaluation of the induction of early and late apoptosis after 24h or 72h treatment time revealed a significant increase in necrotic-late apoptotic and early apoptotic cells at 50 nM after 72h treatment in A-172 and A-375 cells (Fig. 3c,d). Notably, A-172 and A-375 cells did not survive the 72h treatment period with 100 nM bortezomib. Furthermore, no significant increase in necrotic-late apoptotic and early apoptotic cells could not be detected in BT-474 or ZR-75–30 cell lines. As expected, A-172 (24h treatment [IC50 = N/A; AUC = 0.74]; 72h treatment [IC50 = 10.3 nM; AUC = 0.28], A-375 (24h treatment [IC50 = 42 nM; AUC = 0.76]; 72h treatment [IC50 = 11.2 nM; AUC = 0.29], and RPMI-8226 (24h treatment [IC50 = 7.6 nM; AUC = 0.32]; 72h treatment [IC50 = 5.2 nM; AUC = 0.16]) were most sensitive to bortezomib. Although the β5 catalytic site was not fully inhibited, A-172 and A-375 cells still underwent apoptosis.
In vitro analysis confirms that A-375 melanoma, A-172 glioblastoma, and RPMI-8226 multiple myeloma cells are more sensitive to bortezomib than BT-474 and ZR-75–30 breast cancer cells, which is consistent with the GDSC1/GDSC2 dataset. Cell viability analysis depicting the effect of (a) 24h and (b) 72h bortezomib treatment on half-maximal inhibitory concentration (IC50) and area under the curve (AUC) metrics in five cancer cell lines (A-172, A-375, BT-474, RPMI-8226, and ZR-75–30). (c) Representative images showing the effect of different bortezomib concentrations on cell density for the A-375 cell line. (d) Bar chart showing inhibition of the β5 proteasome catalytic activity at different bortezomib concentrations (0–1000 nM) after 2h drug exposure.
In vitro analysis of bortezomib-induced cell cycle arrest and apoptosis in A-375 melanoma cells. (a,b) Cell cycle distribution analysis showing G2/M cell cycle arrest in A-375 cells following 24h treatment with 50 nM bortezomib. The one-way ANOVA test was used to calculate statistically significant differences between the proportion of cells present in different cell cycle phases (G1, S, and G2/M phases). Not significant (P > 0.05); *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001. Only statistically significant differences are shown in the figure. (c,d) Annexin V analysis showing a high proportion of early apoptotic A-375 melanoma cells after 24h bortezomib treatment, and necrotic – late apoptotic cells after 72h treatment with 50 nM bortezomib. The one-way ANOVA test was used to determine statistically significant differences between the proportion of necrotic-late apoptotic or early apoptotic cells at different bortezomib concentrations. Not significant (P > 0.05); *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001. Only bars with significant differences between the same state and at different bortezomib concentrations were shown in the figure.
Mutations in the MUC12, PCDH15, RYR1 and SPTA1 genes were most prevalent in bortezomib-insensitive cell lines
To determine an association between amino acid mutation frequency and bortezomib sensitivity, we then retrieved mutation data for the bortezomib-insensitive and bortezomib-sensitive cell lines from the Sanger COSMIC Cell Lines Project and Sanger Institute Cell Model Passports. Analysis of mutation frequency in both bortezomib-sensitivity groups (bortezomib-sensitive and bortezomib-insensitive) revealed a significantly higher mutation burden in bortezomib-insensitive cell lines (P = 0.02; Fig. 4a). We then determined the 50 most frequently mutated genes in each sensitivity group (Supplementary Fig. 1a-b). By comparing the mutation rate of these genes in the different sensitivity groups, we were able to identify 19 genes (ANKRD36C, FSIP2, HERC2, HMCN1, MGAM, MUC12, MUC16, PCDH15, PDE4DIP, PTPRN2, RIMS2, RYR1-3, SPTA1, TP53, TRRAP, USH2A, and WDFY4) that had significantly elevated mutation rates in the bortezomib-insensitive group (Fig. 4b and Supplementary Tables 2–3; P < 0.05). Gene Ontology analysis showed that these 19 genes play a vital role in DNA repair, gene transcription, and cell cycle. The mutation frequency of the 19 genes was then validated using the Cancer Cell Line Encyclopedia (CCLE) dataset, thereby showing that five of the 19 genes (MUC12, MUC16, RIMS2, RYR2, and SPTA1) were more frequently mutated in the bortezomib-insensitive cell lines (Supplementary Table 4). Nevertheless, there was no significant difference in the frequency of mutated cancer drivers between the two sensitivity groups (P = 0.369; Fig. 4c). KRAS, LRP1B, and TP53 (bortezomib-insensitive cell lines) and CREBBP, KMT2C, KMT2D, and TP53 (bortezomib-sensitive cell lines) were identified as commonly mutated cancer drivers in ≥ 5 cell lines (Supplementary Fig. 2a-b). Taken together, mutations were commonly occurred in all cell lines, but mutation rates for the MUC12, PCDH15, RYR1 and SPTA1 genes were significantly higher in the bortezomib-insensitive group.
Mutation frequency and mutation type in the bortezomib-insensitive and sensitive groups. (a) Box plots depicting differences in mutation frequency between bortezomib-sensitive and bortezomib-insensitive cancer cell lines using the ggstatsplot R package with ggbetweenstats and Welch’s t-test. (b) Waterfall plot depicting the mutation frequency of genes in both sensitivity groups. T-test (P < 0.05) was used to stratify genes with significantly elevated mutation frequencies between the sensitivity groups. The most deleterious mutations are shown if several mutations affected the same gene. (c) Box plots depicting differences in mutation frequency for cancer drivers between bortezomib-sensitive and bortezomib-insensitive cancer cell lines using Welch’s t-test.
Gene expression analysis reveals 33 genes predicted to be involved in bortezomib insensitivity
We then examined gene expression patterns in untreated bortezomib-sensitive (n = 46) and bortezomib-insensitive (n = 37) cell lines using transcriptomics data for 970 cell lines from the Sanger COSMIC Cell Lines Project. Consequently, 41 (27 over- and 14 underexpressed) and 193 (127 over- and 66 underexpressed) dysregulated genes were identified in > 20% of the cell lines in the bortezomib-sensitive and bortezomib-insensitive groups, respectively (Fig. 5a and Supplementary Fig. 3a,b). Genes associated with bortezomib-sensitivity were found to be involved in transmembrane transport of small molecules, metabolism, chromatin organization, while genes associated with bortezomib-insensitivity played a role in the metabolism of proteins, signal transduction, and the cell cycle. Using transcriptomic and survival data from UCSC Xena Browser, the influence of 220/234 genes (missing data for 14 genes) on survival was investigated in 589 bortezomib-treated multiple myeloma (MM) patients from the MMRF-COMMPASS study 23 (Supplementary Table 5). Principal component analysis (PCA) of the transcriptomic data revealed two distinct clusters containing a mix of MM stages in both groups (Supplementary Fig. 4). Furthermore, hierarchical clustering showed that the majority of genes were uniformly expressed in all patients, while 7/220 genes (DDX3Y, EIF1AY, RPS4Y1, SMCY, USP9Y, UTY, and ZFY) showed aberrant gene expression patterns in the two main clusters of MM patients (Supplementary Fig. 5). Kaplan–Meier analysis revealed significantly more unfavorable overall survival for MM patients with low expression of the seven genes (Supplementary Fig. 6).
The effect of bortezomib treatment on gene dysregulation in the two sensitivity groups. (a) Venn diagram illustrating the number of dysregulated genes in > 20% of cell lines in each sensitivity group. The pink circle (middle) shows the number of dysregulated genes (n = 560) identified using “limma” R package, while the green (left) and blue (right) circles represent genes identified by Sanger COSMIC Cell Lines Project that were associated with bortezomib-insensitivity (n = 193) and sensitivity (n = 41), respectively. Of genes identified in the Sanger COSMIC Cell Lines Project, 31 genes out of 193 and 2 genes out of 41 were found among the 560 genes identified by limma. (b) Volcano plot showing the 560 most dysregulated (up or down regulated) genes identified using limma R package. (c) Heatmap illustrating hierarchical clustering for expression of the 560 genes identified using limma with Manhattan distance metric and Ward’s minimum variance method (Ward.D2). (d) Gene Ontology and Reactome enrichment analysis for the 560 dysregulated genes between the bortezomib sensitivity groups.
Moreover, differential expression (DE) analysis using limma identified 560 DE genes between bortezomib-sensitive and bortezomib-insensitive cell lines, of which 292 were overexpressed and 268 were underexpressed (Fig. 5b and Supplementary Table 6). However, 33/560 genes were identified by both Sanger and limma (ALDH18A1, ATAD2, ATP9A, ATXN7, BRMS1L, CCNH, CDC42EP2, CENPX_ENST00000580435, FBXO21, FKBP3, HAUS6, IGFBPL1, LIFR, LRTOMT, MBIP, NDUFB9, NINL, OXSR1, P2RY2, PARP2, POFUT2, PRKG2, PUF60, SCRIB, SHQ1, SLC2A4RG, SLC45A4, TMEM45B, TOX4, UGDH, USP4, WDR36, WDYHV1; Fig. 5a). Hierarchical clustering of the expression patterns for the 560 genes demonstrated relatively good clustering depending on bortezomib sensitivity, tissue type, and TCGA cancer type (Fig. 5c). More specifically, bortezomib-sensitive organ systems (blood, skin, nervous system, and kidney) frequently clustered together, while cell lines in the bortezomib-insensitive organ systems (digestive, lung and urogenital) clustered differently. Furthermore, the 560 genes were found to play a central role in the immune system and cell cycle (Fig. 5d). Using the TIMER2.0 database, we then assessed the relationship between immune infiltrates (B cells, CD4 + T cells, CD8 + T cells, myeloid dendritic cells, macrophages, and neutrophils) and the expression of the four genes (BTN3A1, MAP3K3, PSMB11, and UBA7) involved in immune response (Supplementary Fig. 7). We were thereby able to show a positive correlation between BTN3A1, MAP3K3, and UBA7 expression and the majority of immune infiltrates (B cells, CD4 + T cells, CD8 + T cells, macrophages, and neutrophils) in most cancer types, and a negative correlation with myeloid dendritic cells. However, PSMB11 expression was only weakly correlated with a few cancer types.
Using two different approaches, we were therefore able to identify 33 concordant genes associated with bortezomib sensitivity. We investigated the gene expression patterns for 32/33 concordant genes (one gene missing data) and their influence on overall survival in the Pan-cancer cohort (21 cancer types) using KM plotter. ATAD2, BRMS1L, PUF60, SHQ1, and USP4 were found to influence overall survival in 15, 9, 13, 13, and 12 of the 21 cancer types, respectively (Supplementary Fig. 8).
Discussion
In the current study, we evaluate bortezomib sensitivity in 860 cancer cell lines representing 30 cancer types and identify key genomic and transcriptomic features of bortezomib-sensitive and bortezomib-insensitive cells. Mutational events have previously been correlated with therapy resistance24. Although MM and mantel cell lymphoma are routinely treated with bortezomib, this study reveals that other hematological cancers (ALL, CLL, DLBC, LAML, LCML), as well as cancers of the kidney, nervous system, and skin may also benefit from treatment with bortezomib25. Heterogeneity in response to treatment within organ systems (e.g. lung) was also observed, where several cell lines from the same organ system were classified as bortezomib-sensitive while others were classified as bortezomib-insensitive. When setting up a drug screen, this diversity in sensitivity to treatment must be considered and a wide range of bortezomib concentrations must be used to be able to determine the IC50 and AUC values for all tested samples. The manufacturer Cell Signaling Technology recommends using 1–1000 nM of bortezomib in in vitro studies. However, in our analysis we could not determine the IC50 after 24 h of bortezomib exposure in e.g. breast cancer cell lines (BT-474 and ZR-75–30) even when using up to 10,000 nM. When exposing the cells for 72h with bortezomib, we were able to determine the IC50 values for these cell lines. Therefore, the dose range that should be used when setting up a drug screen for bortezomib will depend on the cell type that will be examined.
Nevertheless, lung cancers were primarily insensitive to treatment with bortezomib. Our in vitro studies confirmed that although bortezomib inhibited the proteasome in all four cell lines (A-172, A-375, BT-474, and ZR-75–30), treatment-induced apoptotic cell death and G2/M cell cycle arrest were primarily shown in A-172 glioblastoma and A-375 melanoma cells. A significant increase in the proportion of necrotic-late apoptotic cells was observed after 72h with 50 nM bortezomib in both A-172 and A-375 cell lines. The breast cancer cell lines (BT-474 and ZR-75–30) were tolerant to up to 100 nM bortezomib with no G2/M arrest or apoptosis observed following 24h or 72h drug exposure. Although 24h treatment with bortezomib is more biologically relevant since its terminal half-life in humans is approximately 15h, we also included the 72h time point in the study in order to evaluate the long-term effect of treatment with bortezomib26,27.
The impact of treatment with bortezomib was revealed when about 40% of the β5 catalytic site was inhibited at a concentration of 100 nM, leading to apoptosis in A-172 and A-375 cells. These findings not only indicate that these cells were sensitive to proteasome inhibition, but that a functional proteasome is important for their survival. Previously, other researchers have also found that the A-375 cell line is sensitive to bortezomib by affecting proliferation and cell survival28. We also determined that bortezomib induced G2/M phase arrest before the cells underwent apoptosis. G2/M arrest induced by bortezomib is a common event, which has also been described in glioblastoma cancer cells29.
Among the 19 genes (ANKRD36C, FSIP2, HERC2, HMCN1, MGAM, MUC12, MUC16, PCDH15, PDE4DIP, PTPRN2, RIMS2, RYR1-3, SPTA1, TP53, TRRAP, USH2A, and WDFY4) determined to be significantly more mutated in bortezomib-insensitive cell lines, only TP53 has previously been described to be involved in treatment resistance30. Furthermore, we identified 33 dysregulated genes (e.g. ALDH18A1, ATAD2, FBXO21, LIFR) potentially involved in therapy resistance, as shown in other studies31,32,33. We demonstrated that bortezomib-insensitive cell lines have significantly higher mutation frequencies than bortezomib-sensitive cells, indicating that the increased mutation frequency confers a reduction in sensitivity to bortezomib treatment. However, mutations in the 19 genes were more recurrent in bortezomib-insensitive cells, thereby suggesting a link with bortezomib resistance. TP53 mutations have previously been correlated with chemo/radio-resistance30. Using the CCLE mutation dataset containing 27/38 bortezomib-insensitive and 22/49 bortezomib-sensitive cancer cell lines, we were able to show consistently elevated mutation frequencies for 18/19 genes (not PDE4DIP) in bortezomib-insensitive cell lines compared to bortezomib-sensitive cell lines. However, only 5/19 genes (MUC12, MUC16, RIMS2, RYR2, and SPTA1) showed significantly higher mutation rates in bortezomib-insensitive cell lines. The fact that we could only validate 5 of the 19 genes in the CCLE dataset could possibly be due to the relatively high number of cell lines from the GDSC dataset that were missing in the CCLE dataset. However, the clinical significance of these mutated genes needs to be investigated further to determine their potential contribution to bortezomib resistance. Interestingly, mutation frequency in cancer drivers was similar in both sensitivity groups.
Using Sanger expression data, we identified a total of 234 genes involved in bortezomib sensitivity across cancer types. Interestingly, external validation showed that low expression of seven genes (DDX3Y, EIF1AY, RPS4Y1, SMCY, USP9Y, UTY, and ZFY) had an adverse effect on survival for MM patients and three genes (BTN3A1, MAP3K3, and UBA7) were correlated with immune infiltrates in multiple cancer types. However, by performing the transcriptomics analysis in two steps, we were able to identify 33 differentially expressed genes (e.g. ALDH18A1, ATAD2, FBXO21, LIFR) between the bortezomib-sensitivity groups. Among these genes, overexpressed LIFR has previously been reported as an important cancer-related gene involved in tumor growth, metastasis, and therapy resistance31. The vast majority of these genes (31/33) were also found in at least 20% of the bortezomib-insensitive cell lines using Sanger gene expression data. Survival analysis showed that several of the dysregulated genes (e.g. ATAD2, BRMS1L, PUF60, SHQ1, and USP4) have an impact on prognosis in multiple cancer types, thereby further highlighting the clinical significance of aberrant gene expression patterns on patient survival. These putative biomarkers need further evaluation to determine their role in treatment sensitivity and clinical utility during cancer treatment decision-making. Furthermore, hierarchical clustering demonstrated that cell lines derived from bortezomib-sensitive organ systems (e.g. blood, kidney, lung, nervous system, and skin) clustered together due to similar transcriptomic profiles even if some of the cancer types were bortezomib-insensitive. However, bortezomib-insensitive organ systems also displayed transcriptomic heterogeneity as some cell lines from the same organ system clustered together while others did not. These findings may be due to inherent differences within specific cancer types, e.g. breast cancer can be stratified into several subtypes (e.g. Luminal, HER2 + , and triple-negative) with varying clinical and biological features. According to the LNIC50 thresholds set in the current study, all nine bortezomib-sensitive breast cancer cell lines were estrogen receptor-negative (5/9 triple-negative), whereas all four bortezomib-insensitive cell lines were estrogen receptor-positive. In previous work, we showed that triple-negative breast cancer cell lines were more sensitive to treatment with bortezomib than estrogen receptor-positive cells34.
Although the current study investigated up to 1,000 cancer cell lines representing various cancer types and organ systems, some cancer types were still underrepresented, e.g. lung cancer was represented by 163 cell lines while pancreas cancer was only represented by 14 cell lines. Moreover, the GDSC datasets interpreted treatment sensitivity using cell viability metrics like IC50 and AUC values. IC50 is known to be influenced by cellular proliferation rates that can differ significantly between laboratories, but these factors could be remedied using e.g. growth rate metrics (e.g. GR50) which normalizes growth rate inhibition34. To circumvent this problem, we used data for both IC50 and AUC values to set “sensitivity” thresholds that could stratify the 860 cancer cell lines into two sensitivity groups (bortezomib-sensitive and bortezomib-insensitive).
In conclusion, our in silico drug repositioning approach revealed a number of cancer types, besides multiple myeloma and mantel cell lymphoma, which may be suitable for treatment with bortezomib. We also showed that bortezomib is a multifaceted and potent drug for several solid tumors from multiple organ systems. By integrating genomic and transcriptomic profiles with corresponding sensitivity data for bortezomib, we were able to identify mutated (e.g. MUC12, PCDH15, RYR1 and SPTA1) and dysregulated genes (e.g. ALDH18A1, ATAD2, FBXO21, LIFR), thereby warranting further evaluation for their role in resistance to bortezomib. Due to their consistent expression patterns in bortezomib-treated samples, these genes may be suitable putative predictive biomarkers to ensure treatment precision for bortezomib and other proteasome inhibitors. However, future studies showing the effect of treatment with bortezomib using animal models and patient-derived samples are warranted.
Methods
Drug sensitivity data
To explore cancer cell line sensitivity to proteasome inhibitors, two publicly available drug sensitivity datasets containing half-maximum inhibitory concentration (IC50) and area under the curve (AUC) values for bortezomib were retrieved from the Genomics of Drug Sensitivity in Cancer (GDSC) database35,36,37. The two datasets consisted of 791 (GDSC1; Sanger) and 756 (GDSC2; Massachusetts General Hospital, MGH) cancer cell lines originating from 13 organ systems (aerodigestive tract, blood, bone, breast, digestive system, kidney, lung, nervous system, pancreas, skin, soft tissue, thyroid, and urogenital system; Fig. 6a), 30 cancer types, and 11 unclassified groups. The datasets were then merged into a single dataset and cell lines existing in both datasets were removed from the GDSC2 dataset. In total, 860 cell lines were included in the study (Supplementary Table 1). Then, logarithmic IC50 (LNIC50) and AUC values were used to stratify the data into two bortezomib sensitivity groups (bortezomib-sensitive and bortezomib-insensitive). The sensitivity groups were classified as follows: a) bortezomib-sensitive for LNIC50 < -6 and AUC < 0.6 and b) bortezomib-insensitive for LNIC50 > -3 and AUC > 0.9 (Table 1 and Supplementary Fig. 7a–f). To be classified in a specific sensitivity group, each cell line needed to be classified as “bortezomib-sensitive” or “bortezomib-insensitive” with both LNIC50 and AUC values. The study design and workflow are shown in Fig. 6b.
Flowchart describing the workflow and study design. (a) Schematic image illustrating the 13 organ systems for the cancer cell lines included in the study. (b) Data sources and cell line availability in the different analyses. External validation was performed using cancer cell lines derived from breast, glioblastoma, melanoma, and multiple myeloma cancer, as well as CCLE mutation data to confirm the mutation frequency of 19 genes. KM-plotter was used to explore the influence of 32 of the 33 dysregulated genes on survival in patient samples representing 21 cancer types (pan-cancer). TIMER2.0 was used to validate gene expression relationship between genes involved in bortezomib-sensitivity and immune cells in different cancer types and genes involved in bortezomib-sensitivity and outcome in myeloma patients using MMRF-COMMPASS study. (c) Flowchart depicting the study design for the drug sensitivity screen, cell cycle distribution analysis, and apoptosis analysis using Annexin V.
Genomics and transcriptomics profiling data
Genomics and transcriptomics data were retrieved from the Sanger COSMIC Cell Line Project (mutation signatures and gene expression profiles for 970 and 1,007 cell lines, respectively)38 and Cell Model Passport (cancer driver mutation frequency data for 1,318 cell lines)39 to evaluate mutations on the protein level and gene expression patterns. First, we explored the frequency of mutated genes (missing data for NCI-H2595 and SK-MEL-1 cell lines) and cancer-driver genes in the bortezomib-sensitive and bortezomib-insensitive groups (missing data for the EHEB and QIMP-WIL cell lines). Second, a total of 16,248 genes were evaluated for up- or downregulation in the bortezomib-sensitive and bortezomib-insensitive groups by stratifying gene expression levels based on Z-scores (downregulation < -2, upregulation > 2, and normal expression between ≥ -2 and ≤ 2). Only genes over- or underexpressed in > 20% of the cell lines in each sensitivity group were included. The HT, MY-M12, NCI-H2595, and NK-92MI cell lines were removed from the analysis due to missing data. Genes of interest were further investigated for pathway involvement using the Reactome interactive database40.
Cell culture
To validate our findings from the publicly available datasets, we used the human glioblastoma (A-172), melanoma (A-375) and breast cancer (MCF-7) cell lines provided by Martin Johansson, Jonas Nilsson, Julie Grantham (University of Gothenburg), as well as breast cancer (BT-474 and ZR-75–30) and multiple myeloma (RPMI-8226) cell lines that were purchased from The American Type Culture Collection (Fig. 6c). The cell lines were cultured in RPMI 1640 (RPMI-8226 and ZR-75–30 cells) supplemented with 2 mM L-glutamine, 2 g/L D-glucose, and 10% fetal bovine serum (FBS; ThermoFisher Scientific), or Dulbecco Modified Eagle´s Medium (DMEM; A-172, A-375, BT-474, and MCF-7 cells) supplemented with 2 mM L-glutamine, 4 g/L D-glucose, and 10% FBS (ThermoFisher Scientific) and maintained at 37°C in a humidified 5% CO2 environment. Cell authentication was performed using the Eurofins Genomics Human Cell Line Authentication service. First, A-172, A-375, BT-474, RPMI-8226, and ZR-75–30 cells were screened for sensitivity to bortezomib, as described elsewhere34. In brief, the cells were seeded on 96-well plates at a density ranging between 3.0 × 103 and 7.5 × 103 cells/well depending on proliferation rates and incubated for 24h. The cells were then exposed to bortezomib (Selleckchem; nine tenfold concentrations between 1 and 10,000 nM) and matched DMSO concentration vehicle controls for 24h and 72h. Cell viability was determined using the resazurin cell viability assay and growth rate metrics assessed (IC50 and AUC) with the GRmetrics (version 1.16.0) package41 in R/Bioconductor (version 4.0.3) as previously described34, and ggplot2 (version 3.3.5) for data visualization42. To determine the proteasome activity of the β5 chymotrypsin-like active site following 2h bortezomib exposure, we used Suc-Leu-Leu-Val-Tyr-AMC (Enzo; Cat. BML-P802) at a concentration of 20 µM per well in 96-well plates for A-172, A-375, BT-474, and ZR-75–30 cells and the fluorescence measured (excitation 355 and emission 460) using a Wallac 1420 VICTOR2 microplate reader (Perkin Elmer).
Annexin V early apoptosis assay and cell cycle analysis
To evaluate apoptotic cell death and cell cycle distribution in bortezomib-treated cell lines (A-172, A-375, BT-474, and ZR-75–30), the cells were first seeded at a density of 5 × 105 (apoptosis) and 1 × 106 (cell cycle) cells/well on 6-well plates and incubated for 24h at 37°C in a humidified 5% CO2 environment. After 24h, the medium was discarded and new medium containing bortezomib (1, 10, and 100 nM) was added to treatment wells and corresponding DMSO (drug solvent) to control wells. Additional bortezomib doses (25, 50 and 75 nM) were evaluated for A-172 and A-375 cells due to extremely low cell viability at 100 nM. Following treatment with bortezomib for 24h or 72h, the cells were collected and washed twice with PBS. In total, 1 × 105 cells were further processed using Annexin V-FITC Early Apoptosis Detection Kit (Cat. 6592, Cell Signaling Technology, Danvers, Massachusetts, USA) according to the manufacturer’s instructions and analyzed 10,000 events using a BD Accuri™ C6 personal flow cytometer (BD Biosciences, Franklin Lakes, New Jersey, USA) with the FL1 (Annexin V-FITC) and FL3 channels (propidium iodide, PI; Fig. 6c). Data analysis was performed using FlowJo™ v10.8.1 Software (BD Life Sciences). For cell cycle analysis, the cells were fixed in pre-chilled 70% ethanol and stored at -20°C for at least three days. The cells were then centrifuged at 220xg, washed twice with PBS, and stained using PI/RNase Staining Solution (Cat. 4087, Cell Signaling Technology, Danvers, Massachusetts, USA). Data analysis was performed for 10,000 events using a BD LSRFortessa™ cell analyzer (BD Biosciences, Franklin Lakes, New Jersey, USA) and FlowJo™ (BD Life Sciences; Fig. 6c).
Quantitative real-time PCR
Total RNA was extracted from untreated BT-474, MCF-7, and ZR-75–30 cells using the RNeasy Lipid Tissue Mini Kit (Qiagen), followed by evaluation of RNA concentration and integrity using Qubit (ThermoFisher Scientific) and TapeStation (Agilent), respectively. Complementary DNA was synthesized with the Superscript III First-Strand Synthesis for qRT-PCR kit (ThermoFisher Scientific). Quantitative real-time PCR (qPCR) was performed using predesigned TaqMan Gene Expression Assays (ThermoFisher Scientific) for SHARPIN (HS00229642_m1) and VPS28 (HS01598026_m1) with a 7500 Fast Real-Time PCR System (Applied Biosystems Inc). The relative gene expression patterns were determined using the ΔCt method after normalizing the data using the geometric mean of three endogenous controls (HPRT1 [Hs02800695_m1], PPIA [Hs99999904_m1], and PUM1 [HS00472881_m1]).
External validation
The 19 genes with significantly elevated mutation rates in the bortezomib-insensitive group were validated using the CCLE mutation dataset downloaded from cBioPortal (https://www.cbioportal.org/). Only 27/38 bortezomib-insensitive and 22/49 bortezomib-sensitive cancer cell lines were available in the CCLE mutation dataset. The clinical significance of aberrant gene expression patterns (high or low expression) for 32 of the 33 dysregulated genes (CENPX_ENST00000580435 gene was excluded due to missing data) were evaluated using the KM plotter interactive website to assess the effect of expression on overall survival in the pan-cancer cohort containing 7,389 cancer samples (RNA-seq) derived from 21 cancer types. The default settings were used for KM plotter. Analysis of gene expression patterns for 220 genes (193 genes identified in bortezomib-insensitive cell lines and 41 genes identified in bortezomib-sensitive cell lines; data for 14 genes were missing) linked to survival for 589 MM patients treated with bortezomib from the MMRF-COMMPASS study was performed using clinical data from the UCSC Xena Browser and KM plotter (overall survival). Gene expression was analyzed using DESeq2 (version 1.0.2), followed by hierarchical clustering using pheatmap (version 1.0.12) with Manhattan distance metric and Ward’s minimum variance method (Ward.D2), ggplot2 (version 3.4.4) for data visualization, and summarization of the dataset with tableone (version 0.13.2). TIMER2.0 was used to assess the relationship between immune infiltrates (B cells, CD4 + T cells, CD8 + T cells, myeloid dendritic cells, macrophages, and neutrophils) and the expression of genes associated with immune response.
Statistical analysis
Statistical analyses were performed in R/Bioconductor (version 4.0.3) and Microsoft Excel 2016/2019. P < 0.05 was considered to be statistically significant. Hierarchical clustering of gene expression patterns was performed using the pheatmap R package (version 1.0.12)43 and the Manhattan distance metric and Ward’s minimum variance method (Ward.D2). Differentially expressed genes between the sensitivity groups were identified using Limma R package (version 3.50.0)44. The mutation frequency between the sensitivity groups was performed using the ggstatsplot R package (version 0.9.1) with ggbetweenstats45. Waterfall plots were used to stratify the 50 most frequently mutated genes in each sensitivity group using maftools package (version 2.10.0) and GenVisR package46,47. If multiple mutations were detected in the same gene, the most deleterious (mutations that affect the protein to become less functional or non-functional) was considered48. A descriptive analysis using one-way ANOVA with the dplyr R package (version 1.0.8) was used to identify significant changes in cell cycle arrest and apoptosis induced by bortezomib49.
Data availability
All data used in this study are included or referred to within this work.
References
Coux, O., Tanaka, K. & Goldberg, A. L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847 (1996).
Jang, H. H. Regulation of protein degradation by proteasomes in cancer. J. Cancer Prev. 23, 153–161 (2018).
Rousseau, A. & Bertolotti, A. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 19, 697–712 (2018).
Sharon, M., Taverner, T., Ambroggio, X. I., Deshaies, R. J. & Robinson, C. V. Structural organization of the 19S proteasome lid: insights from MS of intact complexes. PLoS Biol. 4, e267 (2006).
Concannon, C. G. et al. Apoptosis induced by proteasome inhibition in cancer cells: Predominant role of the p53/PUMA pathway. Oncogene 26, 1681–1692 (2007).
Tanaka, K. The proteasome: Overview of structure and functions. Proc. Japan Acad. Series B 85(12), 36 (2009).
Morozov, A. V. & Karpov, V. L. Proteasomes and several aspects of their heterogeneity relevant to cancer. Front. Oncol. 9, 761 (2019).
Chen, L. & Madura, K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 65, 5599–5606 (2005).
Weyburne, E. S. et al. Inhibition of the proteasome β2 Site sensitizes Triple-negative breast cancer cells to β5 inhibitors and suppresses Nrf1 activation. Cell. Chem. Biol. 24, 218–230 (2017).
Lü, S. & Wang, J. The resistance mechanisms of proteasome inhibitor bortezomib. Biom. Res. 1, 13 (2013).
Nunes, A. T. & Annunziata, C. M. Proteasome inhibitors: Structure and function. Semin. Oncol. 44, 377–380 (2017).
Chen, D., Frezza, M., Schmitt, S., Kanwar, J. & Dou, Q. P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. cancer Drug Targets 11, 239–253 (2011).
Xin, B. T. et al. Structure-based design of inhibitors selective for human proteasome β2c or β2i subunits. J. Med. Chem. 62, 1626–1642 (2019).
Accardi, F. et al. Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: Impact on myeloma-induced alterations of bone remodeling. BioMed. Res. Int. 2015, 172458 (2015).
Kubiczkova, L., Pour, L., Sedlarikova, L., Hajek, R. & Sevcikova, S. Proteasome inhibitors—Molecular basis and current perspectives in multiple myeloma. J. Cell. Mol. Med. 18, 947–961 (2014).
Manasanch, E. E. & Orlowski, R. Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 14, 417–433 (2017).
Park, J. E., Miller, Z., Jun, Y., Lee, W. & Kim, K. B. Next-generation proteasome inhibitors for cancer therapy. Transl. Res. 198, 1–16 (2018).
Pushpakom, S. et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug. Discov. 18, 41–58 (2019).
Ashburn, T. T. & Thor, K. B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3, 673–683 (2004).
Jordan, V. C. Tamoxifen: A most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2, 205–213 (2003).
Quirke, V. M. Tamoxifen from failed contraceptive pill to best-selling breast cancer medicine: A case-study in pharmaceutical Innovation. Front. Pharmacol. 8, 620 (2017).
Garcia-Albeniz, X. & Chan, A. T. Aspirin for the prevention of colorectal cancer. Best Pract. Res. Clin. Gastroenterol. 25, 461–472 (2011).
Keats, J. J. et al. Molecular predictors of outcome and drug response in multiple myeloma: An interim analysis of the mmrf commpass study. Blood 128, 194 (2016).
Kikutake, C., Yoshihara, M., Sato, T., Saito, D. & Suyama, M. Intratumor heterogeneity of HMCN1 mutant alleles associated with poor prognosis in patients with breast cancer. Oncotarget 9, 33337 (2018).
Kondagunta, G. V. et al. Phase II trial of bortezomib for patients with advanced renal cell carcinoma. J. Clin. Oncol. 22, 3720–3725 (2004).
Osawa, T. et al. Blood distribution of bortezomib and its kinetics in multiple myeloma patients. Clin. Biochem. 47, 54–59 (2014).
Levêque, D., Carvalho, M. C. & Maloisel, F. Review Clinical pharmacokinetics of bortezomib. Vivo 21, 273–278 (2007).
Didier, R. et al. Targeting the proteasome-associated Deubiquitinating enzyme USP14 impairs melanoma cell survival and overcomes resistance to MAPK-targeting therapies. Mol. Cancer Ther. 17, 1416–1429 (2018).
Johansson, P. et al. A Patient-Derived Cell Atlas Informs Precision Targeting of Glioblastoma. Cell. Rep. 32, 107897 (2020).
Zhu, G. et al. Mutant p53 in cancer progression and targeted therapies. Front. Oncol. 10, 595187 (2020).
Viswanadhapalli, S., Dileep, K. V., Zhang, K. Y. J., Nair, H. B. & Vadlamudi, R. K. Targeting LIF/LIFR signaling in cancer. Genes. Dis. 9, 973 (2021).
Liu, N. et al. ATAD2 is associated with malignant characteristics of pancreatic cancer cells. Oncol. Lett. 17, 3489–3494 (2019).
Gong, J., Zhou, Y., Liu, D. & Huo, J. F-box proteins involved in cancer-associated drug resistance. Oncol. Lett. 15, 8891–8900 (2018).
Larsson, P. et al. Optimization of cell viability assays to improve replicability and reproducibility of cancer drug sensitivity screens. Sci. Rep. 10, 5798 (2020).
Yang, W. et al. Genomics of Drug Sensitivity in Cancer (GDSC): A resource for therapeutic biomarker discovery in cancer cells. Nucl. Acids Res. 41, D955–D961 (2013).
Iorio, F. et al. A landscape of Pharmacogenomic interactions in cancer. Cell 166, 740–754 (2016).
Garnett, M. J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Sondka, Z. et al. COSMIC: Acurated database of somatic variants and clinical data for cancer. Nucleic. Acid. Res. 52, D1210–D1217 (2024).
van der Meer, D. et al. Cell Model Passports—a hub for clinical, genetic and functional datasets of preclinical cancer models. Nucleic. Acid. Res. 47, D923–D929 (2019).
Gillespie, M. et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 50, D687–D692 (2022).
Clark, N. A. et al. GRcalculator: An online tool for calculating and mining dose-response data. BMC Cancer 17, 698 (2017).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer International Publishing, 2016).
Kolde, R. pheatmap, pretty heatmap, https://CRAN.R-project.org/package=pheatmap, (2019).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl. Acids res. 43, e47–e47 (2015).
Patil, I. Visualizations with statistical details: The “ggstatsplot” approach. J. Open Source Softw. 6, 3167 (2021).
Mayakonda, A., Lin, D. C., Assenov, Y., Plass, C. & Koeffler, H. P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018).
Skidmore, Z. L. et al. GenVisR: Genomic Visualizations in R. Bioinformatics 32, 3012–3014 (2016).
Brooker, R. J. Genetics Analysis and Principles, Fifth Edition. (Mc Graw Hill Education (Uk), 2015).
Wickham, H., François, R., Henry, L., Müller, K. dplyr: A Grammar of Data Manipulation, https://CRAN.R-project.org/package=dplyr, (2022).
Acknowledgements
This work was funded by Assar Gabrielsson Research Foundation for Clinical Cancer Research (P.L.), The Swedish Society of Medicine (T.Z.P.), Åke Wiberg Research Foundation (T.Z.P.), Magnus Bergvall Research Foundation (T.Z.P.), Wilhelm och Martina Lundgren Research Foundation (T.Z.P.), Anna-Lisa och Bror Björnsson Research Foundation (T.Z.P.), Assar Gabrielsson Research Foundation for Clinical Cancer Research (T.Z.P.). We would like to thank Jonas Nilsson and Martin Johansson at Sahlgrenska Center for Cancer Research and Julie Grantham at Department of Chemistry and Molecular Biology (University of Gothenburg) for kindly providing the A-375, A-172 and MCF-7 cell lines used in this study.
Funding
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
T.Z.P., P.L., M.O.: Study concept and experimental design; P.L., S.S., and T.Z.P.: Analysis and interpretation of data; P.L., T.Z.P., M.O., S.S., A.F.B., E.F.-A., A.K., P.K., K.H.: Writing of the manuscript, preparation of figures and statistical analysis; P.L., and T.Z.P.: Acquisition of funding; All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Larsson, P., Olsson, M., Sarathchandra, S. et al. Multi-omics analysis identifies repurposing bortezomib in the treatment of kidney-, nervous system-, and hematological cancers. Sci Rep 14, 18576 (2024). https://doi.org/10.1038/s41598-024-62339-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-62339-x