Abstract
Xenocoumacin 1 (Xcn 1), antibiotic discovered from secondary metabolites of Xenorhabdus nematophila, had the potential to develop into a new pesticide due to its excellent activity against bacteria, oomycetes and fungi. However, the current low yield of Xcn1 limits its development and utilization. To improve the yield of Xcn1, response surface methodology was used to determine the optimal composition of fermentation medium and one factor at a time approach was utilized to optimize the fermentation process. The optimal medium composed of in g/L: proteose peptone 20.8; maltose 12.74; K2HPO4 3.77. The optimal fermentation conditions were that 25 °C, initial pH 7.0, inoculum size 10%, culture medium 75 mL in a 250 mL shake flask with an agitation rate of 150 rpm for 48 h. Xenorhabdus nematophila YL001 was produced the highest Xcn1 yield (173.99 mg/L) when arginine was added to the broth with 3 mmol/L at the 12th h. Compared with Tryptic Soy Broth medium, the optimized fermentation process resulted in a 243.38% increase in Xcn1 production. The obtained results confirmed that optimizing fermentation technology led to an increase in Xcn1 yield. This work would be helpful for efficient Xcn1 production and lay a foundation for its industrial production.
Similar content being viewed by others
Introduction
Xenorhabdus nematophila is an entomopathogenic nematode symbiotic bacteria which belonging to the family Morganellaceae1,2 Generally, X. nematophila has two phenotypic variants, called primary and secondary. Only the primary variant absorbed bromothymol blue in the nutrient agar media supplemented with 25 mg/L bromothymol blue and 40 mg/L triphenyltetrazolium chloride (NBTA)2,3. Significantly, the primary variant produced various antimicrobial secondary metabolites such as nematophin4, odilorhabdins5, pristinamycin6, xenocoumacins7, and xenortides8. Xenocoumacin 1 (Xcn 1), a water-soluble antimicrobial agent produced by Xenorhabdus nematophila, originally identified in the fermentation broth of Xenorhabdus sp. Q1 (ATCC 39497)7. Biosynthetic gene cluster of Xcn1 had been identified that the production of Xcn1 was generated by xcnA-L genes and disintegrated by xcnMN genes9,10.
Pharmacological studies on Xcn1 showed that it had antibacterial and antifungal activity. The minimum inhibitory concentration (MIC) were 0.5 μg/mL and 0.125 μg/mL when Xcn1 against Escherichia coli ESS and Cryptococcus neoformans, respectively7. Xcn1 also showed biological activity with median effect concentrations (EC50) ranging from 0.25 to 4.17 μg/mL against five species of Phytophthora11. Furthermore, Xcn1 had the potential to be developed as an acaricide. The half maximal inhibitory concentration (IC50) of Xcn1 against Tetranychus urticae was 17.71 μg/mL12. Interestingly, Xcn1 was friendly to Neoseiulus californicus, the natural enemy of T. urticae13. Therefore, the development of Xcn1 as a new pesticide was a possibility.
However, the low yield and high cost of chemical synthesis of Xcn1 limit its industrial production. Approximately 7–9 steps were required to chemically synthesize Xcn114. Similarly, the low yield of Xcn1 produced by X. nematophila was a serious limitation to its commercialization. Therefore, it was necessary to improve the production of Xcn1. Several strategies, aiming at improving the biosynthetic efficiency, such as tuning the expression of regulatory genes, increasing the supply of precursors, and over-expressing the biosynthesis and export genes, were used to increase antibiotic yields15,16. Xcn1 was produced in X. nematophila naturally with the help of positive regulatory factors such as FliZ17, Hfq18 and Lrp19. The yield of Xcn1 was increased by deleting or silencing genes of negative regulatory factors such as CpxR20, LeuO19 and OmpR21. In situ product removal method was used to increase the production of Xcn122. Moreover, there were effective tools that block degradation pathways and promoter engineering were employed, which had led to a yield increase of Xcn1 exceeding ten times23,24.
Response surface methodology (RSM) was a powerful methodology that was routinely applied in the optimization of bioprocessing of numerous substances such as enzymes (xylanases25 and proteases26), pecticoligosaccharides27, and biodegradation of antibiotics28. The medium composition of X. nematophila and X. stockiae were optimized by response surface methodology to enhancing the antifungal activity of cell-free broth29,30. Our previous research showed that antimicrobial activity of cell-free broth was enhanced by manipulating pH31, dissolved oxygen3 and other fermentation process32.
Precursors were crucial factors to improving the production of natural products in the fermentation process33,34. The production of antibiotics was increased by adding appropriate precursors. For instance, addition of valine, a biosynthetic precursors of glycopeptide antibiotic A40926, to minimal medium increased A40926 production35. Sodium decanoate was an effective precursor for synthesis of daptomycin from Streptomyces roseosporus NRRL11379 which was increased the daptomycin production remarkably36. There was no precedent that introducing precursor chemicals for secondary metabolites into medium promotes secondary metabolite production in X. nematophila. From the molecular structure of Xcn1, arginine, leucine, urea and acetic acid were identified as precursors of Xcn1 biosynthesis10,37.
In order to obtain higher production of Xcn1, composition and their concentrations of the fermentation medium were optimized by RSM in this research. Furthermore, detailed optimization was carried out on parameters such as temperature, pH, fermentation period, seed quantity, broth loading, and shaking speed in the fermentation process. Moreover, several precursor chemicals were investigated to test their effectiveness for the improvement of Xcn1 yield.
Materials and methods
Strains and culture conditions
Xenorhabdus nematophila YL001 was isolated from entomopathogenic nematode Steinernema sp. YL001 which was obtained from Yangling, China3. It was preserved at − 78 °C in Shaanxi Research Center of Biopesticide Engineering & Technology. The 16S rRNA gene sequence of X. nematophila YL001 obtained accession number EU124381 (https://www.ncbi.nlm.nih.gov/nuccore/EU124381.1) after molecular identification38. For Xcn1 production, X. nematophila YL001 was maintained in primary variant on NBTA medium, distinguished by absorbing bromothymol blue, and used throughout the study.
A single colony of X. nematophila YL001 was transferred into a 250 mL flask containing 100 mL of Luria–Bertani (LB) at 28 °C and 180 rpm for 24 h to seed cultivation. Then, 10 mL of the seed culture was transferred into a 250 mL flask containing 100 mL of fermentation medium and the system was incubated for 48 h with continuous agitation in the constant temperature cultivation oscillator (ZHWY-2112B, Shanghai Zhicheng, China). Aforesaid fermenting procedure was used in all subsequent experiments. The initial fermentation medium, Tryptic Soy Broth (TSB), was composed of the following: 17 g/L Bacto Tryptone, 3 g/L Soy Peptone, 2.5 g/L Glucose, 2.5 g/L K2HPO4, and 5 g/L NaCl in water; pH 7.2.
Purification and identification of Xcn1
McInerney’s method was improved and used for isolating and purifying Xcn17. In brief, fermentation broth of X. nematophila YL001 was centrifuged to obtain cell-free supernatant. 90% of the water was removed by rotary evaporator and then the concentrated supernatant was extracted with petroleum ether, chloroform and ethyl acetate. The aqueous phase was acidified to pH 3.0 with oxalic acid, subsequently the aqueous phase pH was adjusted to 7.0 with sodium hydroxide. Centrifuge again, and then the supernatant was added to the X-5 macroporous resin column (100 cm × 98 cm, Nankai University Chemical Factory, China) and stood for 6 h for adsorption. Next, the column was eluted with water, 25% methanol (v/v), 50% methanol (v/v), 30% acetone (v/v) containing 0.1 mol/L HCl and 50% acetone (v/v) containing 0.2 mol/L HCl in turn at a flow rate of 3 mL/min. Acetone-containing eluate was concentrated and its pH was adjusted to 8.0 with sodium hydroxide and subsequently loaded onto a 110 cation exchange chromatography column (Tianjin Guangfu Fine 195 Chemical Research Institute, Chian) at a flow rate of 5 mL/min, then eluted with 0.2 mol/L NH4Cl. Further purification was conducted by the Sephadex G25 column with 0.1 mol/L NH4Cl eluted. Eventually, eluate was adsorbed by X-5 macroporous resin column and eluted by acetone to obtaining purified Xcn1. 1H NMR and 13C NMR were identified by the nuclear magnetic resonance spectrometer (AVANCE III, 500 MHz, Bruker, Switzerland) (Figs. S1, S2). The structure of Xcn1 was determined by high resolution liquid chromatography mass spectrometry (LC-30A + TripleTOF5600 + , AB SCIEX, USA) (Fig. S3).
One factor at a time approach (OFAT)
To selecting the original medium with the highest yield of Xcn1, ten commonly used bacterial media were investigated. The fermentation medium included LB medium, TSB medium, Beef peptone yeast (BPY) medium (10 g/L Peptone, 5 g/L Beef Extract, 5 g/L Yeast Extract, 5 g/L Glucose, and 5 g/L NaCl in water; pH 7.2), BR medium (10 g/L Beef Peptone, 3 g/L Beef Extract, and 5 g/L NaCl in water; pH 7.2), KB medium (5 g/L Peptone, 3 g/L Yeast Extract, and 2.5 g/L Glucose in water; pH 7.2), NB medium (5 g/L Beef Extract, 6 g/L Peptone, and 15 g/L NaCl in water; pH 7.2), NB + medium (3 g/L Beef Extract, 5 g/L Peptone, and 10 g/L NaCl in water; pH 7.2), PP3 medium (20 g/L Proteose Peptone in water; pH 7.2), PP3 + medium (20 g/L Proteose Peptone and 10 g/L NaCl in water; pH 7.2), YS medium (5 g/L Yeast Extract, 0.5 g/L (NH4)2SO4, 0.5 g/L K2HPO4, 0.2 g/L MgSO4·7H2O, and 5 g/L NaCl in water; pH 7.2).
In the content of 5 g/L, various carbon sources such as glucose, fructose, maltose, starch, dextrin, lactose and sucrose were tested individually. To screen nitrogen sources, 20 g/L concentrations of soy peptone, bacto-tryptone, proteose peptone, beef peptone, yeast extract, potassium nitrate, urea and beef extract were evaluated respectively. To analyze the mineral sources, the inorganic salt such as, magnesium sulfate, sodium chloride, dipotassium hydrogen phosphate and sodium sulfate were tested with content of 10 g/L separately.
The initial pH was set at 5.0, 6.0, 7.0, 8.0 and 9.0. Liquid loading of broth in the 250 mL flask were set at 50, 75, 100, 125 and 150 mL. Seed quantity was set at 4%, 6%, 8%, 10% and 12%. The rotating speeds of the shaker were set at 50, 100, 150 and 200 rpm. Fermentation period were set at 24, 48, 72, 96 and 120 h. And temperatures of fermentation were set at 15, 20, 25, 30 and 35 °C. Several precursors such as arginine, leucine, acetic acid and urea were added to the medium and theirs concentrations were set at 2, 3 and 4 mmol/L. In addition, precursors were added in different time, such as the 0 h, 6 h, 12 h, 18 h, 24 h and 30 h, during fermentation.
Experimental design and optimization by RSM
The fermentation medium was optimized by response surface methodology. In order to enhance the production of Xcn1, central composite design (CCD) was used to optimize the concentrations of the variables such as carbon source(X1), nitrogen source(X2) and mineral source(X3) with the help of Design expert 10 (Stat-Ease, USA). Variables were analyzed at five different levels (− α, − 1, 0, + 1, and + α). Twenty experimental trials have been carried out for the selected three variables (maltose, proteose peptone and K2HPO4). These twenty trials included six centre points, six axial points and eight factorial points. After which, the mean value of the response Y (the yield of Xcn1) was assayed in triplicate analysis. The CCD experiment was not only employed to analyze the interactions among the important three selected variables (proteose peptone, maltose and K2HPO4) but also to find their optimum levels.
Xcn1 contents analysis by high performance liquid chromatography (HPLC)
Previously purified Xcn1 was seen as the pure product to prepare the standard stock solution with a concentration of 2000 μg/mL. Then, the mother liquor was dissolved with ultra-pure water and diluted it to 1000 μg/mL, 800 μg/mL, 600 μg/mL, 400 μg/mL, 200 μg/mL and 100 μg/mL standard solution. Standard curve, represents the linear relationship between peak area and the concentration of Xcn1, was drawn with Xcn1 concentration as abscissa and absorption peak area as ordinate. The linear regression equation was showed in Eq. (1) and the correlation coefficient was R2 = 0.9993 (Fig. S4).
The broth of X. nematophila YL001 was centrifuged and the supernatant was added to the activated X-5 macroporous resin column for chromatographic separation. The macroporous resin column was eluted in five times of the volume with deionized-water, 50% methanol (v/v), 30% acetone (v/v) with 0.01 mol/L HCl and 50% acetone (v/v) with 0.02 mol/L HCl successively. The eluent of 30% acetone (v/v) with 0.01 mol/L HCl was collected and dried. Dried samples were dissolved in 10 mL 50% methanol for HPLC detection. Twenty microliters of sample were injected into a reversed-phase C18 column (2.1 × 150 mm), eluted with 30% acetonitrile (v/v) in 0.1% trifluoroacetic acid at a flow rate of 1 mL/min, monitored at 312 nm, and the temperature of the column was 25 °C7.
Statistical analysis
Design expert 10 (Stat-Ease, USA) was used to analyze the variables. The 3D response graph and profile for predicted values and desirability level for factors were plotted using the same software. The statistical analysis of the model was performed in the form of analysis of variance (ANOVA). For each variable, the quadratic models were represented as contour plots. In addition, the fermentation conditions and precursors were analyzed using Origin 9.1 and Microsoft office Excel. Data were given from 3 replications ± SE and data with different letters were significantly different at P < 0.05 by Duncan’s multiple range test.
Results
Identification of Xcn1
The chemical structure of Xcn1 was showed in Fig. 1a. The signals of hydrogen atoms and carbon atoms of Xcn1 were well presented in the nuclear magnetic spectrum, and their chemical shifts and coupling constants are also consistent with the reported data7. The data analysis of 1H NMR and 13C NMR spectra for Xcn1 were showed in Table S1.
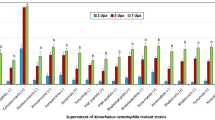
Effects of different medium and nutrient sources on Xcn1 production. (a) The structure of Xenocoumacin 1 (Xcn1). (b) Effects of different media on Xcn1 production. (c) Effects of various nitrogen sources on Xcn1 production. Abbreviation of nitrogen sources (SP: Soy peptone; TP: Tryptone; PP: Proteose peptone; BP: Beef peptone; YE: Yeast extract; PN: Potassium nitrate; UR: Urea; BA: Beef paste). (d) Effects of different carbon sources on Xcn1 production. Abbreviation of carbon sources (GL: Glucose; FR: Fructose; MA: Maltose; ST: Starch; DE: Dextrin; LA: Lactose; SU: Sucrose). (e) Effects of various inorganic salts on Xcn1 production. Abbreviation of inorganic salts (KP: K2HPO4; MS: MgSO4; NS: Na2SO4; NC: NaCl). (f) Effects of various concentrations of proteose peptone, maltose and K2HPO4 on Xcn1 production. The data represent the mean values of three independent replicates, and the error bars represent the standard deviations. Data with different letters are significantly different at P < 0.05 by Duncan’s multiple range tests.
Effect of different media on Xcn1 production
The results of Xcn1 production of X. nematophila YL001 in different media were shown in Fig. 1b and Fig. S5. The results indicated that PP3 medium showed a maximum Xcn1 production (87.21 μg/mL), followed by BR medium (69.71 μg/mL) and KB medium (67.61 μg/mL). Lower Xcn1 production was observed in YS medium (11.12 μg/mL).
Effect of various nutrient sources on Xcn1 production
Among various nitrogen sources mentioned in Fig. 1c and Fig. S6, the X. nematophila YL001 produced the maximum Xcn1 in proteose peptone (81.82 μg/mL), followed by tryptone (69.19 μg/mL) and yeast extract (63.34 μg/mL). Among carbon source, maltose showed a maximum Xcn1 production (106.31 μg/mL) followed by fructose (101.67 μg/mL) and starch (98.57 μg/mL). Lower Xcn1 production was observed with the sucrose (37.06 μg/mL) (Fig. 1d and Fig. S7). Among the various inorganic salt studied (Fig. 1e and Fig. S8), the X. nematophila YL001 produced the maximum Xcn1 (112.30 μg/mL) in K2HPO4 and the minimum level of Xcn1 (71.30 μg/mL) in NaCl. Proteose peptone, maltose and K2HPO4 were chosen as the source of nitrogen, carbon, and inorganic salt for further experiments (Fig. 1f and Fig. S9).
Optimization of medium constituents by response surface method
After determination of nitrogen sources, carbon sources and inorganic salts in the medium, the combined effects of concentration of medium constituents on Xcn1 production were further investigated. Table 1 showed experimental range and levels of the independent variables. The experimental results listed in Table 2 were analyzed using multiple regressions. The production of Xcn1 was maximum at run 11 (113.65 μg/mL). The second-order polynomial model was proposed to evaluate the optimum levels of these selected variables and was shown in Eq. (2).
In this study, the determination coefficient (R2) confirmed the importance of statistical design, exhibiting minor experimental error and a fit regression equation. The R2 of this designed CCD model was 0.9443. The F value of the model is 18.38, while the P value is less than 0.0001, indicating that the model has significance (Table 3). The saddle or elliptical nature of contour plot showed the significance of the good interactions between the respective variables. Figure 2 showed the contour plot and 3D response surface plot for the Xcn1 yield generated by the predicted CCD model. According to the model equation, we predicted that a maximum Xcn1 yield of 113.65 μg/mL could be achieved at 12.74 g/L maltose, 20.83 g/L proteose peptone and 3.77 g/L K2HPO4. By HPLC, the content of Xcn1 was 117.99 μg/mL with the optimized medium, which was close to the predicted response (Table 2 and Fig. S10). Compared with the output of Xcn1 of TSB medium (50.67 μg/mL), the output of Xcn1 of the optimized PP3 medium (113.65 μg/mL) increased by 112.65%.
Optimization of cultural conditions
The production of Xcn1 reached the optimal yeild at 25 °C (Fig. 3a and Fig. S11). It was selected that pH 7 for optimal production (125.72 μg/mL) of Xcn1 (Fig. 3b and Fig. S11). 10% was selected as the optimal inoculum size (Fig. 3c and Fig. S12). 100 rpm was selected as the optimal rotating speed (Fig. 3d and Fig. S12). It showed that the optimal medium loading was 75 mL medium in a 250 mL flask (Fig. 3e and Fig. S13). There was no significant increase in the production of Xcn1 when the fermentation time increased after 48 h (Fig. 3f and Fig. S13). Consequently, 48 h was selected as the optimal fermentation time.
Effects of different fermentation conditions on the production of Xcn1. (a) Effects of various fermentation temperatures on Xcn1 production. (b) Effects of different initial pH of fermentation medium on Xcn1 production. (c) Effects of various inoculum size on Xcn1 production. (d) Effects of different rotating speed of shaker on Xcn1 production. (e) Effects of various volume of liquid medium on Xcn1 production. (f) Effects of different fermentation time on Xcn1 production. The data represent the mean values of three independent replicates, and the error bars represent the standard deviations. Data with different letters are significantly different at P < 0.05 by Duncan’s multiple range tests.
Effect of precursors on Xcn1 production
Markedly, in those tested precursor, arginine was the best for Xcn1 production and its optimal concentration was 3 mmol/L (Fig. 4 and Fig. S14–15).The production of Xcn1 was assessed at 48 h and reached the maximum yield when arginine was added to the medium at the 12 h (Fig. 4e and Fig. S16). With the optimal concentration (3 mmol/L), arginine was added to the fermentation medium at the 12 h.
Effects of different precursor substances concentration and arginine adding time on Xcn1 production. (a) Effects of various concentration of urea on Xcn1 production. (b) Effects of different concentration of acetic acid on Xcn1 production. (c) Effects of various concentration of leucine on Xcn1 production. (d) Effects of different concentration of arginine on Xcn1 production. (e) Effects of adding time of arginine on Xcn1 production. The data represent the mean values of three independent replicates, and the error bars represent the standard deviations. Data with different letters are significantly different at P < 0.05 by Duncan’s multiple range tests.
Discussions
Medium played a decisive role in the whole fermentation process of microorganisms30. By optimizing the nutrition of medium, the yield of Xcn1 was enhanced from 50.73 μg/mL to 117.99 μg/mL. Proteose peptone was the most suitable nitrogen sources for Xcn1 production. Previous studies showed that X. nematophila YL001 produced the most antibiotic activity when proteose peptone was used as nitrogen source30. Going further, it indicated that Xcn1 was the main active component of the antibiotic produced by X. nematophila YL00110,39. In order to verify the accuracy of prediction by RSM, obtained medium formula was used to flask fermentation. The measured yield of Xcn1 was consisted with the predicted result so that RSM was an effective method for optimizing media for antibiotic production.
To ensure accuracy, the factors for RSM optimization were usually limited to 4. Accordingly, optimization of 6 fermentation conditions was carried out by one factor at a time approach. Through the optimization of fermentation conditions, the yield of Xcn1 reached 153.56 μg/mL. Temperature and pH had significant influence on Xcn1 production of X. nematophila YL001, and high or low temperature and pH both led to a sharp decrease in Xcn1 production (Fig. 4). Several other environmental conditions, such as inoculum size, shaking speed, broth loading and fermentation time, could not make such a huge change in Xcn1 production. In this study, 7.0 was the most suitable pH for Xcn1 production. However, this was not consistent with the results obtained in previous studies. The weak alkaline pH environment was found to be beneficial for the production of Xcn1 and the production of Xcn1 were 2.49-fold higher at pH 8.5 relative to that at pH 7.0 in X. nematophila YL0012. To some extent, it may be due to the combined effects of various conditions of fermentation, such as fermentation time and broth loading, on Xcn1 production. For example, previous study showed that 50 mL medium was loaded in a 250 mL flask and incubated for 72 h, which resulted 8.5 was a better pH2. Accordingly, more precise control of pH and temperature would help to increase the yield of Xcn1. The optimal medium and fermentation process were chosen for this study in an effort to enhance the production of Xcn1 in X. nematophila YL001.
Four precursors were involved in the biosynthesis of Xcn1 directly or indirectly. The synthesis of arginine by microorganism needed urea to provide nitrogen. Arginyl thioester was condensed with N-acyl-D-asparaginyl thioetser into the long chain of Xcn1, which occurred in the initial stage of Xcn1 long chain biosynthesis10. Hence, arginine was the most helpful precursor for Xcn1 biosynthesis, which is consistent with the results of our study. Moreover, leucine and acetic acid were involved in the long chain synthesis of Xcn1, respectively, to form peptide bonds and extend the carbon chain10. Arginine was added to the medium to increase the production of Xcn1 in this study. If precursors, such as arginine, leucine and acetic acid, were added in the media with proper proportion at the right time, the production of Xcn1 would be further improved.
Previous studies evaluated the content of antibacterial active substances in fermentation broth of X. nematophila YL001 according to the size of bacteriostatic circle which was not accurate enough30. In this study, HPLC method was provided a more precise and accurate method for the Xcn1 detection and was proved to be a powerful tool for the optimization Xcn1 production by X. nematophila YL001.
In an industrial point of view, to improve the production, various methods and techniques should be screened for Xcn1 production. A series of published research suggested that fermentation optimization had limited effect on improving Xcn1 production compared to modifying the genome of X. nematophila23,24. However, fermentation optimization was still an indispensable part of industrial production. Furthermore, the fermentation of X. nematophila YL001 would be studied in a fermentation facility to further optimize the fermentation conditions at pilot sacle, so as to improve the output of Xcn1 and lay a foundation for its industrial production.
Conclusions
Medium, fermentation conditions and precursors were regulated and used to enhance the production of Xcn1. The production of Xcn1 on the optimal fermentation conditions reached 173.99 μg/mL, which was 3.43 times as much as that on the original conditions (TSB medium). The results will be helpful for the development of X. nematophila YL001 cultivation process for efficient Xcn1 production and lay a foundation for its industrial production, and promotes the application of Xcn1 in sustainable agricultural development.
Data availability
All data generated or analyzed during this study are included in this published article and Supplementary materials.
References
Adeolu, M., Alnajar, S., Naushad, S. & Gupta, R. S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Micr. 66, 5575–5599 (2016).
Guo, S. et al. Regulation of antimicrobial activity and xenocoumacins biosynthesis by pH in Xenorhabdus nematophila. Microb. Cell Fact. 16, 203 (2017).
Wang, Y. & Zhang, X. Influence of agitation and aeration on growth and antibiotic production by Xenorhabdus nematophila. World J. Microb. Biot. 23, 221–227 (2007).
Zhang, S. et al. Nematophin, an antimicrobial dipeptide compound from Xenorhabdus nematophila YL001 as a potent biopesticide for Rhizoctonia solani control. Front. Microbiol. 10, e01765 (2019).
Pantel, L. et al. Odilorhabdins, antibacterial agents that cause miscoding by binding at a new ribosomal site. Mol. Cell 70, 83–94 (2018).
Brachmann, A. O. et al. Reciprocal cross talk between fatty acid and antibiotic biosynthesis in a nematode symbiont. Angew. Chem. Int. Ed. Engl. 51, 12086–12089 (2012).
McInerney, B. V. et al. Biologically active metabolites from Xenorhabdus spp., Part 2. Benzopyran-1-one derivatives with gastroprotective activity. J. Nat. Prod. 54, 785–795 (1991).
Reimer, D. et al. Xenortide biosynthesis by entomopathogenic Xenorhabdus nematophila. J. Nat. Prod. 77, 1976–1980 (2014).
Reimer, D., Luxenburger, E., Brachmann, A. O. & Bode, H. B. A new type of pyrrolidine biosynthesis is involved in the late steps of xenocoumacin production in Xenorhabdus nematophila. ChemBioChem 10, 1997–2001 (2009).
Masschelein, J., Jenner, M. & Challis, G. L. Antibiotics from Gram-negative bacteria: A comprehensive overview and selected biosynthetic highlights. Nat. Prod. Rep. 34, 712–783 (2017).
Yang, X. et al. Antifungal activity of xenocoumacin 1 from Xenorhabdus nematophilus var. pekingensis against Phytophthora infestans. World J. Microb. Biot. 27, 523–528 (2011).
Incedayi, G. et al. Relative potency of a novel acaricidal compound from Xenorhabdus, a bacterial genus mutualistically associated with entomopathogenic nematodes. Sci. Rep. 11, 11253 (2021).
Wei, J. et al. Acaricidal effect of the antimicrobial metabolite xenocoumacin 1 on spider mite control. J. Integr. Agr. 23, 948–959 (2024).
Zumbrunn, C. et al. Synthesis and structure-activity relationship of xenocoumacin 1 and analogues as inhibitors of ribosomal protein synthesis. ChemMedChem 16, 891–897 (2021).
Chen, W. et al. Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat. Commun. 10, 960 (2019).
Weber, T. et al. Metabolic engineering of antibiotic factories: new tools for antibiotic production in actinomycetes. Trends Biotechnol. 33, 15–26 (2015).
Jubelin, G. et al. FliZ is a global regulatory protein affecting the expression of flagellar and virulence genes in individual Xenorhabdus nematophila bacterial cells. Plos Genet. 9, e1003915 (2013).
Neubacher, N. et al. Symbiosis, virulence and natural-product biosynthesis in entomopathogenic bacteria are regulated by a small RNA. Nat. Microbiol. 5, 1481–1489 (2020).
Engel, Y. et al. The global regulators Lrp, LeuO, and HexA control secondary metabolism in entomopathogenic bacteria. Front. Microbiol. 8, e209 (2017).
Zhang, S. et al. CpxR negatively regulates the production of Xenocoumacin 1, a dihydroisocoumarin derivative produced by Xenorhabdus nematophila. MicrobiologyOpen 8, e00674 (2019).
Park, D. et al. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol. Microbiol. 73, 938–949 (2009).
Dong, Y. et al. Improving the yield of Xenocoumacin 1 enabled by in situ product removal. ACS Omega 5, 20391–20398 (2020).
Qin, Y. et al. Improving the yield of Xenocoumacin 1 by PBAD promoter replacement in Xenorhabdus nematophila CB6. Agr. Basel 11, 1251 (2021).
Qin, Y. et al. Enhancing the production of Xenocoumacin 1 in Xenorhabdus nematophila CB6 by a combinatorial engineering strategy. J. Agr. Food. Chem. 71, 8959–8968 (2023).
Matrawy, A. A., Khalil, A. I., Marey, H. S. & Embaby, A. M. Biovalorization of the raw agro-industrial waste rice husk through directed production of xylanase by Thermomyces lanuginosus strain A3–1 DSM 105773: a statistical sequential model. Biomass Conv. Bioref. 11, 2177–2189 (2021).
Matrawy, A. A., Marey, H. S. & Embaby, A. M. The agro-industrial byproduct wheat bran as an inducer for alkaline protease (ALK-PR23) production by pschyrotolerant Lysinibacillus sphaericus strain AA6 EMCCN3080. Waste. Biomass Valor. 15, 1943–1958 (2024).
Embaby, A. M. et al. A novel non-cumbersome approach towards biosynthesis of pectic-oligosaccharides by non-aflatoxigenic Aspergillus sp. section Flavi strain EGY1 DSM 101520 through citrus pectin fermentation. PLoS One 11, e0167981 (2016).
Anan, A. et al. Statistically optimized ceftriaxone sodium biotransformation through Achromobacter xylosoxidans strain Cef6: an unusual insight for bioremediation. J. Basic Microbiol. 58, 120–130 (2018).
Sa-uth, C., Rattanasena, P., Chandrapatya, A. & Bussaman, P. Modification of medium composition for enhancing the production of antifungal activity from Xenorhabdus stockiae PB09 by using response surface methodology. Int. J. Microbiol. 2018, 3965851 (2018).
Wang, Y., Li, Y., Zhang, Q. & Zhang, X. Enhanced antibiotic activity of Xenorhabdus nematophila by medium optimization. Biores. Technol. 99, 1708–1715 (2008).
Wang, Y., Fang, X., Cheng, Y. & Zhang, X. Manipulation of pH shift to enhance the growth and antibiotic activity of Xenorhabdus nematophila. J. Biomed. Biotechnol. 2011, e672369 (2011).
Wang, Y. H., Feng, J. T., Zhang, Q. & Zhang, X. Optimization of fermentation condition for antibiotic production by Xenorhabdus nematophila with response surface methodology. J. Appl. Microbiol. 104, 735–744 (2008).
Hassan, A. et al. Optimization of enhanced microbial production of zinc bacitracin by submerged fermentation technology. J. Basic. Microb. 60, 585–599 (2020).
Teijaro, C. N., Adhikari, A. & Shen, B. Challenges and opportunities for natural product discovery, production, and engineering in native producers versus heterologous hosts. J. Ind. Microbiol. Biot. 46, 433–444 (2019).
Beltrametti, F. et al. Valine influences production and complex composition of glycopeptide antibiotic A40926 in fermentations of Nonomuraea sp. ATCC 39727. J. Antibiot. (Tokyo) 57, 37–44 (2004).
Ng, I. S. et al. Daptomycin antibiotic production processes in fed-batch fermentation by Streptomyces roseosporus NRRL11379 with precursor effect and medium optimization. Bioprocess Biosyst. Eng. 37, 415–423 (2014).
Reimer, D. et al. A natural prodrug activation mechanism in nonribosomal peptide synthesis. Nat. Chem. Biol. 7, 888–890 (2011).
Fang, X. et al. Molecular identification and cultivation characteristics of entomopathogenic bacteria. J. NWAFU. (Nat. Sci. Ed.) 36, 199–204 (2008).
Reimer, D. & Bode, H. B. A natural prodrug activation mechanism in the biosynthesis of nonribosomal peptides. Nat. Prod. Rep. 31, 154–159 (2014).
Acknowledgements
We thank the State Key Laboratory of Crop Stress Biology for Arid Areas (Northwest A&F University, Yangling, China) for assisting in the nuclear magnetic resonance spectrometer analyses. We also thank the Life Science Research Core Services (Northwest A&F University, Yangling, China) for assisting in the high resolution liquid chromatography mass spectrometry analyses.
Funding
This work was supported by the Natural Science Foundation of China (NSFC) (No. 32072474, 31171910).
Author information
Authors and Affiliations
Contributions
YHW, JG and YH conceived and designed the research; YH, SZ, YW, JG, and JH performed the experiments; YH, JG and ZY analyzed the data and wrote the paper; YT and YH revised the article. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Zhang, S., Wang, Y. et al. Enhancing the yield of Xenocoumacin 1 in Xenorhabdus nematophila YL001 by optimizing the fermentation process. Sci Rep 14, 13506 (2024). https://doi.org/10.1038/s41598-024-63794-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-63794-2