Abstract
Intergroup aggression often results in the production of public goods, such as a safe and stable social environment and a home range containing the resources required to survive and reproduce. We investigate temporal variation in intergroup aggression in a growing population of colobus monkeys (Colobus vellerosus) to ask a novel question: “Who stepped-up to produce these public goods when doing so became more difficult?”. Both whole-group encounters and male incursions occurred more frequently as the population grew. Males and females were both more likely to participate in whole-group encounters when monopolizable food resources were available, indicating both sexes engaged in food defence. However, only females increasingly did so as the population grew, suggesting that it was females who increasingly produced the public good of home range defence as intergroup competition intensified. Females were also more active in male incursions at high population densities, suggesting they increasingly produced the public good of a safe and stable social environment. This is not to say that males were chronic free-riders when it came to maintaining public goods. Males consistently participated in the majority of intergroup interactions throughout the study period, indicating they may have lacked the capacity to invest more time and effort.
Similar content being viewed by others
Introduction
Humans have modified the environments we inhabit to an unprecedented extent. We have drastically altered landscapes1, hunted some species to extinction and enabled others to proliferate2 and driven climate change on a global scale3. Consequently, animal populations have experienced dramatic changes in their risk of mortality, and the quality of the habitat they live in. For social species, the resulting changes in population density, food availability, and group composition impact the intensity of intergroup competition experienced4,5. However, we know very little about how group-living individuals behaviourally adapt to such changes in their competitive landscape.
During each encounter with a neighbouring group, every group member must weigh the costs versus benefits of participation6,7 when deciding whether to engage in intergroup aggression. Participants pay an opportunity cost to fight8 and can risk wounds, injuries, or even death9,10,11,12,13. However, there are numerous potential benefits that could incentivize individuals to take this risk, such as maintaining or improving access to important resources like space, mates, food, water, or shelter [e.g.,6,7,14,15]. Individuals may also participate for other reasons like defending themselves or their offspring16,17,18, because they have been forcefully recruited into the fight19, or to obtain a return benefit in the future. For example, they may support group members in the fight with the expectation of a reciprocal investment at a later time20, or use intergroup aggression as a costly and honest signal of their genetic quality to attract more mates21. Lastly, intergroup aggression may function to discourage or prevent extra-group individuals from trying to immigrate into the group22,23. Irrespective of the benefit(s) that motivate participation, intergroup aggression often produces public goods (also referred to as social goods or collective benefits) as a by-product24,25.
Public goods are goods that are non-excludable, meaning that all group members can utilize them, regardless of whether they contributed to their production26. We posit that there are two public goods that intergroup aggression commonly produces: access to a home range containing the resources required to survive and reproduce, and a safe and stable social environment. In territorial species, intergroup aggression can function to delineate territory boundaries13,27. However, even in non-territorial species, the group that wins typically displaces the group that loses28 and groups that consistently win intergroup conflicts can annex space from their neighbours29,30. Consequently, participation in intergroup conflicts can impact the quantity and quality of the resources a group has access to within their home range or territory. Participation in intergroup conflicts can also prevent the forceful immigration of extra-group members31,32,33. When participants fail to repel unwanted immigrants, resident group members may be overthrown and/or evicted22, infanticidal attacks may occur, and group members may emigrate to avoid the period of violence and instability that can follow34,35,36,37,38. Given the social upheaval that can result from such a takeover, intergroup aggression that repels potential immigrants can provide a safe and stable social environment for group members.
Public goods are vulnerable to collective action problems because individuals that pay the costs of producing the public good, obtain lower net benefits than free-riding group members26. These payoff differences disincentivize participation, which can lead to a situation where no one produces the public good and the whole group suffers26. The ubiquity of intergroup conflicts across social species suggests that most social animals overcome collective action problems, at least to some extent. However, a high proportion of free riders during intergroup conflicts can negatively influence a group’s chance of winning24,25,39.
In game theory, the Volunteer’s Dilemma models situations where players can either make a small sacrifice that will benefit everyone in the group or wait and hope to benefit from someone else’s costly action. When the public good(s) produced from intergroup aggression are viewed within the framework of the Volunteer’s Dilemma, the production curve is modelled as a step function40,41. The implication of this is that a threshold number of ‘volunteers’ are required to produce the public good. For example, a group may need to mobilize more active participants than the opposing group to win the fight17,42. In the Volunteer’s Dilemma, the payoffs are such that it is less costly to volunteer to produce the public good than it is to live without it40,41. For example, it is better to fight to maintain a home range than it is to live without consistent access to the resources found within it. When viewing participation in intergroup conflicts as a Volunteer’s Dilemma, the most relevant question to understanding public goods production is ‘Which group members volunteer, and in what conditions do they do so?’. Work to date has highlighted that it is often privileged individuals that have priority-of-access to the public good that are most likely to produce it42,43. For example, individuals who are high-ranking may obtain a disproportionate share of the resources within their home range, and so have the most to gain from volunteering. Quantitative studies support this supposition, as high-ranking individuals have consistently been found to participate in intergroup conflicts more frequently than low-ranking group members [e.g.,7,17,42,44,45,46,47,48,49].
Although we often talk about public goods ‘production’, it is notable that both of the public goods that arise from intergroup aggression need to be ‘maintained’ over time. Social groups must repeatedly engage in intergroup conflicts to maintain home range boundaries, and repeatedly repel intruders to maintain a safe and stable social environment. If changing social (e.g., population density) or ecological conditions (e.g., resource availability) alter the intensity of intergroup competition4,5, more volunteers may be needed to win a fight and/or volunteers may need to engage in intergroup aggression more frequently to maintain these public goods. However, we are aware of no studies to date that have investigated temporal variation in volunteering to ask, “Who volunteers when the public good becomes harder to maintain?”. In this study, we examine participation in intergroup conflicts in a growing population of (ursine or white-thighed) black-and-white colobus monkeys (Colobus vellerosus) to address this knowledge gap.
We examine contributions to two different public goods (food availability via home range defence and a safe and stable social environment) by investigating participation in two types of intergroup conflicts. Whole-group conflicts occur when two groups are in close proximity and one or more individuals from at least one group exhibits intergroup aggression. Because entire groups are involved, the outcome of these encounters likely determines who wins access to the resources currently contested but could also impact home-range boundaries over the long-term. Thus, participation in whole-group conflicts likely produces the public good of a successfully defended home range and access to the resources within it. Notably, home range defence need not be the motivation for participation. For example, a male could fight in one intergroup conflict to defend access to mates, another to protect their offspring and another to gain access to a valuable food patch. If this intergroup aggression causes the opposing group to flee the area, the male will have contributed to home range defence. Incursions are when one individual, or a coalition of same-sexed individuals, approach another group without the bulk of their social group. Although incursions can be neutral/affiliative (i.e., prospecting)31,33,49,50,51,52, they can also be highly aggressive, with one or more individuals attempting to forcefully immigrate into a group31,32,33,34,53. In this colobus population, male incursions can result in take-overs, in which the resident alpha male is evicted, infanticidal attacks occur, and female group members emigrate to avoid the period of instability that can follow35,36. Thus, participation during male incursions likely produces the public good of a safe and stable social environment.
We hypothesized that the intensity of intergroup competition would increase as the population grew (i.e., increased in density), and that both whole-group conflicts and male incursions would increase in frequency over time as a result. We investigated how males and females each modified their propensity to participate in intergroup conflicts (i.e., volunteer) when it became more difficult to maintain public goods. Because female participation in intergroup conflicts often functions as food defence7,45,49,53,54,55,56, we expected that females would be most likely to participate in whole-group conflicts when high-quality, monopolizable food resources (young leaves, fruits, seeds and flowers) were available within their home range, and that they would increasingly participate in food defence as the population size increased. Although male participation in intergroup conflicts is thought to primarily function as mate defence15,57,58,59,60,61, studies on other species of black-and-white colobus have also found evidence for male food defence62,63. Therefore, we also expected males to participate when monopolizable food resources were at stake, and to increasingly do so as the population grew. We further expected males to be the primary participants during male incursions, and to increasingly participate in these as the population grew as this would presumably intensify competition for mates. From these predicted patterns of participation, we hypothesized that male volunteers would contribute to the production of both public goods (i.e., home range defence and a safe and stable social environment), whereas females would primarily volunteer to produce the public good of home range defence.
Methods
Study population
The study population of black-and-white colobus monkeys inhabits a 1.92 km2 semi-deciduous dry forest by the villages of Boabeng and Fiema in central Ghana (70 43′ N and 10 42′ W; ESM Fig. 1). The colobus monkeys at this site were traditionally protected by religious taboos. After a period during which the taboos eroded and only a few dozen monkeys remained, concerned villagers led by D.E.K. Akowuah approached Ghana Wildlife Division for governmental protection. They also started an ecotourism project—the Boabeng-Fiema Monkey Sanctuary—that became one of Ghana’s top tourist destinations64,65. As a result, the colobus population at Boabeng-Fiema has steadily grown over the past 40 years, increasing from a few dozen individuals to about 400 by 202365,66,67,68,69. We used data collected between 2000 and 2009, during which time the population increased from approximately 200–300 individuals (ESM Fig. 2). Because these population estimates have been made in the same geographic area, an increase in population size is synonymous with increasing population density.
Data collection
We observed wild monkeys in field but did not capture or manipulate them in any way. We utilized data collected by four different researchers, working on eight different groups, in three separate study periods (2 study groups from Sep. 2000-Nov. 2001, 4 study groups from Jul. 2004-Aug. 2005 and 8 study groups from May 2008- Jun. 2009). To ensure consistency in behavioural data collection, each researcher overlapped with the next for at least one month in the field. Researchers collected data on group composition, the movements of the study groups, their diet, the distribution of important food trees and their monthly phenology, temperature and rainfall. Researchers also recorded the location of the centre-of-mass of the group every 30 min, and these locations were used to estimate annual home ranges for each study group, each study period (ESM Fig. 1). Researchers also collected aggressive participation ad libitum during two types of intergroup encounters: whole-group encounters, and incursions by one or more males. Two groups were deemed to be having a whole-group encounter when located within 50 m of each other61,70, and in conflict when aggressive behaviours, which included threat displays, chasing, and contact aggression61,70, were observed. Incursions occurred when one individual, or a small number of same-sexed individuals, left their group (if they were from a bi-sexual group) and approached another group by themselves (i.e., their group was > 50 m away). The majority of incursions observed were by one or more males. Female incursions were observed but were at too low of a frequency to be analysed here. All data collection protocols were approved by the Boabeng-Fiema Monkey Sanctuary’s management committee, the Ghana Wildlife Division, and the University of Calgary's Life and Environmental Sciences Animal Care Committee (Protocols BI2000-01, BI2003-02B, BI 2006–28, BI 2009–25) and adhered to the laws of Ghana.
Data analyses
Because not all group members were individually recognized in the early study years, data on aggressive participation during intergroup encounters was summarized at the level of the sexes. These data ware also aggregated on a monthly basis to enable us to calculate encounter rates. We built two generalized linear mixed models (GLMMs) to investigate the factors that influenced the frequency of whole-group encounters, and the frequency of male incursions. The response variable in each model was the number of each type of encounter that each group experienced in a given month. We set the error structure to “Poisson”, used a “log” link function, and also included the observation hours for that group, that month, as an offset to control for differences in sampling effort. Study group was included as a random effect to account for the repeated sampling of groups over time71. We then built four separate GLMMs to examine the factors that drove both male and female participation during whole-group encounters and male incursions. The response variable in each male participation model was the number of encounters that at least one male group member participated aggressively in that month. Similarly, the response variable in each female participation model was the number of encounters that at least one female group member participated aggressively in that month. We set the error structure to “binomial” and used a “logit” link function. We also included the number of encounters each group experienced that month as a weight in each GLMM using a cbind() parameterization, and included study group as a random effect71. We did not include random slopes as our dataset did not support the more complex model structure72,73,74. Predictor variables in all models included estimated population size, monthly rainfall, an index of food availability within the group’s home range, and variables quantifying the numbers of adult males and females in the group. Because the data were summarized by month, we were unable to include predictor variables specific to individual intergroup encounters, such as relative group size, or whether the location was in the core versus periphery of the home range.
We included population size as a predictor variable in each of our GLMMs because the main goals of this study were to (1) determine whether the intensity of intergroup competition increased with population size, and (2) examine how males and females each responded to this. Although the Boabeng-Fiema colobus population have been censused frequently since 1990, censuses were not conducted in every year that researchers were collecting data on intergroup encounters. Therefore, we used the censuses that were completed to create a linear model between year and population size, and then used the estimated population size from this model in our analyses (ESM Fig. 2). Population size was also included as an interaction term with single-male vs. multi-male groups in our GLMM assessing factors affecting rates of male incursions.
The study site experiences a marked dry season (typically November to March) and two peaks in rainfall (May to July and September to October) (ESM Fig. 3a). This seasonal rainfall pattern leads to a peak of young leaves, seeds, fruits, and flowers in the dry season (ESM Fig. 3b)75. The plant part availability is reflected in the colobus diet consisting mostly of mature leaves in the rainy season and more high-quality food items in the dry season75. These high-quality food items, which are also those that tend to be more patchily distributed76, and thus, are expected to increase the intensity of intergroup contest competition during the dry season77,78. Contrastingly, there is also evidence that groups may increase day ranges during the rainy season, which could lead to a higher number of intergroup encounters79. To control for these potential seasonal effects on the frequency of intergroup encounters and/or the participation of individuals in these encounters, we include the long-term average rainfall per calendar month (ESM Fig. 3a) as a predictor variable in all our models. We used long-term average rainfall per calendar month rather than the observed amount of rainfall for each month because rainfall data was not collected throughout the entire study period, but rather in 2001 and from 2003 to 2006.
Although the typical amount of rainfall each month should explain some of the variation in food availability (i.e., more young leaves, flowers, fruits and seeds available in the dry season), we also observed considerable variation in food availability among years, and among the home ranges of each of the study groups. Therefore, we also included a more precise estimate of current food availability for each study group as a predictor variable in our analyses. More specifically, we created an aggregated food-availability index (FAI)80 that approximated the availability of monopolizable foods in the home range of each group, during each study period. This was accomplished by estimating each group’s home range boundaries, and then using data on the distribution of tree species important in their diet and their monthly phenology to calculate the monthly FAI for flower buds, flowers, unripe fruits, ripe fruits, unripe seedpods, ripe seedpods and young leaves (see Electronic Supplementary Materials for additional detail). When examining male and female aggression in whole group encounters, we included food availability by itself in our GLMMs and also as an interaction term with the number of adult females in the group.
We included the number of adult females in each group (range = 2–11) as predictor variables in all our GLMMs, but changed the way we quantified male group composition. We used the number of adult males in our GLMMs examining rates of whole group encounters because here we were primarily interested in how the number of males and/or females, and therefore the level of intragroup competition, influenced the intensity of intergroup contest competition. Conversely, in our GLMMs examining participation and rates of male incursions we used single-male versus multi-male as a predictor variable because previous work has shown differences in intergroup conflict outcome between single-male and multi-male groups79, suggesting participation may also differ between these two types of groups. Six of the eight study groups were single male groups for at least part of the study period, and we observed groups to be single male across 40% of all group-months in the study period.
All predictor variables were z-score transformed prior to analyses81 and we used pair-wise correlation coefficients and variance inflation factors [‘car’ package, version 3.0–7, 82] to ensure there was no multicollinearity among predictors (i.e., all VIF were < 3.0 and correlation coefficients < 0.8)71,81. We built all GLMM models using the ‘lme4’ package [version 1.1–21, 83] in R84. The ‘DHARMa’ package85 was used to ensure that each GLMM did not suffer from over- or under-dispersion. We used the “confint.merMod” function in the ‘lme4’ package83 to derive profile confidence intervals for each model parameter, and used these confidence intervals as well as likelihood ratio tests (comparing the full model to the model with each term removed) to assess the significance of each predictor variable and interaction term. We based our inferences on full models plus important interaction effects rather than using a stepwise procedure86,87, and we did not interpret main effects if that predictor also featured in a significant interaction term. We also used a likelihood ratio test, in which we compared the full model to the null model (model including only the intercept, random effects, weight and offset (where applicable)) to assess the overall significance of each GLMM71,88. Lastly, the “r.squaredGLMM” function from the ‘MuMIn’ package89 was used to obtain delta R2GLMM(C) values for each model, as an estimate of model fit90.
Results
We observed 538 intergroup encounters (N = 409 whole-group encounters; N = 129 male incursions) during our 7582 observation hours and we were able to record participation data during 326 whole-group encounters and 127 male incursions. Males were equally as likely to participate aggressively in whole-group encounters (75% of N = 326) and male incursions (80% of N = 127) (Chi-squared test: N = 453, χ2 = 0.97, p = 0.325). Conversely, females were more likely to participate aggressively during whole-group encounters (35% of N = 376) than they were during male incursions (17% of N = 127) (Chi-squared test: N = 453, χ2 = 13.55, p < 0.001).
Rates of intergroup encounters
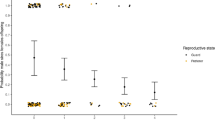
The rate of whole-group encounters was strongly influenced by population size as these occurred at significantly higher frequencies over time (Fig. 1; Table 1). Groups that contained fewer adult females were also significantly more likely to experience whole-group encounters more frequently (Table 1). The effect of population size on the rate of male incursions was more complicated; multi-male groups were more likely to experience male incursions as population size increased, while single-male groups were less likely (Fig. 2; Table 2), likely due to the fact that multi-male groups are less competitive than single-male groups in this species due to lower male quality and collective action problems79,91 Male incursions were also significantly less likely to occur when monopolizable food resources were abundant (Table 2), and a non-significant trend indicates there was a weak tendency for male incursions to occur more frequently in groups that contained few adult females (Table 2).
Predicted male incursion rate as a function of the interaction between population size and whether the group contained a single versus multiple adult males. Expected proportions were obtained by plotting the predictions of the GLMMs (Table 2), setting all additional predictors to their mean values (or median values for categorical variables). Measures of population size have been z-score transformed.
Aggressive participation during whole-group encounters
Females were significantly more likely to participate aggressively in whole-group encounters at higher population sizes/densities (Table 1). Conversely, males showed the opposite pattern of behaviour as population size had a significant negative effect on male participation during whole-group encounters (Table 1). Females were also more likely to participate aggressively during whole-group encounters that occurred during the dry season (Table 1), which is the time of year that patchily distributed, monopolizable food resources were abundant (ESM Fig. 3). We also found that the interaction term between current food availability (i.e., the availability of young leaves, fruits, seeds and flowers) within the group’s home range and the number of adult females in the group to be statistically significant (Table 1). Plotting this interaction effect indicates that groups with many adult females, where intragroup feeding competition should be most intense, were more likely to participate aggressively when these contestable food resources were at stake (Fig. 3a). Conversely, in groups with few adult females, females were most active in the absence of food resources that are likely to induce contest competition (Fig. 3a). The interaction between current food availability and female group size was also a significant predictor of male participation during whole-group encounters (Table 1). Males were more likely to fight in whole-group encounters when patchily distributed, monopolizable food resources were abundant, but this propensity was stronger in groups that contained many adult females (Fig. 3b).
Predicted proportion of intergroup encounters in which (A) female and (B) male group members are expected to participate aggressively as a function of the interaction between the number of adult females in the group and the availability of young leaves, flowers, fruits and seeds within their home range. Expected proportions were obtained by plotting the predictions of the GLMMs (Table 1), setting all additional predictors to their mean values (or median values for categorical variables), and the number of adult females in the group to + 1 SD above the mean (i.e., above average number of adult females in group) versus -1 SD below the mean (i.e., below average number of adult females). Measures of food availability have been z-score transformed.
Aggressive participation during male incursions
Males were most likely to participate aggressively during male incursions if their group contained many adult females (Table 2). Males were also more active during male incursions that took place when monopolizable food resources were abundant within their home range, and showed a weak tendency to participate in male incursions during the dry season when monopolizable food resources were available (Table 2). Notably, population size had no significant impact on male aggression during male incursions (Table 2). Conversely, females were significantly more likely to participate in repelling male intruders when living at a higher population density, as well as when they resided in a single-male group (Table 2).
Discussion
Between the years 2000 and 2009, the population of colobus monkeys living at Boabeng-Fiema grew by ~ 150%, resulting in a significant increase in population density. We found that whole-group encounters became more frequent over time, as did the rate of male incursions, at least in multi-male groups. Thus, our findings indicate that as population density increased, so too did the intensity of intergroup contest competition. Patterns of participation in whole-group encounters suggest that the public good of a successfully defended home range may arise largely through male and female food defence. Both sexes were more likely to participate in whole-group encounters when monopolizable food resources were available within their home range and more likely to be depleted through scramble competition (i.e., when the number of females resident in the group was large). However, females were significantly more likely to engage in food defence as the population grew, whereas males were significantly less likely. Thus, it was females who increasingly volunteered when this public good became harder to maintain. Females were also the sex that increasingly invested in maintaining a safe and stable social environment, as their propensity to participate during male incursions correlated positively with population size. Conversely, male participation in male incursions was not related to population size, indicating that they consistently participated in ~ 80% of male incursions. Thus, males may have hit their capacity to invest time and energy into public goods production, and so the increasing costs of maintaining these public goods in a changing competitive landscape largely fell to females. Indeed, in the first four years of our data set (2001–2005), females participated in 13.7% of between-group encounters and 9.3% of male incursions, while in the last four years (2006–2009), these numbers jumped to 50.8% and 35.7%, respectively.
Aggressive participation in whole-group encounters
Our model of female participation in whole-group encounters showed that female colobus monkeys were most active in whole-group encounters during the dry season, which is the time of year that high-quality, clumped food resources (i.e., young leaves, fruits, seeds and flowers) tended to be available. This was particularly true for females living in groups with many adult females. Because female group size tends to correlate with total group size92,93, groups with many adult females are likely to experience the highest levels of intragroup feeding competition94, giving them the greatest incentive to fight for access to food resources. Female food defence has been found in the majority of studies that have examined female participation in intergroup conflicts7,53,54,55,56, while the other resource that females have been seen to defend is space7,49. In this study, we found that females residing in groups with few adult females were most likely to participate in whole-group encounters when there were no monopolizable food resources at stake. This pattern could arise if females residing in smaller groups were primarily fighting to defend access to space. We observed that newly formed groups tended to be small compared to established groups. Given that the study site appears to be saturated with groups of colobus monkeys (ESM Fig. 1), these small new groups likely had to compete intensely with existing groups to establish and maintain a home range.
As seen in females, we also found evidence for food defence in males. Males were more likely to participate in both whole-group encounters and male incursions when monopolizable foods were available in their home range, and when their group contained many adult females (i.e., when intragroup scramble competition was likely to be high)94. Food defence by males in this species has previously been found61,79 and is also seen in a closely related species of black-and-white colobus (C. guereza)62,63. That males show evidence for food defence during male incursions suggests that in some cases, incursions by a lone male or coalition of males may function in home range defence rather than male prospecting for mating and dispersal opportunities33, even though the bulk of the group was > 50 m away.
Aggressive participation in male incursions
While male participation during male incursions was not impacted by population size, females were significantly more likely to contribute to repelling male intruders over time. Females presumably did so to mitigate the growing risk of infanticide associated with male takeovers35. Notably, in volunteering to maintain a safe and secure social environment, females also provided a mate-defence service to male group members as a by-product. Males able to maintain exclusive access to a group of females may have been more likely to obtain this service, as females showed a weak tendency to participate in male incursions when living in single-male groups. Females may have been more likely to assist in repelling extra-group males when residing in a single-male group, and facing a coalition of males, as the resident male would be at a numeric disadvantage in such cases. Indeed, females are less likely to co-participate in intergroup aggression with other females if their group contains a higher number of males95. Evidence for such a strategy has been found in black howler monkeys (Alouatta pigra), where male takeovers also carry the risk of infanticide. Here, playback studies have shown that females are most likely to participate in intergroup encounters when the males in their group are outnumbered96.
Conclusions
Many populations of social animals have likely experienced drastic changes in the intensity of intergroup competition, either because they live in an area that has suffered habitat loss or degradation, and/or their population has increased. However, only a couple of studies have examined how such changes impact competitive dynamics between social groups5,29, and none have examined how patterns of volunteering versus free-riding change in response to this evolving competitive landscape. Here, we use data collected on a growing population of black-and-white colobus monkeys to ask a novel question: when intergroup competition intensifies, such that it becomes more difficult to maintain home range boundaries and prevent immigration, who steps up and volunteers to produce these public goods? We found that it was females who increasingly fought for the resources that are limiting to their own fitness (i.e., food and space), and they also increased the time and effort invested in preventing male takeovers. This is not to say that males were free riders who exploited the efforts of their female group members. Males participated in the majority of intergroup encounters experienced, while females only participated in 35% of whole-group encounters and 17% of male incursions. Thus, while females were the sex who increasingly invested in home range defence and the maintenance of a safe and stable social environment when it became more difficult to maintain these public goods, this may have been because they were the sex with the greatest capacity to do so.
Data availability
The raw data supporting the conclusions of this article are available as Electronic Supplementary Material.
References
Scanes, C. G. 2017. Human activity and habitat loss: Destruction, fragmentation, and degradation. In Animals and Human Society (eds Scanes, C. G. & Toukhsati, S. R.) 451–482 (Academic Press, 2017). https://doi.org/10.1016/B978-0-12-805247-1.00026-5.
Ceballos, G. et al. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 1(5), e1400253. https://doi.org/10.1126/SCIADV.1400253 (2015).
Stocker, T. F. et al. Climate change 2013: The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change (Cambridge University Press, 2013).
Cheney, D. L. & Seyfarth, R. M. The influence of intergroup competition on the survival and reproduction of female vervet monkeys. Behav. Ecol. Sociobiol. 21, 375–386. https://doi.org/10.1007/BF00299932 (1987).
Thompson, F. J., Marshall, H. H., Vitikainen, E. I. K. & Cant, M. A. Causes and consequences of intergroup conflict in cooperative banded mongooses. Anim. Behav. 126, 31–40. https://doi.org/10.1016/j.anbehav.2017.01.017 (2017).
Kitchen, D. M. & Beehner, J. C. Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144, 1551–1581. https://doi.org/10.2307/4536533 (2007).
Arseneau-Robar, T. J. M., Taucher, A. L., Schnider, A. B., van Schaik, C. P. & Willems, E. P. Intra- and interindividual differences in the costs and benefits of intergroup aggression in female vervet monkeys. Anim. Behav. 123, 129–137. https://doi.org/10.1016/j.anbehav.2016.10.034 (2017).
Mares, R., Young, A. J. & Clutton-Brock, T. H. Individual contributions to territory defence in a cooperative breeder: Weighing up the benefits and costs. Proc. R. Soc. B. 279, 3989–3995. https://doi.org/10.1098/rspb.2012.1071 (2012).
Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior (Harvard University Press, 1986).
Mech, L. D. Buffer zones of territories of gray wolves as regions of intraspecific strife. J. Mammal. 75, 199–202. https://doi.org/10.2307/1382251 (1994).
Mills, M. G. L. Behavioural mechanisms in territory and group maintenance of the brown hyaena, Hyaena brunnea, in the southern Kalahari. Anim. Behav. 31, 503–510. https://doi.org/10.1016/S0003-3472(83)80072-4 (1983).
Hölldobler, B. & Lumsden, C. J. Territorial Strategies in Ants. Science 1979(210), 732–739. https://doi.org/10.1126/science.210.4471.732 (1980).
Mosser, A. & Packer, C. Group territoriality and the benefits of sociality in the African lion. Panthera leo. Anim. Behav. 78, 359–370. https://doi.org/10.1016/j.anbehav.2009.04.024 (2009).
Koch, F., Signer, J., Kappeler, P. M. & Fichtel, C. Intergroup encounters in Verreaux’s sifakas (Propithecus verreauxi): who fights and why?. Behav. Ecol. Sociobiol. 70, 797–808. https://doi.org/10.1007/s00265-016-2105-3 (2016).
Mirville, M. O. et al. Factors influencing individual participation during intergroup interactions in mountain gorillas. Anim. Behav. 144, 75–86. https://doi.org/10.1016/j.anbehav.2018.08.003 (2018).
Arseneau, T. J. M., Taucher, A., van Schaik, C. P. & Willems, E. P. Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim. Behav. 110, 39–50. https://doi.org/10.1016/j.anbehav.2015.09.006 (2015).
Kitchen, D. M. Alpha male black howler monkey responses to loud calls: effect of numeric odds, male companion behaviour and reproductive investment. Anim. Behav. 67, 125–139. https://doi.org/10.1016/j.anbehav.2003.03.007 (2004).
Wich, S. A., Assink, P. R. & Sterck, E. H. M. Thomas langurs (Presbytis thomasi) discriminate between calls of young solitary versus older group-living males: A factor in avoiding infanticide?. Behaviour 141, 41–51. https://doi.org/10.2307/4536111 (2004).
Arseneau-Robar, T. J. M. et al. Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. R. Soc. B. https://doi.org/10.1098/rspb.2016.1817 (2016).
Trivers, R. L. The evolution of reciprocal altruism. Quart. Rev. Biol. 46, 35–57 (1971).
Zahavi, A. Mate selection—A selection for a handicap. J. Theor. Biol. 53, 205–214. https://doi.org/10.1016/0022-5193(75)90111-3 (1975).
Teichroeb, J. A. & Jack, K. M. Alpha male replacements in nonhuman primates: Variability in processes, outcomes, and terminology. Am. J. Primatol. 79, e22674. https://doi.org/10.1002/ajp.22674 (2017).
Scarry, C. J. & Tujague, M. P. Consequences of lethal intragroup aggression and alpha male replacement on intergroup relations and home range use in tufted capuchin monkeys (Cebus apella nigritus). Am. J. Primatol. 74, 804–810. https://doi.org/10.1002/ajp.22030 (2012).
Nunn, C. L. & Lewis, R. J. Cooperation and collective action in animal behaviour. In Economics in Nature: Social dilemmas, mate choice and biological markets (eds Van Hooff, J. A. R. A. M. & Hammerstein, P.) 42–66 (Cambridge University Press, 2001).
Nunn, C. L. Collective benefits, free-riders, and male extra-group conflict. In Primate males: Causes and consequences of variation in group composition (ed. Kappeler, P. M.) 192–204 (Cambridge University Press, 2000).
Olson, M. The Logic of Collective Action: Public Goods and the Theory of Groups (Harvard University Press, 1965).
Lemoine, S. et al. Group dominance increases territory size and reduces neighbour pressure in wild chimpanzees. R. Soc. Open. Sci. https://doi.org/10.1098/RSOS.200577 (2020).
Crofoot, M. C. The cost of defeat: capuchin groups travel further, faster and later after losing conflicts with neighbors. Am. J. Phys. Anthropol. 152, 79–85. https://doi.org/10.1002/ajpa.22330 (2013).
Isbell, L. A., Cheney, D. L. & Seyfarth, R. M. Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya. Behav. Ecol. Sociobiol. 27, 351–358. https://doi.org/10.1007/BF00164006 (1990).
Mitani, J. C., Watts, D. P. & Amsler, S. J. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508. https://doi.org/10.1016/j.cub.2010.04.021 (2010).
Doolan, S. P. & Macdonald, D. W. Dispersal and extra-territorial prospecting by slender-tailed meerkats (Suricata suricata) in the south-western Kalahari. J. Zool. 240, 59–73. https://doi.org/10.1111/j.1469-7998.1996.tb05486.x (1996).
Steenbeek, R. Tenure related changes in wild Thomas’s langurs I: Between-group interactions. Behaviour 136, 595–625. https://doi.org/10.1163/156853999501487 (1999).
Teichroeb, J. A., Wikberg, E. W. & Sicotte, P. Dispersal in male ursine colobus monkeys (Colobus vellerosus): Influence of age, rank and contact with other groups on dispersal decisions. Behaviour 148, 765–793. https://doi.org/10.1163/000579511X577157 (2011).
Kowalewski, M. M. & Garber, P. A. Solving the collective action problem during intergroup encounters: The case of black and gold howler monkeys (Alouatta caraya). In Howler Monkeys Behavior, Ecology, and Conservation (eds Kowalewski, M. M. et al.) 165–189 (Springer, 2015). https://doi.org/10.1007/978-1-4939-1960-4_7.
Teichroeb, J. A. & Sicotte, P. Infanticide in ursine colobus monkeys (Colobus vellerosus) in Ghana: New cases and a test of the existing hypotheses. Behaviour 145, 727–755. https://doi.org/10.1163/156853908783929160 (2008).
Sicotte, P. et al. The influence of male takeovers on female dispersal in Colobus vellerosus. Am. J. Primatol. 79, e22436. https://doi.org/10.1002/ajp.22436 (2017).
Sterck, E. H. M. Determinants of female dispersal in Thomas langurs. Am. J. Primatol. 42, 179–198. https://doi.org/10.1002/(SICI)1098-2345 (1997).
Amann, A. L., Pines, M. & Swedell, L. Contexts and consequences of takeovers in hamadryas baboons: Female parity, reproductive state, and observational evidence of pregnancy loss. Am. J. Primatol. https://doi.org/10.1002/AJP.22649 (2017).
Bornstein, G. The free-rider problem in intergroup conflicts over step-level and continuous public goods. J. Personality Soc. Psych. 62, 597–606. https://doi.org/10.1037/0022-3514.62.4.597 (1992).
Diekmann, A. Volunteer’s dilemma. J. Conflict Resolut. 29, 605–610 (1985).
Archetti, M. Cooperation as a volunteer’s dilemma and the strategy of conflict in public goods games. J. Evol. Biol. 22, 2192–2200. https://doi.org/10.1111/j.1420-9101.2009.01835.x (2009).
Willems, E. P., Arseneau, T. J. M., Schleuning, X. & van Schaik, C. P. Communal range defence in primates as a public goods dilemma. Phil. Trans. R. Soc. Lond. B. https://doi.org/10.1098/rstb.2015.0003 (2015).
Gavrilets, S. & Fortunato, L. A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 1–11. https://doi.org/10.1038/ncomms4526 (2014).
Cooper, M. A., Aureli, F. & Singh, M. Between-group encounters among bonnet macaques (Macaca radiata). Behav. Ecol. Sociobiol. 56, 217–227. https://doi.org/10.1007/s00265-004-0779-4 (2004).
Nunn, C. L. & Deaner, R. O. Patterns of participation and free riding in territorial conflicts among ringtailed lemurs (Lemur catta). Behav. Ecol. Sociobiol. 57, 50–61. https://doi.org/10.1007/s00265-004-0830-5 (2004).
Majolo, B., Ventura, R. & Koyama, N. F. Sex, rank and age differences in the Japanese macaque (Macaca fuscata yakui) participation in inter-group encounters. Ethology 111, 455–468. https://doi.org/10.1111/j.1439-0310.2005.01087.x (2005).
Willems, E. P., Hellriegel, B. & van Schaik, C. P. The collective action problem in primate territory economics. Proc. R. Soc. B. 280, 1–7. https://doi.org/10.1098/rspb.2013.0081 (2013).
Lazaro-Perea, C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21. https://doi.org/10.1016/anbe.2000.1726 (2001).
Boydston, E. E., Morelli, T. L. & Holekamp, K. E. Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369–385. https://doi.org/10.1046/j.1439-0310.2001.00672.x (2001).
Arseneau-Robar, T. J. M. et al. Male monkeys use punishment and coercion to de-escalate costly intergroup fights. Proc. R. Soc. B. https://doi.org/10.1098/rspb.2017.2323 (2018).
Pisor, A. C. & Surbeck, M. The evolution of intergroup tolerance in nonhuman primates and humans. Evol. Anthropol. 28, 210–223. https://doi.org/10.1002/evan.21793 (2019).
Yao, H. et al. Male dispersal in a provisioned multilevel group of Rhinopithecus roxellana in Shennongjia Nature Reserve. China. Am. J. Primatol. 73, 1280–1288. https://doi.org/10.1002/AJP.21000 (2011).
Harris, T. R. Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav. Ecol. Sociobiol. 61, 317–329. https://doi.org/10.1007/s00265-006-0261-6 (2006).
Kinnaird, M. F. Variable resource defense by the Tana River crested mangabey. Behav. Ecol. Sociobiol. 31, 115–122. https://doi.org/10.1007/bf00166344 (1992).
Payne, H., Lawes, M. & Henzi, S. P. Competition and the exchange of grooming among female samango monkeys (Cercopithecus mitis erythrarchus). Behaviour 140, 453–471. https://doi.org/10.1163/156853903322127931 (2003).
Zhao, Q. & Tan, C. L. Inter-unit contests within a provisioned troop of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains. China. Am. J. Primatol. 73, 262–269. https://doi.org/10.1002/ajp.20892 (2010).
Sicotte, P. Inter-group encounters and female transfer in mountain gorillas: Influence of group composition on male behavior. Am. J. Primatol. 30, 21–36. https://doi.org/10.1002/ajp.1350300103 (1993).
Sugiura, H. et al. Variation in intergroup encounters in two populations of Japanese macaques. Int. J. Primatol. 21, 519–535. https://doi.org/10.1023/a:1005448120967 (2000).
Cant, M. A., Otali, E. & Mwanguhya, F. Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555. https://doi.org/10.1046/j.1439-0310.2002.00795.x (2002).
Yi, Y., Fichtel, C., Ham, S., Jang, H. & Choe, J. C. Fighting for what it’s worth: participation and outcome of inter-group encounters in a pair-living primate, the Javan gibbon (Hylobates moloch). Behav. Ecol. Sociobiol. 74, 96–111. https://doi.org/10.1007/s00265-020-02879-0 (2020).
Sicotte, P. & Macintosh, A. J. Inter-group encounters and male incursions in Colobus vellerosus in central Ghana. Behaviour 141, 533–553. https://doi.org/10.1163/1568539041166717 (2004).
Fashing, P. J. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): Evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 50, 219–230. https://doi.org/10.1007/s002650100358 (2001).
Harris, T. R. Multiple resource values and fighting ability measures influence intergroup conflict in guerezas (Colobus guereza). Anim. Behav. 79, 89–98. https://doi.org/10.1016/j.anbehav.2009.10.007 (2010).
Fargey, P. J. Boabeng-Fiema monkey sanctuary—An example of traditional conservation in Ghana. Oryx 26, 151–156. https://doi.org/10.1017/S0030605300023589 (1992).
Saj, T. L., Teichroeb, J. A. & Sicotte, P. The population status of the ursine colobus (Colobus vellerosus) at Boabeng-Fiema, Ghana. In Commensalism and conflict: The human-primate interface (eds Paterson, J. D. & Wallace, J.) 350–375 (American Society of Primatologists, 2005).
Kankam, B. O., Saj, T. L. & Sicotte, P. How to measure “success” in community-based conservation projects: The case of the Boabeng-Fiema Monkey Sanctuary in Ghana. In The Public Sphere and Politics of Survival in Ghana (eds Puplampu, K. P. & Tettey, W. J.) 115–141 (Woeli Publishers, 2010).
Wong, S. N. P. & Sicotte, P. Population size and density of Colobus vellerosus at the Boabeng-Fiema Monkey Sanctuary and surrounding forest fragments in Ghana. Am. J. Primatol. 68, 465–476. https://doi.org/10.1002/ajp.20242 (2006).
Kankam, B. O., Antwi-Bosiako, P., Addae-Wireko, L. & Dankwah, C. Growing population of the critically endangered white-thighed colobus monkey (Colobus vellerosus) from forest fragments in Ghana. J. Trop. Ecol. 39, 1–6. https://doi.org/10.1017/S0266467423000214 (2023).
Wikberg, E. C. et al. Predictors of population estimates in a critically endangered species (Colobus vellerosus). Primate Conserv. 38, 2023–08 (2024).
Oates, J. F. The social life of a black-and-white colobus monkey. Colobus guereza. Z. Tierpsychol. 45, 1–60. https://doi.org/10.1111/j.1439-0310.1977.tb01007.x (1977).
Zuur, A. F., Leno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed Effects Models and Extensions in Ecology with R (Springer, 2009).
Bates, D., Kliegel, R., Sasishth, S., & Baayen, H. Parsimonious mixed models. https://arxiv.org/abs/1506.04967. (2015).
Harrison, X. A. et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, e4794. https://doi.org/10.7717/PEERJ.4794/FIG-3 (2018).
Matuschek, H., Kliegl, R., Vasishth, S., Baayen, H. & Bates, D. Balancing Type I error and power in linear mixed models. J. Mem. Lang. 94, 305–315. https://doi.org/10.1016/J.JML.2017.01.001 (2017).
Saj, T. L. & Sicotte, P. Predicting the competitive regime of female Colobus vellerosus from the distribution of food resources. Int. J. Primatol. 28, 315–336. https://doi.org/10.1007/s10764-007-9124-x (2007).
Johnson, D. D. P., Kays, R., Blackwell, P. G. & Macdonald, D. W. Does the resource dispersion hypothesis explain group living?. Trends Ecol. Evol. 17, 563–570. https://doi.org/10.1016/S0169-5347(02)02619-8 (2002).
Isbell, L. A. & Young, T. Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139, 177–202. https://doi.org/10.1163/156853902760102645 (2002).
Koenig, A. Competition for resources and its behavioral consequences among female primates. Int. J. Primatol. 23, 759–783 (2002).
Teichroeb, J. A. & Sicotte, P. Cascading competition: the seasonal strength of scramble influences between-group contest in a folivorous primate. Behav. Ecol. Sociobiol. 72, 6. https://doi.org/10.1007/s00265-017-2418-x (2018).
Dasilva, G. L. Diet of Colobus polykomos on Tiwai Island: Selection of food in relation to its seasonal abundance and nutritional quality. Int. J. Primatol. 15, 655–680. https://doi.org/10.1007/BF02737426 (1994).
Field, A., Miles, J. & Field, Z. Discovering Statistics using R (SAGE Publications, 2012).
Fox, R. & Weisberg, S. An R Companion to Applied Regression (Sage Publications, 2019).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. https://doi.org/10.18637/jss.v067.i01 (2015).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. See https://www.r-project.org/. (2017).
Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. https://cran.r-project.org/web/packages/DHARMa/index.html (2021).
Forstmeier, W. & Schielzeth, H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 65, 47–55. https://doi.org/10.1007/s00265-010-1038-5 (2011).
Mundry, R. & Nunn, C. L. Stepwise model fitting and statistical inference: Turning noise into signal pollution. Am. Nat. 173, 119–123. https://doi.org/10.1086/593303 (2009).
Bolker, B. M. et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. https://doi.org/10.1016/j.tree.2008.10.008 (2009).
Bartoń, K. Mu-MIn: Multi-model inference. http://R-Forge.R-project.org/projects/mumin/ (2009).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x (2013).
Teichroeb, J. A., Wikberg, E. C., Bădescu, I., MacDonald, L. J. & Sicotte, P. Infanticide risk and male quality influence optimal group composition for Colobus vellerosus. Behav. Ecol. 23, 1348–1359. https://doi.org/10.1093/beheco/ars128 (2012).
Lindenfors, P., Fröberg, L. & Nunn, C. L. Females drive primate social evolution. Biol. Lett. 271, S101–S103. https://doi.org/10.1098/rsbl.2003.0114 (2004).
Mitani, J. C., Gros-Louis, J. & Manson, J. H. Number of males in primate groups: Comparative tests of competing hypotheses. Am. J. Primatol. 38, 315–332. https://doi.org/10.1002/(SICI)1098-2345 (1996).
Teichroeb, J. A. & Sicotte, P. Test of the ecological-constraints model on ursine colobus monkeys (Colobus vellerosus) in Ghana. Am. J. Primatol. 71, 49–59. https://doi.org/10.1002/ajp.20617 (2008).
Wikberg, E. C., Gonzalez, S., Rodriguez, C. & Sicotte, P. Joint intergroup aggression in female colobus monkeys (Colobus vellerosus) is associated with grooming bonds, male participation, and group size. Am. J. Primatol. 84, e23355. https://doi.org/10.1002/ajp.23355 (2022).
van Belle, S. Female participation in collective group defense in black howler monkeys (Alouatta pigra). Am. J. Primatol. 77, 595–604. https://doi.org/10.1002/ajp.22380 (2015).
Acknowledgements
We thank the BFMS management committee, Ghana Wildlife Division, and the University of Calgary's Life and Environmental Sciences Animal Care Committee for the permission to conduct this study; Robert Koranteng, Peprah Samuel, Teresa Holmes, and Fernando Campos for assistance collecting and/or organizing the ranging data; and Alberta Innovates Technology Futures (grant number 200600343), American Society of Primatologists, Animal Behavior Society, International Primatological Society, Leakey Foundation, Natural Sciences and Engineering Research Council of Canada (grant number RGPIN‐203059‐2006), Sweden-America Foundation, Research Services at the University of Calgary, and Wenner-Gren Foundation (grant number 8172) for funding.
Author information
Authors and Affiliations
Contributions
E.C.W., P.S. and T.J.M.A.-R. designed the study; E.C.W. and P.S. funded the study. J.A.T., A.J.J.M., T.L.S. and E.C.W. collected the data; T.J.M.A.-R., E.C.W., E.G. and S.G.L. analyzed the data, T.J.M.A.-R., E.C.W., E.G. and S.G.L. wrote the manuscript; all authors edited the manuscript; T.J.M.A.-R. and J.A.T. revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arseneau-Robar, T.J., Teichroeb, J.A., Macintosh, A.J.J. et al. When population growth intensifies intergroup competition, female colobus monkeys free-ride less. Sci Rep 14, 14363 (2024). https://doi.org/10.1038/s41598-024-64188-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-64188-0