Abstract
The purpose of this research was to examine the potential effects of bentonite (BN) supplemented diets on growth, feed utilization, blood biochemistry, and histomorphology of Dicentrarchus labrax. Six treatments in triplicate were tested: B0, B0.5, B1.0, B1.5, B3.0, and B4.5, which represented fish groups fed diets supplemented with 0, 0.5, 1, 1.5, 3, and 4.5% BN, respectively. For 84 days, juveniles’ seabass (initial weight = 32.73 g) were fed diets containing 46% protein, three times daily at 3% of body weight. With a 5% daily water exchange, underground seawater (32 ppt) was used. Findings revealed significant improvements in water quality (TAN and NH3), growth (FW, WG and SGR) and feed utilization (FCR, PER and PPV) in fish fed BN-supplemented diets, with the best values in favor of the B1.5 group. Additional enhancements in kidney function indicators (urea and uric acid) and liver enzymes were observed in fish of the BN-treated groups along with a decrease in cholesterol level in the B1.5 group. Further improvements in fish innate immunity (hemoglobin, red blood cells, glucose, total protein, globulin, and immunoglobulin IgM), antioxidant activity (total antioxidative capacity and catalase), and decreased cortisol levels in fish of the BN-treated groups. Histological examinations of the anterior and posterior intestines and liver in groups B1.5 and B3 revealed the healthiest organs. This study recommends BN at a concentration of 1.5% as a feed additive in the Dicentrarchus labrax diet.
Similar content being viewed by others
Introduction
Nowadays, the aquaculture industry is critical due to its vital role in supplying approximately half of the world's animal protein needs1,2 and participating in the protection of imperiled species like sturgeons3. The prevalence of aquaculture worldwide, especially in the form of intensive and super intensive farms, and their deleterious discharges of organic and inorganic metabolites have prompted efforts to mitigate these damages and improve water quality in the surrounding environment2,4. Due to their unique structure, chemical composition, exchangeable ions, and particle size, a variety of clay minerals are now used as additives in the water where aquatic animals are raised to improve water quality and promote the removal of organic compounds5,6,7).
Clay minerals have been used in animal/fish feed due to their sorption/absorption properties, which contribute greatly to the health of organisms because they bind to harmful compounds and remove them from the body8, play a crucial role in detoxifying anti-nutritional compounds in food and relieving gastrointestinal ailments, as well as acting as mycotoxin adsorbents9. Bentonites are clays that naturally occur from silicate minerals and have been used to eliminate ammonium from fish tanks due to their lower cost, greater market availability, and superior selectivity10. They belong to the stratum troupe of aluminosilicates with extending frames that characterize their great absorbency and swelling7. As a mineral generated from dust, BN has an additional significant non-nutritive component that is employed as a powerful binding agent and adsorbent for mycotoxins, enzymes, and pathogenic microorganisms in animal feed and the intestines of animals11,12,13. BN clays could enhance the sorption exchange capacity of the acid-salt-soda activation string 7,14. Two types of bentonites are known: sodium BN, which typically has a highly turbulent type and is derived from volcanic ash that precipitated in marine environments, and calcium BN, which has a low-tumescence type and is derived from volcanic ash that precipitated in freshwater environments6,10.
Previous studies reported that BN has a good influence on fish performance when employed as a feed additive. According to Ellis et al.15 the addition of sodium BN to the diet of trout significantly reduces aflatoxin levels in the digestive tract. In addition, Ayoola12 observed that the addition of BN to various rations greatly increased the growth, feed consumption, specific growth rate, red blood cells, and hematocrits of the African catfish, Clarias gariepinus. Furthermore, the growth, survival, dietary consumption, and hematological indicators of the hybrid grouper (Epinephelus fuscoguttatus x Epinephelus lanceolatus), fed diets supplemented with varying concentrations of sodium BN for 8 weeks, were significantly enhanced in comparison to the control group16. In addition, the previous data demonstrated that Nile tilapia fed lead-contaminated diets exhibited a significant decrease in growth, blood parameters compared to fish fed the same diets with BN as a feed additive17. Also, tilapia fed aflatoxin-contaminated feed in the presence of BN had much higher concentrations of albumin, total protein, erythrocytes, and hemoglobin, and lead residues were significantly lower17.
The European seabass D. labrax is an important aquaculture species in Europe and the Mediterranean region, with a 2.9% share of the global aquaculture harvest in 20201. The global harvest of D. labrax has continuously increased from approximately 60,000 tonnes (T) in 2003 to 243,900 T in 20201. Egypt ranks third among the world's largest producers with a total of 34,477 T, after Turkey and Greece1,18. However, no studies have been published on the use of BN clay as a feed additive for the most important marine species cultivated in Egypt, such as gilthead seabream Sparus aurata and D. labrax1,19. Domestic BN has a high capacity for cation exchange, binding capacity, plasticity, impermeability, hydration, swelling, thixotropy, and a tendency to react with organic chemicals20,21,22, can protect the intestinal tract by rapidly binding toxins in the gut, in addition to providing the appropriate minerals to improve the nutritional efficiency, growth, and overall health of D. labrax. This study evaluates the potential ameliorative influences of using BN clay as a feed additive on the growth, immunology, antioxidants, and histology of D. labrax grown in groundwater.
Materials and methods
At El-Max Station for Applied Research of NIOF, Alexandria, Egypt, this experiment was carried out in cooperation with Alexandria University, Faculty of Agriculture (Saba Basha)—Department of Animal and Fish Production.
Experimental design
Six treatments were tested in triplicate to determine the potential benefits of adding raw BN to D. labrax aquafeed, as follows: (B0) = control without BN addition, (B0.5) = adding BN at a level of 0.5%, (B1) = adding BN at a level of BN 1% , (B1.5) = adding BN at a level of BN 1.5%, (B3) = adding BN at a level of BN 3%, and (B4.5) = adding BN at a level of BN 4.5%. The levels studied were chosen based on earlier research17,23. The duration of the experimental period was 84 days. During the experiment period, D. labrax fingerlings were raised in underground saltwater with a salinity of 32 ppt.
Experimental fish and rearing techniques
The D. labrax juveniles used in this study were obtained from the Kilo-21 marine hatchery (GAFRD), Alexandria, Egypt. The juveniles were produced using Mediterranean Sea water. After arriving the experimental station, fish were acclimatized to the new water conditions for two weeks and fed commercial pelleted feed (46/16) protein/fat, prior to the start of the experiment. One hundred and eighty juvenile D. labrax L. with an average initial weight of 32.73 ± 0.04g/fish, and an averae initial length of 13.93 ± 0.2 cm/fish, were used. This experiment utilized six cement tanks (each 3m × 8m and containing 24 m3 water) and eighteen net enclosures (experimental hapa: 1m x 1m x 1m, each containing 0.5 m3 of water). Fish were placed at a density of 10 fish/hapa, and every three hapas were housed in a cement tank, representing one treatment.
Diet preparation and feeding regime
Six levels of local BN (0, 0.5, 1, 1.5, 3, and 4.5% BN /kg feed) were supplemented with a commercial diet used for marine carnivorous fish. The abbreviations B0, B0.5, B1, B1.5, B3, and B4.5 represent diets supplemented with 0, 0.5, 1, 1.5, 3, and 4.5% BN per kilogram of diet, respectively. The desired amount of very fine BN was carefully mixed with the basic diet, which had been finely ground (Moulinex stand mixer—Qa205127). Following the addition of BN and hot water, the diets were pressed using an electric kitchen meat grinder (Moulinex 1600 W, France). The feed strands were then dried at 45 degrees Celsius for 12 h. Using various feed sieves, the pellets were sieved to achieve the desired particle sizes. The pellets were packed and kept at a temperature of − 20°C until use. The fish were fed to satiation four times a day, six days a week. Table 1 shows the chemical composition of the commercial diet and BN from Egypt's Central Eastern Desert. High purity BN purchased from an Egyptian distributor and collected from El Qoseir area in the central eastern desert, Egypt, located on the coast of the Red Sea, was used in the present study. Dardir et al.22 investigated the physicochemical properties of the BN used in the experiment and determined that the average particle size is 8 um, the average pore diameter is 12.6 nm, the specific surface area is 102.5 m2/g, and the cation exchange capacity is 127 meq/100 g.
Water quality analyzes
Throughout the experiment, temperature, dissolved oxygen, pH, total ammonia nitrogen (TAN), and un-ionized ammonia (NH3) were monitored twice weekly for all treatments. SensoDirect 150 (Multiparameter, portable photometer) was used to measure ph/Redox, conductivity, dissolved oxygen, TDS, and temperature (°C) for water analysis. TAN was determined using the Hanna HI-97715 model (portable photometer, Medium-range ammonia, Hanna Instruments, Romania). Using recorded data for pH, temperature, salinity, and TAN, NH3 was calculated.
Fish and feed analytical methods
The chemical composition of both commercial diet and fish was performed at the start of the experiment using ten fish, and at the end with nine fish per treatment (3 per replicate) to estimate the moisture, crude protein, crude fat, fiber and seabass ash, according to Cunniff, Pand Washington24.
Growth performance and feed utilization indices
The fish were collected, counted, and weighed at the end of the experiment. The following growth performance and feed utilization measurements were determined:
Weight gain (g/fish), WG = Wt − W0; Average daily gain, ADG (g∕fish∕day) = Wt − W0∕days; Specific growth rate (%∕day): SGR = 100 × (lnWt − lnW0)∕days, where: W0: initial fish weight (g); Wt: final fish weight (g); ln: natural logarithm; Survival rate (%) = 100 x (Final number of fish ∕ initial number of fish); Condition factor = 100 × ((BW (g)/L3 (cm)); Condition factor (CF, g/cm3) = final body weight (g) / final body length (cm)3.
Feed conversion ratio (FCR)-based on dry matter (DM) = feed intake (g) as DM ∕ weight gain (g); Protein efficiency ratio PER = weight gain (g) ∕protein intake (g); Protein productive value PPV% = 100 × (protein gain (g) ∕protein intake (g)); Energy gain (EG)Kcal = Et − E0, where: Et: Energy content in the fish carcass (Kcal) at the end, E0: Energy content in the fish carcass (Kcal) at the start; Energy utilization (EU%) = 100 x (Energy gain (kcal) ∕Energy intake (kcal)). Viscerosomatic index (VSI, %) = 100 × (viscera weight (g) / fish body weight (g)); Hepatosomatic index (HSI, %) = 100 × (liver weight (g) / fish body weight (g));
Hematological analysis
Blood sampling
At the end of the experiment, blood samples were drawn from the caudal vertebral vein of an anesthetized fish with MS222 at 100 mg/L of three animals per triplicate and nine fish per treatment.
Blood analysis
An automatic blood cell counter (Exigo-Vet., Boule Medical AB Inc., Stockholm, Sweden) was used to measure the number of red blood cells (RBCs), hemoglobin concentration, packed cell volume (PCV), and total and differential white blood cells (WBCs)25 .
Serum biochemical analysis
Serum triglyceride (TG) level was analyzed using the TG quantification Kit (MAK266, Sigma-Aldrich, St Louis, MO, USA). In this assay, TG is converted to free fatty acids and glycerol26. The total cholesterol (CHO) concentration is determined by free CHO and cholesteryl esters enzyme assays26. Total protein (TP) and albumin (ALB) were determined using commercially available kits (Bio-diagnostics, Giza, Egypt), and the difference between TP and ALB content was reported as globulin (GLO) content27. Methods described by Trinder28 were used to evaluate serum glucose (GLU). Serum total immunoglobulin (IgM) was determined by precipitating Ig with polyethylene glycol and subtracting the initial and final total protein according to Siwicki29. Serum digestive enzymes (amylase and lipase) were evaluated according to Zamani et al. (2009). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed as described by Bergmeyer, et al.30 with 0.2 M DL-aspartic acid and 20 mM L-ketoglutarate as substrate and 0.2 M DL-alanine and 2 mM L ketoglutarate, respectively. Alkaline phosphatase (ALP) was assayed as described by Bessey et al.31. Using a colorimetric method of Heinegrd and Tiderstrom32, the serum creatinine (CRE) concentration was determined. The concentration of uric acid (UA) was determined using the method described by Whitehead et al.33. Cortisol (CORT) activity was assayed according to Foster and Dunn34. The catalase (CAT) enzyme was measured using Aebi's35 method. Using an automatic biochemical analyzer, the activities of total antioxidant capacity (TAC) were assessed (Hitachi 7600D, Hitachi, Tokyo, Japan) according to the method of Koracevic et al.36. Glutathione peroxidase (GPx) levels were detected using the respective techniques of Paglia and Valentine37. Malonaldehyde (MDA) levels were determined using Uchiyama and Mihara's38 methodology.
Histology of liver, and intestine
Liver and intestine (anterior and posterior) samples were obtained using three fish per replicate and nine fish per treatment, from all groups, then fixed in 10% neutral buffered formalin. An automated tissue processor was used to process the formalin-preserved intestinal and hepatic tissues from seabass fish. An automated tissue processor was used to process the formalin-preserved intestinal and hepatic tissues from seabass. The procedure began with a two-step fixation and dehydration. The tissue was fixed by immersion in 10% buffered formalin for 48 h, then removing the fixative in distilled water for 30 min. The tissues were then dehydrated by passing through a graded sequence of alcohol (70%, 90%, and 100%). The tissue was first subjected to 70% alcohol for 120 min, then 90% alcohol for 90 min, and then to two cycles of one hour of absolute alcohol. Following dehydration, the samples were cleared in numerous changes of xylene. It involved immersing the tissue for one hour in a mixture of 50% alcohol and 50% xylene, followed by one and a half hours in pure xylene. After that, the samples were impregnated with molten paraffin wax, imbedded, and blacked out. On a Leica Rotary Microtome (RM 2145, Leica Microsystems, Wetzlar, Germany), paraffin longitudinal Sects. (4–5 um) were cut and mounted on glass slides. According to Feldman and Wolfe (2014), slides are then commonly stained with Hematoxylin and Eosin (H&E). Image J analysis software (National Institutes of Health, MD, USA) was used for histomorphometric analysis, and the length, width of intestinal villi, and Crypt's length, as well as muscle layer thickness, were measured.
Statistical analyses
The results are expressed as mean SEM. The data meet the homogeneity of variance assumption. Data were statistically analyzed with a one-way analysis of variance (ANOVA) using SPSS software (Standard Version 26.0 SPSS Inc. Chicago, Illinois). Duncan's multiple range test was utilized to compare differences among treatment means at the P < 0.05 level.
Ethical declaration
The research experiment was approved by the University of Alexandria, Institutional Animal Care and Use Committee, IACUC approval AU19/22/10/20/3/26, Alexandria University, Egypt. The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations, and that the study is reported in accordance with the ARRIVE guidelines.
Results
Influences of dietary BN on water quality
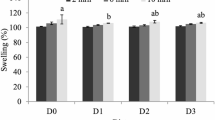
Throughout the period of the experiment, all water quality parameters remained within acceptable ranges (Boyd and Tucker 2012). The average water temperature, dissolved oxygen concentration, nitrite concentration and pH were 25.5°C, 6.1 mg/L, 0.027 mg/L and 7.92, respectively. During the 12-week test period, total TAN and NH3 concentrations changed significantly (P < 0.05) among the six groups, as shown in Fig. 1. The highest concentrations of TAN and NH3 were found in the B0 group, while the lowest levels were found in group B1.5. Additionally, the TNA and NH3 concentrations in all groups treated with BN were significantly lower than in the B0 group.
Growth performance and feed utilization indices of D. labrax
The level of BN in the diet had a significant effect on growth parameters, as determined by a mean ANOVA (Table 2). The growth indices of the fish fed the BN-containing diets were significantly (P < 0.05) superior to those fed a control diet. At the end of the experiment, the fish in group B 1.5 grew significantly more and had enhanced growth characteristics (higher FW, WG, SGR and CF) than those fed the control diet or other diets supplemented with BN. Overall, the addition of BN improved the performance of the fish in all treated groups, with group B1.5 showing the highest significant increase in SGR and FW, followed by groups B1 and B3 with no significant differences between them. The feed utilization parameters of D. labrax fingerlings are presented in (Table 2). FCR varied significantly among the six treatments, with the best value (1.43) recorded for fish in group B1.5 and the worst value (2.03) recorded for fish in group B0. Increasing the amount of BN in the diet to 3% and 4.5% adversely affected the FCR value. The values of the indices PER, PPV, EG, and EU% formed a fluctuating curve, with group B0 being the starting point (lowest value) and B 4.5 being the endpoint (lowest value), while group B1.5 represented the top of the curve.
Whole-body chemical composition
The effects of dietary BN levels on the chemical composition of D. labrax's whole body are described in Table 3. Statistical analysis found that the proportions of protein and dry matter in flesh did not vary significantly between the six treatments. Furthermore, there were no significant variations (P < 0.05) variations in the fat content of fish carcasses, between the BN-treated groups and the control, except for fish from the B3 group. Regarding fish body ash, the addition of BN up to 1% reduced the ash content in the carcass of the fish that recorded the lowest value with fish of the B1 group, while an increase in the ash level occurred with increasing the dietary BN to 1.5, 3, and 4.5%, as these groups did not differ significantly from the control.
Effects on blood hematological and biochemical parameters
The addition of BN to fish diets significantly (P < 0.05) improved the blood biochemical parameters of D. labrax (Table 4). Compared to the control diet, fish-fed BN-supplemented diets demonstrated higher values of blood parameters such as hemoglobin, RBCs and WBCs. Supplementation of the D. labrax diet with BN at various levels resulted in significant changes in the serum lipid profile. Significant decreases in CHO, HDL, and LDL levels were recorded for B0.5 and B1.5 compared to B0. Groups B0 and B0.5 had the lowest TG value. Groups B3 and B4.5 had significantly (P < 0.05) higher values of CHO, TG and HDL, while the B1 group had the highest level of LDL (Table 5).
The addition of BN to the diet had a significant effect (P < 0.05) effect on the indicators of kidney function of D. labrax (Table 6). The levels of urea and UA decreased significantly in the B1 and B1.5 groups compared to the B0 group. Meanwhile, the addition of BN to the seabass diet did not affect CRE levels while the B4.5 group had a significantly higher ammonia value than the B0 group. Serum liver enzymes (ALT, AST, and ALP) showed significant decreases (P < 0.05) in BN-treated fish diets compared to those fed the control diet. The lowest AST values were observed in the B0.5, B1, B1.5, and B3 groups, respectively, while the lowest ALT value was recorded in the B0.5, B1 and B1.5 groups, with no significant differences between them. Except for the B4.5 group, ALP levels decreased significantly in all groups treated with BN compared to the control, with the lowest value in the B0.5 group.
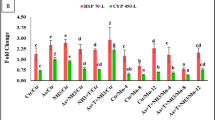
Regarding the immune parameters of D. labrax, the BN-supplemented diets of fish had significantly (P < 0.05) higher levels (P < 0.05) of GLU (group B4.5), TP (groups B1, B1.5 and B3) and GLO (groups B0.5, B1, B1.5 and B3) (Table 7). Furthermore, an improvement in IgM level was observed in all groups supplemented with BN with a significant increase (P < 0.05) for the B.05 and B3 groups compared to the control (Fig. 2). Furthermore, an improvement in digestive enzymes (amylase and lipase) occurred in BN-fortified fish feed diets, as exhibited in Table 8. The highest amylase activity was recorded for fish in the B3 and B4.5 groups, while the highest lipase activity was reported for fish in group B1.5.
Regarding the activities of cortisol and antioxidants (CAT, TAC, MDA, and GPx), Fig. 2 shows that their concentrations were significantly affected by the addition of BN to the diet. Compared to the control group, fish fed BN-fortified diets at different concentrations showed a significant (P < 0.05) decrease in cortisol levels. Furthermore, the addition of BN to the diet made the CAT levels significantly (P < 0.05) higher in all treated groups compared to the control group. The addition of BN did not improve GPx levels, which decreased significantly (P < 0.05) compared to the B0 group. Supplementation of the D. labrax diet with BN improved TAC activity in all treated groups except for the B0.5 group which did not differ significantly from B0. However, with the exception of the B0.5 group, MDA levels increased significantly (P < 0.05) in the BN-treated groups compared to the control.
Influences of dietary BN on the histology of the intestine and liver
Different levels of BN supplementation in the diet of D. labrax had a positive effect and enhanced histological characteristics (Fig. 3). For all groups, microscopic examination of the intestine revealed normal histological characteristics with branched intestinal villi. Compared to the control group, the fish in group B1.5 exhibited more goblet cells, more active pancreatic acini, and relatively lengthy, branching intestinal villi. Furthermore, the fish-fed diet B1.5 had higher villi length values, while the fish-fed diets B3 and B0.5 had the highest villi width values, respectively, with no significant differences between them (Table 9). Furthermore, the B1 diet fed fish exhibited the longest crypts. Diets supplemented with BN at concentrations ranging from 0.5 to 3% improved villi length (B1.5 group), villi width (B3 group) and crypt length (B1 group) relative to the control diet (B0) (Table 9). Normal histological features were observed in the livers of the six experimental groups, with hepatocellular vacuolations in groups B0.5 and B1, interlobular hepatic vascular dilation in group B3, and periportal hepatic vascular dilation in group B4.5 (Fig. 3).
Photomicrograph of the anterior intestine and liver of European sea bass (Dicentrarchus labrax L.) fed raw bentonite at levels of 0, 0.5, 1, 1.5, 3, and 4.5% Per kg feed for 84 days. Where B0 = 0% bentonite, B0.5 = 0.5% bentonite, B1 = 1% bentonite, B1.5 = 1.5% bentonite, B3.0 = 3.0% bentonite, and B4.5 = 4.5% bentonite. H&E, Scale bars 25 um and 10 um. Group B0 demonstrates normal histological characteristics, including villi with branching (green arrow), normal villous length (green arrow), villous width (black arrow), crypt length (yellow arrow), and muscle coat thickness (red arrow) (red arrow). Group B0.5 demonstrates normal histological characteristics, including relatively short, branched intestinal villi (green arrow), normal villous breadth (black arrow), crypt length (yellow arrow), and muscle coat thickness (red arrow) (red arrow). There is an increase in the number of goblet cells (orange arrows), short, thick celli (brown arrows), and hepatocellular vacuolations (light blue arrows). Group B1.0 demonstrates normal histological characteristics, including villi with branching (green arrow), normal villous length (green arrow), villous width (black arrow), crypt length (yellow arrow), and muscle coat thickness (red arrow) (red arrow). There is a normal population of goblet cells (orange arrow), long, thick celli (brown arrow), and hepatocellular vacuolations (light blue arrow). Group B1.5 demonstrates normal histological characteristics, including relatively long, branched intestinal villi (green arrow), normal villous width (black arrow), crypt length (yellow arrow), and muscle coat thickness (red arrow) (red arrow). Increased goblet cells (orange arrow) and more active pancreatic acini are shown. Group B3 demonstrates normal histological features, including villi with branching (green arrow) and increased villous width (black arrow). The length of the crypt (yellow arrow) and thickness of the muscular coat (red arrow) appear normal. There is a modest amount of goblet cells (orange arrow), highly branching big intestine folds (orange arrows), and interlobular hepatic vascular dilatation (red arrow). Group B4.5 demonstrates normal histological characteristics, including relatively short branched intestinal villi (green arrow) and increased villous width (black arrow). The length of the crypt (yellow arrow) and thickness of the muscular coat (red arrow) appear normal. There are a moderate number of immune cells (yellow star) and periportal hepatic vascular dilatation (red arrow).
Discussion
Due to the rapid global expansion of aquaculture to generate large amounts of animal protein and the scarcity of land and water resources, intensification of aquaculture operations is one of the viable options to meet the global demand for seafood (Kurian et al. 2020). The quality of the feed, the aquatic environment, and the culture system used directly affect the growth performance and health of cultured organisms4,39.
The present experiment demonstrates a clear improvement in water quality. The results for TAN and NH3 values in the six groups demonstrated that the addition of BN to the diets decreased TAN and NH3 excretion and that there was a direct correlation between an increase in BN of up to 1.5% in the diet and ammonia depletion. This finding is consistent with the findings of Ayoola12 who discovered that the addition of BN to the diets of African catfish (Clarias gariepinus) decreased the levels of ammonia in the rearing water. Unconsumed feeds contributed to an increase in bad water quality, but the high water stability of clay-supplemented diets maintained good water quality compared to the control diet. On the other hand, several previous studies5,6,7,16,40,41 reported that when BN was added to fish farming water, toxic compounds (TAN and NH3) were reduced and the rate of starch and protein degradation increased due to absorption and ion exchange. The adsorption process is the time during which solution molecules aggregate on the outer and/or interior surface of a porous substance42,43. Both absorption and ion exchange are diffusion processes, and combine for a uniform treatment known as the sorption process, which is the transfer of aqueous molecules into a solid mass41,42.
This decrease in NH3 could be due to the ion exchange process that takes place between BN molecules and organic compounds, during which ammonium is recovered and reused for other purposes, such as a N fertilizer41. Furthermore, BN, which is a silicate sheet clay with high cation exchange and ion adsorption capacity, has a higher selectivity for ammonium removal due to its large surface areas of a net negative charge; as a result, inorganic and organic cations (NH4+, Pb+2, Cu+2, K+, etc.) can be retained in the water2,41,44. The total cation exchange capacity of BN ranges from 40 to 130 meq/100 g45.
Preceding studies have shown that including clay minerals in the diet of fish improves growth, immunity, and disease resistance11,15,46,47. Compared to the control group in the present study, the inclusion of BN in the diet of D. labrax significantly increased growth indices and feed efficiency in the present experiment. The best indices favored the BN groups, as their results displayed a peak in group B 1.5, which had the highest FW, WG and SGR and the best FCR. The beneficial effects of dietary BN are consistent with previous studies on Nile tilapia Oreochromis niloticus17, African catfish, Clarias gariepinus12, rainbow trout, Oncorhynchus mykiss11,15,46,47, and hybrid grouper Epinephelus fuscoguttatus x Epinephelus lanceolatus16. In addition, Kurian et al.48 reported that the combination of Leucas Aspera, Oxy-cyclodextrin and sodium BN improved growth and digestive enzyme, preserved liver tissue, and stimulated the innate immunity of Nile tilapia. As a clay mineral, BN had a significant effect on the growth performance and FCR of D. labrax, indicating that the capacity of dietary BN to improve growth performance is dose dependent.
For marine species, the results herein are consistent with those of Arshad et al.16 who discovered that hybrid grouper fed a 1.5% sodium BN supplemented diet for 8 weeks exhibited significant growth and feeding utilization improvements, recording the highest weight gain and the best FCR (1.54). The scientists attributed these results to the fact that BN improved the usage of nutrients by slowing the transit of predigested feed through the intestines of fish. This resulted in a greater utilization of nutrients, especially protein, which led to greater growth47.
Current findings can be attributed to a variety of variables, (1) BN inclusion improved food intake by modifying conditions in the digestive tract, such as pH, buffer capacity, feed dilution, and osmotic pressure21; (2) BN consumption lowers or eliminates aflatoxin49, and its protective effects against toxicity are associated with toxin absorption50; (3) BN is an effective binding factor and plays a role in the absorption of heavy metal ions and mycotoxins from feeds51; and (4) as a clay mineral, BN stabilizes the intestinal barrier and is capable of absorbing pathogenic microbes, enzymes and toxins, making it a useful treatment for gastrointestinal diseases52. In the current experiment, compared to the B1.5 group, the B3 and B4.5 groups showed a significant decrease in SGR and FCR. High levels of BN inclusion resulted in a high viscosity of the food, decreased digestive enzyme mixing, increased endogenous nutritional losses, and increased the thickness of the whirlpool water stratum close to the mucus, which hindered the digestion process53. Furthermore, large quantities of BN may promote toxic fermentation in the fish intestines.
Blood quality measurements represent biochemical changes that occur in animals, revealing their metabolic and physiological status in general. Therefore, blood biochemistry measures are often used as diagnostic tools in biomonitoring, allowing the detection of pathophysiological changes attributed to nutrition54. The increase in the number of red blood cells (RBCs) in diets containing BN, particularly diet B1.5, is consistent with the findings of the researchers16,55. According to Radu et al.55 a high level of RBCs reflects a healthy physiological state, which is reflected in growth performance. However, in the present study, higher concentrations of BN (above 1.5%) resulted in a reduced content of RBC. This result is consistent with those of Arshad et al.16. According to Ivanc et al.56 a decrease in RBCs may indicate a disturbance in the consumption or quality of the diet in fish. The decrease in WBC of fish fed BN-enriched diets, particularly diet B1.5, is consistent with the findings of previous studies16. White blood cells (WBCs) are a heterogeneous class of nucleated cells that play a crucial role in phagocytosis and immunity, and therefore in defense against infection and foreign substances57. Increases in WBC counts may suggest a disturbance in the normally healthy immune system58. Similarly, Jawahar et al.13 observed that the increasing inclusion of zeolite as a feed additive led to an increase in white blood cell counts in several fish. Previous studies have proven the correlation between increased hemoglobin concentration and dietary BN addition, as this present study demonstrates17.
The decrease in serum CHO values in fish fed diets containing BN in the B.05 and B1.5 groups is a significant indicator of fish health. This is consistent with Karimi et al.23. The CHO and TG values obtained in the current study were remarkably similar to those seen in Montmorillonite-fed rainbow trout, the component that constitutes about 80% of the BN. Increased CHO (B3 and B4.5 groups) and TG (B1, B1.5, B3 and B4.5 groups) may be expressed as particularly elevated BN concentrations led to increased viscosity and thickness of the feed and thickness of the whipped layer close to the mucus, and could also cause baleful leavening in fish guts53,59. On the other hand, the addition of BN to the diet improved HDL levels (groups B3 and B4.5) and decreased LDL values (groups B.5 and B1.5) and their values obtained here were lower than those reported in the previous study on rainbow trout. As important diagnostic indicators, serum liver enzymes (AST, ALT, and ALP) and serum kidney indicators (CRE, UA, and urea) are frequently used to assess fish health and condition60. In the present study, fish fed the BN-supplemented diets had lower levels of urea (B1, B1.5, B3, and B4.5) and UA (B1 and B1.5) than fish fed the control and other treated diets. Furthermore, with the exception of the B4.5 group, an overall improvement in liver function occurred in fish of the BN-treated groups, particularly (B1.5), where lower enzyme levels were recorded. These enhancements can be expounded as BN has a detoxifying task that drives the absorption of venoms and harmful substances. Also, it has lubricity, moldability, low permeability, and high dry bond strength, making it an extraordinary substitute for adsorbents for dismissing toxins and contaminants61. This was consistent with the findings of previous studies that fish in good health condition had low levels of previous enzymes and vice versa4,61.
The importance of digestive enzymes in nutrient absorption and feed utilization is crucial. In line with the findings of Kurian et al.48, the current study showed a significant increase in the concentration of digestive enzymes (lipase and amylase). This improvement may be attributed to the ability of BN to remove harmful gases and toxins from the intestine2,62, which results in improved absorption through the intestinal tract63,64,65, leading to the increased metabolic activity of digestive enzymes.
Innate immunity is the major form of defense in fish. Lysozyme and immunoglobulins are two of the most commonly studied biomarkers of the innate immune response66. Existing research on the effect of BN on the immunological and antioxidant characteristics of D. labrax is extremely limited. Serum TP is a significant clinical indication of the nutritional state, stress level, health, and liver function of fish. Furthermore, serum TP contains nonspecific immunological factors, including immunoglobulins64. The TP values in the current study were almost identical to those obtained in the previous rainbow trout study23. This indicates superior immune system development in the BN-supplemented groups. Furthermore, similar to the present findings, serum TP levels of Channa striatus fed Zeolite-supplemented diets were markedly elevated13. Immunoglobulins or antibodies, which are important for adaptive immune responses, belong to the Ig superfamily67. IgM is the most dominant Ig in fish plasma and has been regarded as the oldest antibody class for a very long time. In this Ig-mediated humoral defense, the complement system is activated, viruses and poisons are neutralized, and pathogens are opsonized for phagocyte destruction68. In this study, diets supplemented with BN considerably increased seabass plasma IgM levels. The results of the previous parameters (TP and IgM) in the current study are very compatible with those of other published studies4,69. Cortisol has been proven to be a reliable stress biomarker and is considered the primary stress hormone70. In the current study, all diets supplemented with BN decreased cortisol levels compared to the control diet. Cortisol levels in fish are affected by feed type71, stocking density72, and water recirculation73. Significantly negative effects of the chronic increase in plasma cortisol on fish appetite, growth performance, condition factor, and feed conversion were reported74.
Fish have both enzymatic and nonenzymatic antioxidant defense systems that protect them from the effects of oxidative stress. Antioxidant defense systems contain antioxidant enzymes that defend against tissue oxidative damage75. In the current study, the activity of oxidative enzymes (TAC, CAT, and GPx) was assessed, with a substantial improvement in CAT and TAC. To assess the process of oxidative stress in an aquatic animal, CAT, TAC, and GPX are important relevant antioxidant factors that are palpably noted to provide defense against oxidative stress and distortions resulting from their interaction with fish exposed to various environmental contaminants, and their activity is evidence of a strong protection system in fish61,75. The increase in CAT is consistent with earlier studies on Nile tilapia76, and seabass larvae75, but the increase in TAC is consistent with other studies on Japanese seabass69. According to Gupta et al.77 fish treated with probiotics had higher levels of catalase and superoxide dismutase (SOD) activity. Higher CAT and TAC activities in BN-fed fish in the present study may indicate a healthy hepatopancreas with little damage. BN may also play an important role in the increased antioxidant capacity of seabass, as found by Abbas et al.61.
Growth, disease resistance, and food utilization are significantly influenced by the healthy development of fish internal organs, especially the gut and liver4,65. Light microscopy in this study showed that the intestine and liver of BN-treated D. labrax had normal and healthy morphology, and the B1.5 group showed the greatest improvement in both organs compared to the control group. The improved intestinal morphology, goblet cell abundance and microvilli length of seabass fed a diet supplemented with BN and normal liver tissue (B1.5) are in line with the outcomes of Abbas et al.61 who found that supplementing the Nile tilapia diet with BN significantly improved liver and kidney functions, as evidenced by a decrease in plasma values of liver enzymes and kidney damage indices. BN defends the liver and intestinal tract of fish by increasing the activity of the microintestinal flora, which aids in the absorption of nutrients, along with many inorganic and organic compounds in the digestive system78, boosting vitamin B and K synthesis and promoting bile acid and xenobiotic metabolism79,80. BN was shown to improve fish liver function12,79, and the hepatorenal function61.
Conclusion
Many countries with lower standards for the quality of fish and/or aquafeed seek efficient and cost-effective feed additives to counteract the presence of various toxins and/or nutrient deficiencies in the feed ingredients they use. From this perspective, the search for natural, inexpensive, effective, and readily available feed additives is a challenge in these countries. In this study, six different levels of BN, which is abundant and inexpensive in Egypt, were evaluated as feed additives in seabass farming using underground saltwater. The results showed that the diet supplemented with 1.5% BN improved juvenile seabass growth performance, feed utilization, blood biochemical analyzes, enzyme activities, antioxidant activity, immune response, and liver and intestinal histology. Therefore, the recommended concentration of BN in the seabass diet formulation is 1.5%.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lotfy , A. M. & et al. Growth, Feed Utilization, Blood Biochemical Variables, Immunity, Histology of the Intestine, Gills and Liver Tissues, and Carcass Composition of the European Seabass (Dicentrarchus labrax) Raised Using Different Water Sources. Egypt. J. Aquat. Biol. Fish. 27, 687–711 (2023).
Abdel-Rahim, M. M. et al. Potential benefits of natural bentonite as a water clarifier on ammonia removal, performance, immunity, antioxidant, and histology of Dicentrarchus labrax. Egypt. J. Aquat. Res. 49, 253–260 (2023).
Elhetawy, A. I., Vasilyeva, L. M., Sudakova, N. & Abdel-Rahim, M. M. Sturgeon aquaculture potentiality in Egypt in view of the global development of aquaculture and fisheries conservation techniques: an overview and outlook. Aquat. Sci. Eng. 38, 222–231 (2023).
El-Kady, A. A., Magouz, F. I., Mahmoud, S. A. & Abdel-Rahim, M. M. The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 546, 737249 (2022).
Sahin, D. et al. Evaluation of natural minerals (zeolite and bentonite) for nitrogen compounds adsorption in different water temperatures suitable for aquaculture. Int. Lett. Natl. Sci. 71 (2018).
Şahin, D. et al. Ammonium removal in aquatic conditions using different levels of calcium bentonite. Gaziosmanpaşa Bilimsel Araştırma Dergisi 7, 61–69 (2018).
Tlupov, T., Bambetova, K., Magomedov, K., Kumykov, R. & Khalko, N. in E3S Web of Conferences. 02021 (EDP Sciences).
Dominy, N. J., Davoust, E. & Minekus, M. Adaptive function of soil consumption: an in vitro study modeling the human stomach and small intestine. J. Exp. Biol. 207, 319–324 (2004).
Ambula, M., Oduho, G. & Tuitoek, J. Effects of high-tannin sorghum and bentonite on the performance of laying hens. Trop. Anim. Health Prod. 35, 285–292 (2003).
Enyidi, U. & Emeaso, B. A. Effects of African bentonite on feed mycotoxigenic fungi and growth of African Catfish Clarias gariepinus. Aquac. Stud. 20, 121–131 (2020).
Eya, J. C., Parsons, A., Haile, I. & Jagidi, P. Effects of dietary zeolites (bentonite and mordenite) on the performance juvenile rainbow trout Onchorhynchus myskis. Austr. J. Basic Appl. Sci. 2, 961–967 (2008).
Ayoola, M. O. Application of dietary bentonite clay as feed addictive on feed quality, water quality and production performance of African catfish (Stellenbosch University, 2016).
Jawahar, S. et al. Dietary supplementation of Zeolite on growth performance, immunological role, and disease resistance in Channa striatus against Aphanomyces invadans. Fish Shellfish Immunol. 51, 161–169 (2016).
Jaynes, W., Zartman, R. & Hudnall, W. Aflatoxin B1 adsorption by clays from water and corn meal. Appl. Clay Sci. 36, 197–205 (2007).
Ellis, R., Clements, M., Tibbetts, A. & Winfree, R. Reduction of the bioavailability of 20 μg/kg aflatoxin in trout feed containing clay. Aquaculture 183, 179–188 (2000).
Arshad, S. E., Jeffrey, F. N. M., Amin, Z. & Shapawi, R. Effects of sodium bentonite clay as a feed additive on the growth and haematology parameters of hybrid grouper, Epinephelus fuscoguttatus x Epinephelus lanceolatus. Songklanakarin J. Sci. Technol. 43 (2021).
Ayyat, M. S., Ayyat, A. M., Naiel, M. A. & Al-Sagheer, A. A. Reversal effects of some safe dietary supplements on lead contaminated diet induced impaired growth and associated parameters in Nile tilapia. Aquaculture 515, 734580 (2020).
Gafrd. (Ministry of Agriculture and Land Reclamation Cairo, Egypt, 2014).
Lotfy, A. M., Elhetawy, A., Habiba, M. M. & Abdel-Rahim, M. M. A comparative study on the effects of seawater and underground saltwater on water quality, growth, feed utilization, fish biomass, digestive system development, and blood health in gilthead seabream, Sparus aurata. AACL Bioflux 14, 1609–1621 (2021).
Hassan, M. & Abdel-Khalek, N. Beneficiation and applications of an Egyptian bentonite. Appl. Clay Sci. 13, 99–115 (1998).
Slamova, R., Trckova, M., Vondruskova, H., Zraly, Z. & Pavlik, I. Clay minerals in animal nutrition. Appl. Clay Sci. 51, 395–398 (2011).
Dardir, F. M., Mohamed, A. S., Abukhadra, M. R., Ahmed, E. A. & Soliman, M. F. Cosmetic and pharmaceutical qualifications of Egyptian bentonite and its suitability as drug carrier for Praziquantel drug. Eur. J. Pharm. Sci. 115, 320–329 (2018).
Karimi, M., Mousavi, S. M., Zolgharnain, H. & Zakeri, M. Dietary montmorillonite as growth promoter and immunomodulator in rainbow trout (Oncorhynchus mykiss). Chemosphere 252, 126459 (2020).
Cunniff, P. & Washington, D. Official methods of analysis of AOAC international. J. AOAC Int. 80, 127A (1997).
Thrall, M. A., Weiser, G., Allison, R. W. & Campbell, T. W. Veterinary Hematology and Clinical Chemistry. (John Wiley & Sons, 2012).
Yarahmadi, P. et al. Protective effects of the prebiotic on the immunological indicators of rainbow trout (Oncorhynchus mykiss) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 54, 589–597 (2016).
Hedayati, S. A., Farsani, H. G., Naserabad, S. S., Hoseinifar, S. H. & Van Doan, H. Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 222, 100–107 (2019).
Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6, 24–27 (1969).
Siwicki, A. i. Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and monocytes, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. Fish Dis. Diagn. Prev. Methods (1993).
Bergmeyer, H. U., Scheibe, P. & Wahlefeld, A. W. Optimization of methods for aspartate aminotransferase and alanine aminotransferase. Clin. Chem. 24, 58–73 (1978).
Bessey, O. A., Lowky, O. & Brock, M. J. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. Biol. Chem. 164, 321–329 (1946).
Heinegård, D. & Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta 43, 305–310 (1973).
Whitehead, T., Bevan, E., Miano, L. & Leonardi, A. Defects in diagnostic kits for determination of urate in serum. Clin. Chem. 37, 879–881 (1991).
Foster, L. B. & Dunn, R. T. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin. Chem. 20, 365–368 (1974).
Aebi, H. in Methods in enzymology Vol. 105 121–126 (Elsevier, 1984).
Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S. & Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 54, 356–361 (2001).
Paglia, D. E. & Valentine, W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169 (1967).
Uchiyama, M. & Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86, 271–278 (1978).
Elhetawy, A. I. et al. Effects of the rearing system of the Russian sturgeon (Acipenser gueldenstaedtii) on growth, maturity, and the quality of produced caviar. Aquac. Aquar. Conserv. Legis. 13, 3798–3809 (2020).
Martin, S. A. & Król, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 75, 86–98 (2017).
Seruga, P. et al. Removal of ammonia from the municipal waste treatment effluents using natural minerals. Molecules 24, 3633 (2019).
Gupta, V. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 90, 2313–2342 (2009).
Kammerer, J., Carle, R. & Kammerer, D. R. Adsorption and ion exchange: Basic principles and their application in food processing. J. Agric. Food Chem. 59, 22–42 (2011).
König, T. N., Shulami, S. & Rytwo, G. Brine wastewater pretreatment using clay minerals and organoclays as flocculants. Appl. Clay Sci. 67, 119–124 (2012).
Wilson, I. (Clay Minerals Society, 2007).
Ucar, A. et al. Effects of dietary bentonite on improvements in hema-tology and enzyme in copper exposed rainbow trout (Oncorhynchus mykiss). J. Appl. Phys 5, 67–72 (2019).
Danabas, D. Effects of zeolite (Clinoptilolite) on some water and growth parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792). (2011).
Kurian, A., Lakshmi, S., Fawole, F. J., Faggio, C. & Elumalai, P. Combined effects of leucas aspera, oxy-cyclodextrin and bentonite on the growth, serum biochemistry, and the expression of immune-related gene in nile tilapia (Oreochromis niloticus). Turk. J. Fish. Aquat. Sci. 21, 147–158 (2021).
Dixon, J., Kannewischer, I., Arvide, M. T. & Velazquez, A. B. Aflatoxin sequestration in animal feeds by quality-labeled smectite clays: An introductory plan. Appl. Clay Sci. 40, 201–208 (2008).
Kreulen, D. Lick use by large herbivores: A review of benefits and banes of soil consumption. Mammal Rev. 15, 107–123 (1985).
Magnoli, A. P. et al. Commercial bentonites as detoxifier of broiler feed contaminated with aflatoxin. Appl. Clay Sci. 40, 63–71 (2008).
González, R. et al. Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br. J. Pharmacol. 141, 951–960 (2004).
Rosas, C. et al. Effect of type of binder on growth, digestibility, and energetic balance of Octopus maya. Aquaculture 275, 291–297 (2008).
Lu, J., Zhang, M. & Lu, L. Tissue metabolism, hematotoxicity, and hepatotoxicity of trichlorfon in Carassius auratus gibelio after a single oral administration. Front. Physiol. 9, 551 (2018).
Radu, D., Oprea, L., Bucur, C., Costache, M. & Oprea, D. Characteristics of haematological parameters for carp culture and Koi (Cyprinus carpio Linneaus, 1758) reared in an intensive system. Bull. UASVM Anim. Sci. Biotechnol. 66, 336–342 (2009).
Ivanc, A., Hasković, E., Jeremić, S. & Dekić, R. Hematological evaluation of welfare and health of fish. Praxis veterinaria 53, 191–202 (2005).
Hassaan, M. S. et al. The effect of dietary sericite on growth performance, digestive enzymes activity, gut microbiota and haematological parameters of Nile tilapia, Oreochromis niloticus (L.) fingerlings. Anim. Feed Sci. Technol. 262, 114400 (2020).
Delves, P. J., Martin, S. J., Burton, D. R. & Roitt, I. M. Roitt's Essential Immunology. (John Wiley & Sons, 2017).
Dias, J., Huelvan, C., Dinis, M. T. & Métailler, R. Influence of dietary bulk agents (silica, cellulose and a natural zeolite) on protein digestibility, growth, feed intake and feed transit time in European seabass (Dicentrarchus labrax) juveniles. Aquat. Liv. Resour. 11, 219–226 (1998).
Coz-Rakovac, R. et al. Blood chemistry and histological properties of wild and cultured sea bass (Dicentrarchus labrax) in the North Adriatic Sea. Vet. Res. Commun. 29, 677–687 (2005).
Abbas, E. A., Mowafy, R. E., Khalil, A. A. & Sdeek, F. A. The potential role of the dietary addition of bentonite clay powder in mitigating diazinon-induced hepatorenal damage, oxidative stress, and pathological alterations in Nile tilapia. Aquaculture 533, 736182 (2021).
Boonanuntanasarn, S., Khaomek, P., Pitaksong, T. & Hua, Y. The effects of the supplementation of activated charcoal on the growth, health status and fillet composition-odor of Nile tilapia (Oreochromis niloticus) before harvesting. Aquac. Int. 22, 1417–1436 (2014).
Abdel-Rahim, M. M. et al. Effect of long-term dietary supplementation with lavender, Lavandula angustifolia, oil on European seabass growth performance, innate immunity, antioxidant status, and organ histomorphometry. Aquac. Int. 1–19 (2023).
Elhetawy, A. I. et al. Dietary wood and activated charcoal improved ammonium removal, heavy metals detoxification, growth performance, blood biochemistry, carcass traits, and histopathology of European Seabass. Aquac. Nutr. 2023 (2023).
Elhetawy, A. I. et al. Interactive impacts of rosemary oil and amylase-lipase enzymes on Liza ramada performance, ammonia excretion, digestion, serum biochemistry and intestinal histomorphology. Egypt. J. Aquat. Res. 50, 154–161 (2024).
Ellis, A. Innate host defense mechanisms of fish against viruses and bacteria. Dev. Comp. Immunol. 25, 827–839 (2001).
Uribe, C., Folch, H., Enríquez, R. & Moran, G. Innate and adaptive immunity in teleost fish: A review. Veterinarni medicina 56, 486–503 (2011).
Mashoof, S. & Criscitiello, M. Fish Immunoglobulins. Biology (Basel) 215(4), 45 (2016).
Wang, C. Y. et al. Effects of Chinese herbal medicines mixture on growth performance digestive enzyme activity immune response of juvenile Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 24, 683–693 (2018).
Sadoul, B. & Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 94, 540–555 (2019).
Sadoul, B. et al. Adaptive capacities from survival to stress responses of two isogenic lines of rainbow trout fed a plant-based diet. Sci. Rep. 6, 35957 (2016).
McKenzie, D. J. et al. Effects of stocking density and sustained aerobic exercise on growth, energetics and welfare of rainbow trout. Aquaculture 338, 216–222 (2012).
Colson, V. et al. Welfare assessment of rainbow trout reared in a recirculating aquaculture system: Comparison with a flow-through system. Aquaculture 436, 151–159 (2015).
Gregory, T. R. & Wood, C. M. The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol. Biochem. Zool. 72, 286–295 (1999).
Shahin, S. A. et al. Silymarin, supplemented weaning diet boosted survival, growth, antioxidant status, and fatty acids profile of Seabass. Ann. Anim. Sci. 23, 253–264 (2023).
Elkaradawy, A., Abdel-Rahim, M. M. & Mohamed, R. A. Quillaja saponaria and/or linseed oil improved growth performance, water quality, welfare profile and immune-oxidative status of Nile tilapia, Oreochromis niloticus fingerlings. Aquac. Res. 53, 576–589 (2022).
Gupta, A., Gupta, P. & Dhawan, A. Paenibacillus polymyxa as a water additive improved immune response of Cyprinus carpio and disease resistance against Aeromonas hydrophila. Aquac. Rep. 4, 86–92 (2016).
Schell, T., Lindemann, M., Kornegay, E. & Blodgett, D. Effects of feeding aflatoxin-contaminated diets with and without clay to weanling and growing pigs on performance, liver function, and mineral metabolism. J. Anim. Sci. 71, 1209–1218 (1993).
Phuong, N. M. et al. Novel removal of diazinon pesticide by adsorption and photocatalytic degradation of visible light-driven Fe-TiO 2/Bent-Fe photocatalyst. J. Chem. 2019 (2019).
Clarke, G. et al. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 28, 1221–1238 (2014).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A. A. E: Methodology, Conception, Supervision, Manuscript revision. A. I. G. E: Methodology, manuscript drafting, Conceptualization and reviewing. W. M. R: Methodology, laboratory work, samples analysis. S. Y. E: Conception, Investigation, Software. E. H. E: Resources, Methodology, Validation. A. M. L: methodology, samples analysis, software, investigation. M. M. A: The experimental idea, Formal analysis, Data Curation, Visualization, Manuscript revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Dahhar, A.A., Elhetawy, A.I.G., Refaey, W.M.A. et al. Bentonite-supplemented diets improved fish performance ammonia excretion haemato-biochemical analyses immunity antioxidants and histological characteristics of European seabass Dicentrarchus labrax. Sci Rep 14, 13868 (2024). https://doi.org/10.1038/s41598-024-63936-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-63936-6
Keywords
This article is cited by
-
Dietary rosemary oil with/without zymogen forte improves water quality, growth hormones, immune-physiological response, stress resilience, and health status of Chelon ramada grown in groundwater
BMC Veterinary Research (2025)
-
Long term dietary Moringa oleifera leaf extract to Florida red tilapia Oreochromis sp improves performance immunity maturation and reproduction in saltwater
Scientific Reports (2025)
-
The effect of aqueous application of probiotics on growth, heavy metal accumulation, blood biochemistry, and histological alterations of Dicentrarcus labrax
Aquaculture International (2025)
-
Integrated aquaculture of whiteleg shrimp Litopenaeus vannamei and European seabass Dicentrarchus labrax: impacts on performance, welfare, blood physiological response, carcass traits, productivity, and farm profitability
Aquaculture International (2025)