Abstract
Aflatoxins (AFs) are hazardous carcinogens and mutagens produced by some molds, particularly Aspergillus spp. Therefore, the purpose of this study was to isolate and identify endophytic bacteria, extract and characterize their bioactive metabolites, and evaluate their antifungal, antiaflatoxigenic, and cytotoxic efficacy against brine shrimp (Artemia salina) and hepatocellular carcinoma (HepG2). Among the 36 bacterial strains isolated, ten bacterial isolates showed high antifungal activity, and thus were identified using biochemical parameters and MALDI-TOF MS. Bioactive metabolites were extracted from two bacterial isolates, and studied for their antifungal activity. The bioactive metabolites (No. 4, and 5) extracted from Bacillus cereus DSM 31T DSM, exhibited strong antifungal capabilities, and generated volatile organic compounds (VOCs) and polyphenols. The major VOCs were butanoic acid, 2-methyl, and 9,12-Octadecadienoic acid (Z,Z) in extracts No. 4, and 5 respectively. Cinnamic acid and 3,4-dihydroxybenzoic acid were the most abundant phenolic acids in extracts No. 4, and 5 respectively. These bioactive metabolites had antifungal efficiency against A. flavus and caused morphological alterations in fungal conidiophores and conidiospores. Data also indicated that both extracts No. 4, and 5 reduced AFB1 production by 99.98%. On assessing the toxicity of bioactive metabolites on A. salina the IC50 recorded 275 and 300 µg/mL, for extracts No. 4, and 5 respectively. Meanwhile, the effect of these extracts on HepG2 revealed that the IC50 of extract No. 5 recorded 79.4 µg/mL, whereas No. 4 showed no cytotoxic activity. It could be concluded that bioactive metabolites derived from Bacillus species showed antifungal and anti-aflatoxigenic activities, indicating their potential use in food safety.
Similar content being viewed by others
Introduction
Fungi can damage agricultural commodities during harvest, transport, and storage, resulting in decreased production and value1,2. Filamentous fungi are the most harmful microorganisms to agricultural products' quality and safety3.
Mycotoxins produced by filamentous fungi have major economic impacts on crops such as cereals, almonds, tea, pistachios, and cotton seeds4. Cereals and their products are an essential source of nutrients for human consumption in developing and developed countries and are considered one of the world's most critical food and feedstuffs5. Most cereals are susceptible to various fungal invasions during pre- and post-harvest and storage, resulting in massive yield loss, reduced seed quality, nutritional values, and germination6,7.
Many filamentous fungi, viz., Alternaria, Aspergillus, Fusarium, and Penicillium species, are primarily attached to cereal grains, making them unsuitable for human consumption by impeding their nutrient content followed by the secreting number of toxic secondary metabolites called mycotoxins8. As stated by the Food and Agricultural Organization of the United Nations (FAO), approximately 25% of the world's food grains are contaminated with mycotoxins at levels more remarkable than the prescribed level9.
Aflatoxins (AFs) represent the most widespread and common mycotoxins in food and feed, and they are primarily produced by species belonging to the Aspergillus section Flavi: A. flavus, A. parasiticus, and A. nomius10,11, whereas these fungi are considered a predominant contaminant in food grains, mainly maize12. Aflatoxin B1 (AFB1) is the most destructive of the known AFs, and it has been classified as a Group I human carcinogen by the International Agency for Research on Cancer13.
For many decades, synthetic antifungal agents or preservatives have been used to control fungal pathogens and mycotoxin contamination in foodstuffs, but some commonly used synthetic chemicals have proved to be hazardous to consumers and the environment1,14. To ensure consumer safety, additional control strategies that are effective, non-hazardous, and environmentally friendly are required. As a result, working to develop (micro) biological detoxifying techniques to enhance the safety of these foods for direct utilization is vital. Consequently, the biological control of toxigenic fungi and mycotoxins is regarded as one of the most contemporary means. Various microorganisms have been identified to eliminate or break down mycotoxins in food and feed. These include Bacillus spp.15,16, Brevibacterium spp.17, Eubacterium spp.18, Flavobacterium aurantiacum19, Rhodococcus erythropolis20, Saccharomyces cerevisiae21, lactic acid bacteria22, and others23. According to Schallmey et al.24, Bacillus spp. were extensively evaluated as biological agents, possibly because they proliferated, produced a wide range of antimicrobial compounds, and were generally regarded as safe (GRAS). The antimicrobial compounds produced by Bacillus spp. include lipopeptides, protease antibiotics, and bacteriocin25. Bacillus spp. are also known to produce bioactive secondary metabolites including polypeptides, macro-lactones, fatty acids, polyketides, and isocoumarins, and they have a broad range of biological capabilities, including antibacterial, anticancer, and anti-algal properties26.
Bioactive secondary metabolites are useful natural chemicals generated by numerous microorganisms, many of which have strong bioactive characteristics in the biological control of aflatoxin-producing fungi27. They are expelled after primary growth and throughout the stationary phase, and are among the most commercially significant industrial products and are of great interest. Bioactive metabolites, now dubbed specialized metabolites, frequently have odd structures and have shown substantial implications on the health, nutrition, and economy of our society28. Approximately 53% of the FDA-approved natural products-based medicines originated from microorganisms29.
In two previous studies, bioactive metabolites were extracted from lactic acid bacteria30, and Saccharomyces cerevisiae31. Therefore, the goal of this study was to isolate and identify endophytic bacteria, extract and characterize bioactive secondary metabolites, and assess their antifungal, antiaflatoxigenic, and cytotoxic activity against A. salina and hepatocellular carcinoma.
Results
Antifungal activity of cell-free supernatant (CFS) of bacterial isolates
Thirty-six bacterial isolates were isolated from rice grains. The CFS of ten bacterial isolates showed variable degrees of antifungal activity (Table 1). Results revealed that CFS of the bacteria No. RQ1 and RQ16 showed antifungal activity against A. flavus with a zone inhibition of 12.0, and 13.0 mm respectively. Data also showed that CFS of the bacteria No. RQ1 and RQ16 inhibited A. parasiticus with a zone inhibition of 20.0 mm, for both bacterial isolates. Meanwhile, the CFS of bacteria No. RQ2 showed antifungal activity against Fusarium spp., A. flavus, and A. parasiticus with a zone inhibition of 20, 11, and 17.0 mm respectively.
Data showed that CFS of bacteria No. RQ5 and RQ14 exhibited lower antifungal activity and inhibited one fungal species. On the other hand, the CFS of bacteria No. RQ7 and RQ15 inhibited three fungal species only. The CFS of bacteria No. RQ13 inhibited A. parasiticus, A. niger, A. ochraceus, and Fusarium spp. by 21.0, 15.0, 22.5, and 23.5 mm, respectively. Meanwhile, CFS of bacteria No. RQ22 inhibited A. flavus, A. parasiticus, A. niger, and Fusarium spp. by 20.0, 27.0, 20.0, and 29.0 mm, respectively. It was clear that the CFS of the bacteria No. RQ13 and RQ22 exhibited higher antifungal activity than other bacterial isolates. Therefore, ten bacterial isolates No. RQ1, RQ2, RQ5, RQ7, RQ8, RQ13, RQ14, RQ15, RQ16, and RQ22 showing antifungal activity were selected for their identification using biochemical analyses and MALDI TOF.
Identification of bacterial isolates
The isolated bacteria No. RQ1, RQ2, RQ5, RQ7, RQ8, RQ13, RQ14, RQ15, RQ16, and RQ22 were identified following biochemical analyses (Table 2). Cell morphology examination and biochemical analysis revealed that all bacteria were rod-shaped (bacilli), gram-positive, and oxidase-negative. Data in Table 2 showed the MALDI-TOF MS result scores of the bacterial isolates showing antifungal activity, whereas the bacterial isolates No. RQ1, RQ5, RQ8, RQ13, and RQ16 were identified as Bacillus cereus DSM 31T DSM, with NCBI identifier 1396. The bacterial isolates No. RQ2, RQ7, and RQ15 were identified as Bacillus cereus with NCBI identifier 1396. On the other hand, the bacterial isolates RQ14 and RQ22 were identified as Bacillus thuringiensis DSM 2046T DSM with NCBI identifier 1428. The bacterial isolates No. RQ13 and RQ22 showing high antifungal activity were selected for the extraction of bioactive metabolites. A flow chart explaining the selection of bacterial isolates is shown in Fig. 1. Bioactive secondary metabolites were extracted from Bacillus thuringiensis (No. RQ22) (extracts No. 1, 2, and 3) and from Bacillus cereus (No. RQ13) (extracts No. 4, 5, and 6). A flow chart explaining the selection of extracts is shown in Fig. 2.
Antifungal activity of bioactive metabolites
The six extracts of the two bacterial isolates, Bacillus cereus (No. RQ13) and Bacillus thuringiensis (No. RQ22) were used to investigate the antifungal activity against different fungal species (Table 3). Results showed that extract No. 4 exhibited the highest antifungal activity against A. flavus, A. parasiticus, A. niger, A. ochraceus, F. oxysporum, Fusarium spp., and Penicillium spp., recording a zone inhibition of 11.00, 12.33, 11.67, 20.00, 16.00, 11.67, and 19.00 mm, respectively. On the other hand, extract No. 5 exhibited antifungal activity against A. flavus, A. parasiticus, A. niger, A. ochraceus, and Penicillium spp. with a zone inhibition of 21.00, 9.33, 16.67, 15.67, and 19.00 mm, respectively. Data also revealed that extract No. 4 inhibited F. oxysporum, whereas other extracts could not inhibit F. oxysporum. Thus, extracts No. 4 and 5 were selected for further analysis, as they showed the highest antifungal activity.
Data in Table 3 clearly showed that extracts No. 2 and 5 of bacteria grown on rice medium showed higher antifungal activity against A. flavus, A. niger, and Penicillium spp. On the other hand, extracts No. 1 and 4 of bacteria grown on tryptic soy broth showed antifungal activity against A. flavus and A. ochraceus. Results showed that extracts No. 3 and 6 of bacteria grown on rice yeast extract medium showed low antifungal activity.
Volatile organic compounds (VOCs) in bioactive metabolites
Twelve VOCs, representing 99.96%, were identified in extract No. 4 (Table 4, Figure S1), whereas the major components were butanoic acid, 2-methyl (37.64%) (Figure S2), iso-valeric acid (21.62%) (Figure S3), and propanoic acid, 2-methyl (19.13%) (Figure S4). Seven VOCs, representing 100%, were identified in extract No. 5 (Table 5, Figure S5), whereas the major components were 9, 12-Octadecadienoic acid (Z,Z)—(81.29%) (Figure S6). Hexadecanoic acid (7.75%) and 3, 6-Octadecadiynoic acid methyl ester (4.86%) were detected at a lower percentage. It could be observed that extract No. 5 contained high amounts of 9, 12-Octadecadienoic acid (Z,Z) -.
Polyphenols in bioactive metabolites
The ability of the two extracts No. 4 and 5 to produce polyphenols was investigated. Nine phenolic acids were detected (Table 6, Figure S7) in extract No. 4, whereas cinnamic acid and syringic acid were the most abundant phenolic acids at concentrations of 19.57 and 15.18 µg/g, respectively. Extract No. 4 reflected a distinguished content of flavonoids, with the detection of three compounds (Table 6). Daidzein and naringenin were detected at 24.10 and 14.38 µg/g, respectively. On the other hand, rutin was detected in relatively low concentrations recording 0.01 µg/g.
Nine phenolic acids have been detected (Table 6, Figure S8) in extract No. 5, whereas 3, 4-dihydroxybenzoic acid was the most abundant phenolic acid at a concentration of 560.22 µg/g. Cinnamic acid and coumaric acid were also detected at high concentrations recording 57.33 and 50.92 µg/g, respectively. A distinguished content of flavonoids was also detected in extract No. 5, with three compounds (Table 6), whereas naringenin was detected at a concentration of 9.22 µg/g. On the other hand, daidzein and rutin were detected at lower concentrations recording 1.60 and 0.02 µg/g, respectively.
Scanning electron microscope (SEM)
The effect of extracts No. 4 and 5 on A. flavus structure was determined using a SEM. Figure 3A shows typical conidia and conidiospores of untreated A. flavus. The effect of extract No. 4 on A. flavus spores followed with less deformation but also caused the loss of some fungal spores (Fig. 3B). On the other hand, extract No. 5 had a similar effect on the conidiophores and conidiospores of A. flavus (Fig. 3C).
Scanning electron microscope of (A) untreated A. flavus (control) showing typical conidia and conidiospores; (B) A. flavus after treatment with extract No. 4 (extracted from Bacillus cereus grown on tryptic soy broth) showing loss and mutation of fungal conidiospores; and (C) A. flavus after treatment with extract No. 5 (extracted from Bacillus cereus grown on rice medium) showing loss and mutation of fungal conidiospores.
Antifungal and antiaflatoxigenic activity of bioactive metabolites
The effect of different concentrations of extracts No. 4 and 5 on the fungal growth of A. flavus after 10 days of incubation was studied (Table 7). Results revealed that extracts No. 4 and 5 at 9 mg/mL concentrations reduced fungal growth by 57.32% and 35.98%, respectively. On the other hand, data indicated that different concentrations of extract No. 4 showed variable degrees of antifungal activity, whereas extract No. 5 at concentrations 1 and 3 mg/mL stimulated fungal growth. Inhibition of fungal growth occurred by 21.70%, 30.66%, and 35.98% at concentrations 5, 7, and 9 mg/mL, respectively. It was also noticed that the inhibition of fungal growth increased by increasing the concentration of extracts.
The effect of different concentrations of extracts No. 4 and 5 on aflatoxin production was investigated (Table 7). The data indicated that extract No. 4 at a 1 mg/mL concentration, reduced AFB1, AFB2, and AFG2 by 99.53%, 99.68%, and 100%, respectively. At concentrations of 7 and 9 mg/mL AFB2 production was completely prevented. On the other hand, AFG1 production was completely prevented at concentrations 1, 3, 5, 7, and 9 mg/mL. Results also revealed that at a concentration of 9 mg/mL, an increase in AFG2 production was observed.
In studying the effect of extract No. 5 on aflatoxin production, results indicated that AFB1 was reduced by 83.41% at a concentration of 1 mg/mL, and AFB1 production was continuously reduced by increasing the concentration of the extract. For AFB2 production, the extract at a concentration of 1 mg/mL reduced AFB2 by 90.73%, whereas at concentrations 7 and 9 mg/mL, AFB2 was not detected. Data also showed that the extract, at a concentration of 1 mg/mL, reduced AFG1 by 83.90%, and the reduction increased by increasing the extract concentration to 9 mg/mL to reach 95.70%. On the other hand, AFG2 was reduced by 70.92% at a concentration of 1 mg/mL, whereas the reduction increased by increasing the concentration to 9 mg/mL to reach 100%.
Brine shrimp lethality bioassay
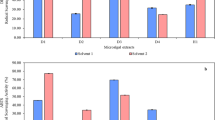
The effect of extracts No. 4 and 5 on the mortality percentage values of the A. salina are presented in Fig. 4. The toxicity test results revealed the highest percentage of mortality for larvae, which was recorded at a concentration of 600 µg/mL for extract No. 5. On the other hand, extract No. 4 showed the lowest percentage of mortality for larvae, which was recorded at a concentration of 1000 µg/mL. The LC50 value was obtained based on the mortality of A. salina larvae induced by the extracts, with extract No. 5 showing the highest LC50 at 300 µg/mL, followed by extract No. 4 at 275 µg/mL.
Cytotoxicity of human cell line
Extract No. 4 showed no anticancer activity; nevertheless, extract No. 5 produced cell toxicity in a concentration-dependent manner (Fig. 5). The IC50 values were used to express anti-proliferative activity, whereas lower IC50 values indicated more significant cell growth-inhibiting activity. Data showed that extract No. 5 had an IC50 value of 79.4 µg/mL, whereas doxorubicin, which was used as a positive control, had an IC50 value of 21.6 µg/mL (Figure S9). It was observed that the extracts tested were not active as doxorubicin.
Discussion
One of the most vital points of this research is the screening of novel microorganisms for antifungal activity. The CFS of thirty-six bacterial isolates were tested for their antifungal activity in this study, whereas the CFS of the following bacterial isolates No. RQ2, RQ7, RQ13, RQ15, and RQ22, were the most promising inhibiting Fusarium spp. to a varying degree. Similarly, several authors established the ability of Bacillus strains to successfully biocontrol Fusarium strains32,33,34. In another study, eight newly grown Bacillus isolates (GB31, GB41X, XJ11, XG11, B. sub2, XJ2, XJ8, and XI4) effectively inhibited the growth of more than two F. graminearum strains in vitro35.
Aspergillus flavus is a microscopic saprophyte fungus that is both pathogenic and toxic and is common in nature and reduces the quality and safety of raw materials and products. The CFS of the bacterial isolates No. RQ1, RQ2, RQ5, RQ15, RQ16, and RQ22 inhibited A. flavus. In agreement, Idiyatov et al.36 stated that isolates EFS10, EFS14, and EFS17 from the B. subtilis species had the most pronounced antifungal activity against A. flavus. On the other hand, A. parasiticus was inhibited by CFS of the bacterial isolates No. RQ1, RQ2, RQ7, RQ13, RQ15, RQ16, and RQ22. Siahmoshteh et al.15 revealed that both B. subtilis and B. amyloliquefaciens demonstrated significant antifungal activity against a wide range of filamentous fungi, and they were successful in suppressing the growth of A. parasiticus by up to 92%.
Also, the CFS of the bacterial isolates No. RQ8 and RQ13 only inhibited A. ochraceus. In agreement, Zhao et al.37 reported that the antifungal activity of the B. subtilis CW14 supernatants was the best, inhibiting mycelial growth and spore germination of A. ochraceus. The antifungal activity of Bacillus isolates could be attributed to the generation of antifungal chemicals that influence the morphological development of fungal structures, such as hyphae growth, morphology, and spore germination15.
The bacterial isolates showing high antifungal activity were identified using biochemical parameters and MALDI-TOF MS analysis. The MALDI-TOF MS has been used as a quick, high-throughput bacterial identification technology in diagnostic microbiology laboratories38. However, this identification technique's efficacy depends on the reference strains obtained in the mass spectral database39. As previously stated, spectral analysis with MALDI-TOF MS can complete identifications as high as 16S rRNA sequences due to its ability to identify at the species level40,41.
The bacterial isolates No. RQ13 and RQ22 which showed higher antifungal activity were thus selected to extract and characterize their bioactive secondary metabolites, whereas VOCs were identified using gas chromatography-mass spectrometry (GC/MS). Our results revealed twelve VOCs were detected in extract No. 4, whereas seven VOCs were detected in extract No.5. Our results were considered lower than those reported by Gao et al.42, who isolated and identified B. subtilis CF-3, and later found that during the fermentation process, a total of 74 potential VOCs were identified43. Similar observations were reported by Rajaofera et al.44, who identified nineteen different VOCs from B. atrophaeus HAB-5. In a similar study, twenty-nine VOCs were identified from B. methylotrophicus BCN2. Meanwhile, thirty VOCS were identified from B. thuringiensis BCN1045. Furthermore, the results agree with Zhang et al.46, who showed that B. subtilis might produce antibiotics, antifungal proteins, and a variety of VOCs47.
The main components in extract No. 4 were butanoic acid, 2-methyl (37.64%), and iso-valeric acid (21.62%). On the contrary, Sadiq and Jamil48 revealed that the extract of intracellular compounds produced by B. cereus showed the presence of VOCs such as toluene, acetic acid butyl ester, 2-Pentanol acetate, and propanoic acid. The VOC hexadecanoic acid was also detected at low concentrations. Similar observations were reported by Rajaofera et al.44, who noted that hexadecanoic acid is one of the compounds produced by B. atrophaeus strain HAB-5. The most critical components identified in extract No. 5, were 9, 12-Octadecadienoic acid (Z,Z)—(81.29%), and hexadecanoic acid (7.75%). Similar results were reported by Jangir et al.49, who identified the following compounds; namely 9,12-octadecadienoic acid (Z,Z)-, and hexadecanoic acid from B. subtilis.
One of the most significant antifungal compounds detected at a high percentage in extract No. 4 was butanoic acid 2-methyl-50. Similar observations were reported by Xu et al.51, who stated that VOCs 2 methylbutanoic acid and iso-valeric acid released by B. tequilensis XK29 inhibited the growth of Ceratocystis fimbriata. On the other hand, the VOC 9, 12-octadecadienoic acid (Z,Z) detected at a high percentage in extract No. 5 was confirmed to be an excellent antifungal agent52.
Polyphenols are the most abundant type of natural antioxidant, flavonoids, hydrolyzable and condensed tannins, phenolic acids, stilbenes, lignans, and phenolic aldehydes53. The LC/MS detected nine phenolic acids in extract No. 4, whereas cinnamic and syringic acids were the most abundant. Extract No. 4 reflected a distinguished content of flavonoids, with the detection of daidzein, naringenin, and rutin. In agreement, Hassan et al.54 found syringic acid, among other phenolic acids. produced by several Bacillus spp., whereas rutin was the only flavonoid produced.
Nine phenolic acids in extract No. 5 were detected. The phenolic acids 3, 4-dihydroxybenzoic acid, and cinnamic acid were the most abundant. On the other hand, a distinguished content of flavonoids was also detected in extract No. 5, with the detection of naringenin, daidzein, and rutin. Similar observations were reported by Zhao et al.55, who successfully applied to an in vitro study in which catechin/epicatechin-broth samples were anaerobically fermented with gut microbes obtained from healthy human donors, and all bacteria used demonstrated outstanding ability in metabolizing grape polyphenols, whereas 3, 4‑dihydroxybenzoic acid was detected among other phenolic acids.
Recently, phenolic compounds have garnered much attention due to their antioxidant activity; additionally, these compounds have been reported to be potential candidates in lowering cardiovascular diseases56 and anti-carcinogenic activities, antiallergenic, anti-atherogenic, anti-inflammatory, antimicrobial, and antithrombotic effects57. Phenolics are gaining popularity in the food sector because they slow the oxidative breakdown of lipids, improving food quality and nutritional content58,59.
In the setting of developing antibiotic resistance, there is a need for active chemicals that inhibit disease propagation, infection, and virulence. The food production industry also relies heavily on developing novel food preservatives that are less damaging to human and environmental health. This prospective sector of microbial VOC application has yet to be investigated60.
Results showed that extracts No. 4 and 5 extracted from B. cereus showed higher antifungal activity against various fungal species. Hathout et al.61 reported similar results and found that Bacillus extract inhibited the growth of Candida albicans and Aspergillus niger. Miljaković et al.62 reported similar observations, stating that B. cereus MH778713's antifungal activity could be linked to the generation of diffusible metabolites and hydrolyzing enzymes. Although only one Bacillus extract had antifungal activity on F. oxysporum, Ramírez et al.63 revealed that B. cereus MH778713 VOCs reduced the mycelial radial growth of F. oxysporum by 38%.
Later, different concentrations of extract No. 4 and 5 were studied on A. flavus fungal growth, and aflatoxin production after 10 days of incubation. Data showed extracts No. 4 and 5 reduced fungal growth. It was also noticed that increasing the concentration of extracts increased the inhibition of fungal growth. Similar results were reported by Siahmoshteh et al.15, who revealed that culture filtrates of soil strains of B. subtilis and B. amyloliquefaciens showed antifungal activity against A. parasiticus NRRL2999. In agreement, Khan et al.34 reported that 100 µg/mL of 1-butanol extract of d B. subtilis 30VD-1 cell-free culture filtrate caused about 40% inhibition of Fusarium spp. The antifungal activity of extracts No. 4 and 5 might be due to bioactive secondary metabolites such as VOCs and polyphenols, known as antimicrobial compounds.
Data also showed that extracts No. 4 and 5 at a 9 mg/mL concentration reduced AFB1 production by 99.99% and 99.97%, respectively. On the other hand, extract No. 4 at a concentration of 9 mg/mL completely prevented AFG1 and reduced AFG2 output by 99.01%, whereas extract No.5 reduced AFG1 by 89.76% and prevented AFG2 production. In experimental animals, Abdel-Wahhab et al.64 revealed that Bacillus extract at the tested doses improved all biochemical parameters and the histological image in rats that received AFB1. In vitro, Pereyra et al.65 indicated that Bacillus spp. reduced AFB1 production by A. parasiticus, whereas extracellular metabolites extracted from the Bacillus species inhibited AFB1 production to non-detectable levels. In another study, the CFS of B. licheniformis CFR1 was able to degrade AFB1 more efficiently than the cell lysate66. Recently, L. rhamnosus bioactive secondary metabolites at a 9 mg/mL concentration reduced AFB1 production by 99.98%30.
The brine shrimp lethality bioassay was initially employed as a simple test to determine toxicity; nevertheless, it is not a specific test. The brine shrimp lethality bioassay is a quick, inexpensive, and straightforward method for assessing the biological activities of extracts and is considered an initial screening method used to evaluate bioactive compounds or compounds assumed suitable as anticancer drugs. The brine shrimp lethality bioassay has been widely used as a preliminary screening of bioactive metabolites to assess their toxicity to A. salina, which could potentially indicate the potential cytotoxic qualities of the test materials67. Extract No. 5 showed the highest LC50, recording 300 µg/mL, followed by extract No. 4, recording an LC50 of 275 µg/mL.
The current investigation revealed that the degree of mortality of A. salina larvae was proportional to the concentration of the extracts. That could be because the higher the concentration, the more potent bioactive contents can be produced. As there is a correlation between cytotoxicity and activity against A. salina nauplii, the cytotoxic effects of the extracts were chosen for subsequent cell line assay68. The extracts showing toxicity against A. salina were studied against HepG2. Results revealed that extract No. 4, which displayed low toxicity against A. salina, showed no anticancer activity. On the other hand, extract No. 5, which showed high toxicity against A. salina, was effective against HepG2 cells by 64.3% at a concentration of 100 µg/mL and recorded an IC50 of 79.4 µg/mL. Similar results were reported by Haneen et al.69, who studied the effect of B. cereus and B. subtilis extracts against human breast adenocarcinoma (MCF-7) and found that at a concentration of 100 µg/mL, the extracts were effective against MCF-7 cells for both B. cereus (48.8%) and B. subtilis (63.8%). In agreement, Ganguly et al.70 stated that the MTT experiment demonstrated significant cytotoxic activity against MCF-7 with an IC50 value of 46.64 µg/mL.
This study indicated that bioactive metabolites derived from Bacillus cereus dramatically inhibited AFs production showing a high potential for managing AFs contamination in the food and feed industries. Cytotoxicity experiments using the brine shrimp lethality assay and the HepG2 cell line revealed that Bacillus cereus bioactive metabolites had tolerable toxicity levels, thus indicating that the bioactive metabolites are safe to use in applications involving human or animal food items.
The various biological activities of bacterial bioactive metabolites make them useful in a variety of industrial applications, as they could be utilized to manufacture antibiotics, antifungals, anticancer agents, and antivirals71. Bacterial bioactive metabolites could be used in wastewater treatment to eliminate contaminants72. They are also essential in the production of bio-fertilizers, biofuels, cosmetics, and biopolymers72. Bacterial bioactive metabolites could have considerable therapeutic potential because they include antibacterial, antifungal, antiviral, and antioxidant properties, which are vital in the face of growing drug-resistant microbial infections71.
Materials and methods
Chemicals
Ethyl acetate and chloroform HPLC grade were purchased from Merck KGaA (Darmstadt, Germany), whereas dimethyl sulfoxide (DMSO) was purchased from Research Lab Fine Chem Industries (Mumbai, 400 002, India). Sodium sulfate anhydrous and yeast extract were purchased from Loba Chemie (Mumbai 400 005, India). The mediums potato dextrose agar, nutrient agar, and tryptic soy broth were purchased from Neogen (Lansing, MI 48912, USA).
Sampling
Rice grains (1 kg) were gathered from retail stores in Egypt's Qalyubia governorate, and they were transported to the Food Toxicology and Contaminants lab and kept in polythene bags refrigerated (< 10 °C) until examination.
Microorganisms
The genome sequence of the aflatoxin-producing Aspergillus flavus utilized in this work was submitted to the GenBank database as A. flavus AAM2020 (Accession No. OP942201) and was isolated from Egyptian maize samples73. From several grains in Egypt, the fungi A. parasiticus, A. niger, A. ochraceus, Fusarium oxysporum, Fusarium spp., and Penicillium spp. were isolated74.
Isolation of endophytic bacteria
The rice grains were washed in running water for ten minutes and then washed two times with sterile distilled water for one minute; after that, they were immersed in 3% (v/v) sodium hypochlorite and 70% (v/v) ethanol for three minutes. The rice was washed thrice with sterilized distilled water for two minutes to finish cleaning. Sterilized rice grains were placed on sterilized filter paper before implanted on nutrient agar Petri dishes and incubated at 30 °C for 2 to 7 days75. Sub-culturing was employed to select and purify morphologically distinct colonies. Pure cultures were used to evaluate their antifungal activity.
Antifungal activity of CFS of bacterial isolates
Endophytic bacterial isolates were tested for their antifungal activity using the agar well diffusion technique76. Isolated endophytic bacteria strains were cultured on tryptic soy broth for 24 h at 37 °C. Following incubation, the broth media were centrifuged (Thermo Fisher Scientific, USA) at 10,000 rpm for 10 min at 4 °C to collect the CFS, which was then filtered using a 0.2 mm sterile Millipore filter (Millex-GS, Millipore, USA). On Petri dishes, potato dextrose agar media was placed, and different fungal isolates (50 µL, 106 spores/mL) were dispersed completely over the agar surface with a sterile cotton swab. Wells were cut with a sterile cork borer and each well-received 100 µL of CFS of bacterial isolates. The Petri dishes were incubated for 48 h at 28 °C, and the inhibition zones (mm) were measured to assess the antifungal activity. Biochemical analysis and MALDI-TOF were used to identify endophytic bacterial isolates with antifungal activity.
Identification of bacterial isolates
The bacterial isolates showing antifungal activity were identified using biochemical parameters (Gram staining77 and oxidase test78) and MALDI-TOF MS. In brief, a loop of freshly bacterial cultures was placed in 300 µL of water and 900 µL of ethanol. The pellets were mixed with equivalent formic acid (70%) and acetonitrile (100%) after centrifugation for 2 min at 13,000 rpm. Using the MALDI-TOF MS technology, the supernatant (extracted bacterial proteins) was utilized for protein identification and profiling (Microflex LT, Bruker, Billerica, MA 01821, USA)79.
For identification, the sample (1 µL) was placed on a MALDI bio target plate and left to dry at room temperature. The dried sample spot was then covered with a (1 µL) matrix of α-cyanohydroxy cinnamic acid (CHCA) to enable the proteins in the sample to crystallize. The MALDI bio target plate was then put into the MALDI-TOF apparatus. The identification score (from 0 to 3) was then used to characterize the amount of mass spectral concordance with the database. The MBT Compass and Flex Analysis tools were used to analyze and process these data.
Extraction of bioactive metabolites
The bacterial isolates No. RQ13 and RQ22 were grown on rice medium (100 g rice in 100 mL distilled water) and rice and yeast medium (100 g rice, 0.5 g yeast extract, in 100 mL distilled water). The rice mediums were incubated at 35 °C in static for 7 days. Simultaneously, the bacterial isolates No. RQ13 and RQ22 were cultured in tryptic soy broth and incubated at 35 °C with shaking at 120 rpm for 3 days80. After incubation, the broth media were centrifuged at 10,000 rpm (Thermo Fisher Scientific, USA) for 10 min at 4 °C to collect the CFS, which was then filtered through sterilized 0.22 µm pore-size filters (Millex-GS, Millipore, USA). Ethyl acetate was added to the CFS and rice medium in a 1:1 (v/v) ratio and vigorously agitated for a few minutes. The ethyl acetate mixtures were put into separating funnels and let to stand until the organic and aqueous phases separated, at which point the organic phase was collected and passed through anhydrous sodium sulfate. This procedure was performed three times before the organic phase was dried off using a rotary evaporator (Heidolph Instruments GmbH & Co. KG, Germany) to produce extracts44.
Determination of antifungal activity of bioactive metabolites
The antifungal potential of the extracts was assessed using an agar well diffusion experiment, as reported by Salman et al.81. On Petri dishes, potato dextrose agar medium was applied, and various fungal isolates (100 µL, 106 spores/mL) were dispersed equally across the agar surface with a sterile cotton swab, followed by wells cut with a sterile cork borer. Then, 100 µL of extracts No. 4 and 5 were applied to each well at a 5 mg/mL concentration. The Petri plates were incubated for 48 h at 28 °C, and the inhibition zones (mm) were measured to assess the antifungal activity.
Determination of volatile organic compounds in bioactive metabolites
The gas chromatography/mass spectrometer (GC/MS) technology (Agilent Technologies, USA) was used to undertake a qualitative and quantitative analysis of volatile organic molecules. A gas chromatograph (7890B) and a mass spectrometer detector (5977A) were part of the GC/MS system. The GC was outfitted with an HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 m film thickness). The carrier gas in the analyses was hydrogen, with a flow rate of 1 mL/min at a splitless injection volume of 1 µL and the following temperature program: 50 °C for 1 min; 5 °C/min rise to 100 °C and hold for 0 min; 10 °C/min rise to 300 °C and hold for 5 min. The injector and detector were held at temperatures of 250 °C and 260 °C, respectively. With a 6-min solvent delay, electron ionization (EI) at 70 eV produced mass spectra with a spectral range of m/z 50–550. Different components were found by comparing the spectrum fragmentation pattern to those in the Wiley and NIST Mass Spectral Library data.
Determination of polyphenols in bioactive metabolites
Extracts were analyzed to assess polyphenols using liquid chromatography-electrospray ionization-tandem mass spectrometry (LC–ESI–MS/MS), with an Exion LC AC system for separation and a SCIEX Triple Quad 5500 + MS/MS system with electrospray ionization (ESI) for detection. A ZORBAX SB-C18 Column (4.6 X 100 mm, 1.8 µm) (Agilent Technologies, USA) was employed for the separation. Two eluents were used in the mobile phases: A (0.1% formic acid in water) and B (acetonitrile). The mobile phase was set at 2% B from 0 to 1 min, 2% B from 1 to 21 min, 60% B from 21 to 25 min, and 2% B from 25 to 28 min. The injection volume was 3 µL, and the flow rate was 0.8 µL/min. The following parameters were employed for MRM analysis of the chosen polyphenols in both positive and negative ionization modes: Ion spray voltages for the positive and negative modes were 4500 and -4500, respectively; 400 °C source temperature; 25 psi curtain gas; 55 psi ion source gases with a declustering potential of 50; 25 psi collision energy; and 10 psi collision energy spread.
Evaluation of the effect of the bioactive metabolites on the microstructure of Aspergillus flavus using a SEM
The effect of extracts on A. flavus microstructure was investigated. Twenty-five milliliters of molten potato dextrose agar were put in Petri plates and permitted to solidify. A fungal spore suspension of A. flavus (100 µL, 106 spores/mL) was distributed evenly over the agar surface, after which wells were cut using a sterile cork borer. Each well received 100 µL of extracts Nos. 4 and 5, and the plates were incubated at 28 °C for 5 days. Mycelium segments (1 cm2) were cut and deposited at room temperature in vials containing 3% glutaraldehyde in 0.05 M phosphate buffer (pH 6.8), as stated by Mims82 and Gong et al.83. An ethanol series was used after chemical fixation, culminating in total ethanol. Fungal cultures were freeze-dried after samples were dried in liquid carbon dioxide. The samples were put in Petri plates, and an unfilled portion of the plate was filled with a vial cap holding 4% osmium tetraoxide in water. Then, segments were coated with 20 to 30 nm of 60:40 gold palladium. All materials were examined in a 20.00 kV electron probe micro-analyzer SEM (Quanta FEG 205, FEI Company, Hillsboro, OR, USA).
Effect of bioactive metabolites on fungal growth and aflatoxin production
The impact of extracts No. 4 and 5 on mycelial dry weight (MDW) and AFs production was determined using the method described by Roshan et al.5 with a few adjustments (using potato dextrose broth instead of sucrose malt-yeast extract-broth). The appropriate amounts of extracts were added to reach final concentrations of 1, 3, 5, 7, and 9 mg/mL of growth media to various flasks containing 20 mL of potato dextrose broth. Each flask was filled with a fungal spore solution (100 µL, 106 spores/mL) from a 7-day-old culture of the aflatoxigenic isolate. For 10 days, cultures were incubated at 28 °C. After incubation, the culture medium was filtered (Whatman No. 1), and the mycelia were washed with water and dried in a hot air oven (110 °C, 12 h). In a separating funnel, the filtrate was extracted twice with 20 mL chloroform, and then the extract was passed through anhydrous sodium sulfate and evaporated to dryness. HPLC was used to identify aflatoxins. The percentage of inhibition was calculated using the following equation:
Brine shrimp lethality bioassay
The in vivo mortality of extracts No. 4 and 5 were estimated using nauplii of the A. salina. Twenty-seven g of the commercially available salt was dissolved with 900 mL of distilled water to make artificial saltwater. The A. salina eggs were placed in a small commercial tank with artificial seawater for nauplii hatching and incubated for 48 h under a halogen lamp, which provided direct light and warmth.
Twenty mg of extracts No. 4 and 5 were diluted in 2 mL of ethyl acetate, and then concentrations of 50, 100, 200, 400, 600, 800, and 1000 g/mL were made. The tubes were allowed to dry out completely. Following that, each tube received 4.5 mL of artificial seawater, and ten nauplii were counted macroscopically and transferred to test tubes using the stem of a graduated Pasteur pipette against an illuminated backdrop. With artificial seawater, the final volume in each tube was adjusted to 5 mL after introducing the nauplii. Two separate counters counted and recorded the quantity of surviving nauplii in each tube after 24 h84,85. The experiment included three replicates for each treatment and 10 nauplii per replication. The LC50 values were calculated with 95% confidence intervals using data analysis and interpreted using the Reed-Muench technique. The Reed-Muench technique presupposes that an animal that survives a particular dose will likewise survive any lower dose and that an animal that dies at a specific dose would similarly die with any higher dose. Thus, throughout the range of doses investigated, information from any group may add to knowledge from other groups86,87.
Cytotoxicity of human cell line
The impact of extracts No. 4 and 5 on HepG2 was investigated. At 37 °C and 5% CO2, cells were suspended in DMEM-F12 media supplemented with 1% antibiotic–antimycotic combination (10,000 U/mL potassium penicillin, 10,000 g/mL streptomycin sulfate, and 25 g/mL amphotericin B) and 1% L-glutamine.
Cell viability was determined using the mitochondrial-dependent reduction of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to purple formazan88. Cells were cultivated for 10 days before being seeded at a density of 10 × 103 cells/well in fresh complete growth medium on 96-well microtiter plates at 37 °C for 24 h in a water-jacketed carbon dioxide incubator (Sheldon, OR 97113, USA).
The cells were cultivated alone (control) or with different extract concentrations to obtain a final concentration of (0.78, 1.56, 3.125, 6.25, 12.50, 25.00, 50.00, and 100.00 µg/mL). After 48 h, the medium was sucked; 40ul MTT salt (2.5 µg/mL) was added to each well and incubated for another four hours at 37 °C with 5% CO2. Each well received 200 µL of deionized water containing 10% sodium dodecyl sulfate (SDS) and was incubated overnight at 37 °C to terminate the reaction and dissolve the generated crystals. A positive control of Adriamycin (Doxorubicin), a recognized cytotoxic natural chemical with a 100% death rate under identical circumstances, was used at a 100 µg/mL concentration.
Using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA), absorbance was measured at 595 nm and a reference wavelength of 620 nm. The extracts were dissolved in DMSO, with a final concentration of less than 0.2% on the cells. The following equation was used to compute the percentage of viability:
The degree of selectivity of the synthesized compounds is stated in the current study as SI = IC50 of the pure compound in a normal cell line/IC50 of the same pure compound in a cancer cell line, where IC50 is the concentration necessary to kill 50% of the cell population. In vitro, bioassay on human tumor cell line test was conducted by the Bioassay-cell culture Laboratory, National Research Centre, Cairo, Egypt.
Statistical analysis
The statistical analyses were conducted using the SPSS 26 (IBM, USA) software. The studies were carried out in triplicate, and the differences between control and treatment groups were assessed using the Student's t-test, while the differences across the groups were examined using the one-way ANOVA test. The significance threshold was chosen at P ≤ 0.05.
Conclusion
The isolated and identified Bacillus species showed variable degrees of antifungal activity. The bioactive metabolites extracted from these Bacillus species produced volatile organic compounds and polyphenols and exhibited antifungal and antiaflatoxigenic activity. These bioactive metabolites induced toxicity against A. salina and against hepatocellular carcinoma. This study is considered the first to report Bacillus bioactive metabolites' ability to reduce and prevent aflatoxin production.
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Mutungi, C. et al. Physical quality of maize grain harvested and stored by smallholder farmers in the Northern Highlands of Tanzania: Effects of harvesting and pre-storage handling practices in two marginally contrasting agro-locations. J. Stored Prod. Res. 84, 101517 (2019).
Russo, M. L. et al. Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. growth and yield. J. King Saud. Univ. Sci. 31(4), 728–736 (2019).
Adetunji, M. C., Ezeokoli, O. T., Ngoma, L. & Mwanza, M. Phylogenetic diversity and prevalence of mycoflora in ready-to-eat supermarket and roadside-vended peanuts. Mycologia 113(1), 1–11 (2020).
Alshannaq, A. & Yu, J. H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 14, 6 (2017).
Roshan, A. B., Venkatesh, H. N. & Mohana, D. C. Chemical Characterization of Schefflera actinophylla (Endl.) harms essential oil: Antifungal and antimycotoxin activities for safe storage of food grains. J. Biol. Act. Prod. Nat. 11(1), 60–69 (2021).
Abhishek, R. U., Thippeswamy, S., Manjunath, K. & Mohana, D. C. Antifungal and antimycotoxigenic potency of Solanum torvum Swartz. leaf extract: Isolation and identification of compound active against mycotoxigenic strains of Aspergillus flavus and Fusarium verticillioides. J. Appl. Microbiol. 119(6), 1624–1636 (2015).
Tian, F. & Chun, H. S. Natural products for preventing and controlling aflatoxin contamination of food. Aflatoxin Control Anal. Detect. Heal. Risks 8, 13–44 (2017).
Thippeswamy, S., Abhishek, R. U., Manjunath, K., Raveesha, K. A. & Mohana, D. C. Antifumonisin efficacy of 2-hydroxy-4-methoxybenzaldehyde isolated from Decalepis hamiltonii. Int. J. Food Prop. 18(9), 2002–2008 (2015).
Adams, M. & Motarjemi, Y. Basic Food Safety for Health Workers World Health Organization Basic Food Safety for Health Workers (1999).
Ehrlich, K. C., Kobbeman, K., Montalbano, B. G. & Cotty, P. J. Aflatoxin-producing Aspergillus species from Thailand. Int. J. Food Microbiol. 114(2), 153–159 (2007).
Singh, P. & Cotty, P. J. Characterization of Aspergilli from Dried Red Chilies (Capsicum spp.): Insights into the Etiology of Aflatoxin Contamination, vol. 289 (2019).
Venkatesh, H. N. et al. Antifungal and antimycotoxigenic properties of chemically characterised essential oil of Boswellia serrata Roxb. ex Colebr. Int. J. Food Prop. 20(2), 1856–1868 (2017).
Ostry, V., Malir, F., Toman, J. & Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 33(1), 65–73 (2017).
Muhialdin, B. J., Saari, N. & Hussin, A. S. M. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules 25, 11 (2020).
Siahmoshteh, F., Hamidi-Esfahani, Z., Spadaro, D., Shams-Ghahfarokhi, M. & Razzaghi-Abyaneh, M. Unraveling the mode of antifungal action of Bacillus subtilis and Bacillus amyloliquefaciens as potential biocontrol agents against aflatoxigenic Aspergillus parasiticus. Food Control 89, 300–307 (2018).
Peles, F. et al. Biological control and mitigation of aflatoxin contamination in commodities. Toxins (Basel) 13(2), 1–19 (2021).
Rodriguez, H. et al. Degradation of ochratoxin a by Brevibacterium species. J. Agric. Food Chem. 59(19), 10755–10760 (2011).
Fuchs, E., Binder, E. M., Heidler, D. & Krska, R. Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Addit. Contam. 19(4), 379–386 (2002).
Line, J., Brackett, R. & Willikinson, R. Evidence for degradation of aflatoxin B1 by Flavobacterium aurantiacum. J. Food Prot. 57, 788–791 (1994).
Alberts, J. F., Engelbrecht, Y., Steyn, P. S., Holzapfel, W. H. & van Zyl, W. H. Biological degradation of aflatoxin B1 by Rhodococcus erythropolis cultures. Int. J. Food Microbiol. 109(1–2), 121–126 (2006).
Hathout, A. & Abdel-Nasser, A. The efficiency of Saccharomyces cerevisiae as an antifungal and antimycotoxigenic agent. Biointerface Res. Appl. Chem. 13(4), 354 (2023).
Nasrollahzadeh, A., Mokhtari, S., Khomeiri, M. & Saris, P. Mycotoxin detoxification of food by lactic acid bacteria. Int. J. Food Contam. 9(1), 1–9 (2022).
Hathout, A. S. & Aly, S. E. Biological detoxification of mycotoxins: A review. Ann. Microbiol. 64(3), 905–919 (2014).
Schallmey, M., Singh, A. & Ward, O. P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 50(1), 1–17 (2004).
Abriouel, H., Franz, C. M. A. P., BenOmar, N. & Galvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35(1), 201–232 (2011).
Mondol, M. A. M., Shin, H. J. & Islam, M. T. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Mar. Drugs 11(8), 2846–2872 (2013).
Ren, X., Zhang, Q., Zhang, W., Mao, J. & Li, P. Control of aflatoxigenic molds by antagonistic microorganisms: Inhibitory behaviors, bioactive compounds, related mechanisms, and influencing factors. Toxins (Basel) 12(24), 1–21 (2020).
Monciardini, P., Iorio, M., Maffioli, S., Sosio, M. & Donadio, S. Discovering new bioactive molecules from microbial sources. Microb. Biotechnol. 7(3), 209–220 (2014).
Patridge, E., Gareiss, P., Kinch, M. S. & Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 21(2), 204–207 (2016).
Abdel-Nasser, A., Hathout, A. S., Badr, A. N., Barakat, O. S. & Fathy, H. M. Extraction and characterization of bioactive secondary metabolites from lactic acid bacteria and evaluating their antifungal and antiaflatoxigenic activity. Biotechnol. Rep. 38(2022), e00799 (2023).
Hathout, A. S., Ghareeb, M. A., Abdel-Nasser, A. & Abu-Sree, Y. Saccharomyces cerevisiae bioactive metabolites: Characterization and biological activities. ChemistrySelect 9, 11 (2024).
Sakalauskas, S., Kačergius, A., Janušauskaite, D. & Čitavičius, D. Bacteria with a broad spectrum of antagonistic activity against pathogenic fungi of cereals. Zemdirbyste 101(2), 185–192 (2014).
Kim, Y. G. et al. Antagonistic activities of novel peptides from Bacillus amyloliquefaciens PT14 against Fusarium solani and Fusarium oxysporum. J. Agric. Food Chem. 63(48), 10380–10387 (2015).
Khan, N. et al. Antifungal activity of bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front. Microbiol. 9, 1–12 (2018).
Stumbriene, K. et al. Screening of new bacterial isolates with antifungal activity and application of selected Bacillus sp. cultures for biocontrol of Fusarium graminearum under field conditions. Crop Prot. 113, 22–28 (2018).
Idiyatov, I. I., Eroshin, A. I., Yusupov, S. A., Tremasova, A. M. & Biryulya, V. V. Endophytic bacteria antagonists of the micromycete Aspergillus flavus: The prospect of improving the quality of food raw materials and food products. IOP Conf. Ser. Earth Environ. Sci. 949, 1 (2022).
Zhao, M., Liu, D., Liang, Z., Huang, K. & Wu, X. Antagonistic activity of Bacillus subtilis CW14 and its β-glucanase against Aspergillus ochraceus. Food Control 131, 108475 (2022).
Bizzini, A. et al. Matrix-assisted laser desorption ionization—Time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J. Clin. Microbiol. 49(2), 693–696 (2011).
Rahi, P., Prakash, O. & Shouche, Y. S. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) based microbial identifications: Challenges and scopes for microbial ecologists. Front. Microbiol. 7, 1–12 (2016).
Stets, M. I. et al. Rapid identification of bacterial isolates from wheat roots by high-resolution whole cell MALDI-TOF MS analysis. J. Biotechnol. 165(3–4), 167–174 (2013).
Calderaro, A. et al. Identification of Borrelia species after creation of an in-house MALDI-TOF MS database. PLoS One 9, 2 (2014).
Gao, H., Xu, X., Dai, Y. & He, H. Isolation, identification, and characterization of Bacillus subtilis CF-3, a bacterium from fermented bean curd for controlling postharvest diseases of peach fruit. Food Sci. Technol. Res. 22(3), 377–385 (2016).
Gao, H., Xu, X., Zeng, Q. & Li, P. Optimization of headspace solid-phase microextraction for GC-MS analysis of volatile compounds produced by biocontrol strain Bacillus subtilis CF-3 using response surface methodology. Food Sci. Technol. Res. 23(4), 583–593 (2017).
Rajaofera, M. J. N. et al. Volatile organic compounds of Bacillus atrophaeus HAB-5 inhibit the growth of Colletotrichum gloeosporioides. Pestic. Biochem. Physiol. 156(February), 170–176 (2019).
He, C. N., Ye, W. Q., Zhu, Y. Y. & Zhou, W. W. Antifungal activity of volatile organic compounds produced by Bacillus methylotrophicus and Bacillus thuringiensis against five common spoilage fungi on loquats. Molecules 25, 3360 (2020).
Zhang, B. et al. High-cell-density culture enhances the antimicrobial and freshness effects of Bacillus subtilis S1702 on table grapes (Vitis vinifera cv. Kyoho). Food Chem. 286, 541–549 (2019).
Leelasuphakul, W., Hemmanee, P. & Chuenchitt, S. Growth inhibitory properties of Bacillus subtilis strains and their metabolites against the green mold pathogen (Penicillium digitatum Sacc.) of citrus fruit. Postharvest. Biol. Technol. 48(1), 113–121 (2008).
Sadiq, H. & Jamil, N. Antagonistic behaviour of organic compounds from Bacillus species and Brevundimonas species. Pak. J. Pharm. Sci. 31(3), 919–926 (2018).
Jangir, M., Pathak, R., Sharma, A., Sharma, S. & Sharma, S. Volatiles as strong markers for antifungal activity against Fusarium oxysporum f. sp. lycopersici. Indian Phytopathol. 72(4), 681–687 (2019).
Surya, M., Thiruvudainambi, S., Ebenezar, E. G., Vanniarajan, C. & Kumutha, K. GC-MS analysis of antimicrobial compounds produced by Bacillus spp. against rice sheath rot pathogen Sarocladium oryzae. J. Entomol. Zool. Stud. 8(1), 1417–1423 (2020).
Xu, M. et al. Antibiotic effects of volatiles produced by Bacillus tequilensis XK29 against the black spot disease caused by Ceratocystis fimbriata in postharvest sweet potato. J. Agric. Food Chem. 69(44), 13045–13054 (2021).
Yassein, A. S. & Elamary, R. B. Efficacy of soil paraburkholderia fungorum and Bacillus subtilis on the inhibition of Aspergillus niger growth and its ochratoxins production. Egypt. J. Bot. 61(1), 319–334 (2021).
Beres, C. & Ignacio Cabezudo, N. M. M. Metabolites of polyphenols produced by probiotic microorganisms and their beneficial effects on human health and intestinal microbiota. In Lactic Acid Bacteria A Functional Approach, 1st edn (2020).
Hassan, A. H. A. et al. Salinity stress enhances the antioxidant capacity of Bacillus and Planococcus species isolated from saline lake environment. Front. Microbiol. 11, 1–15 (2020).
Zhao, D. et al. Development and validation of an ultra-high performance liquid chromatography/triple quadrupole mass spectrometry method for analyzing microbial-derived grape polyphenol metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1099, 34–45 (2018).
Huxley, R. R. & Neil, H. A. W. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 57, 904–908 (2003).
Huang, W.-Y., Cai, Y.-Z. & Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 62(1), 1–20 (2010).
Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 11(2–3), 153–177 (2012).
Lattanzio, V. Phenolic compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes 1543–1580 (2013).
Weisskopf, L., Schulz, S. & Garbeva, P. Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 19(6), 391–404 (2021).
Hathout, A. et al. Novel Egyptian bacterial strains exhibiting antimicrobial and antiaflatoxigenic activity. J. Appl. Pharm. Sci. 6, 12 (2016).
Miljaković, D., Marinković, J. & Balešević-Tubić, S. The significance of Bacillus spp. In disease suppression and growth promotion of field and vegetable crops. Microorganisms 8(7), 1–19 (2020).
Ramírez, V. et al. Bacillus cereus MH778713 elicits tomato plant protection against Fusarium oxysporum. J. Appl. Microbiol. 132, 1–13 (2021).
Abdel-Wahhab, M. A. et al. Secondary metabolites from Bacillus sp. MERNA97 extract attenuates the oxidative stress, genotoxicity, and cytotoxicity of aflatoxin B1 in rats. Food Chem. Toxicol. 141, 111399 (2020).
Pereyra, M. L., Martínez, M. P., Petroselli, G., Balsells, R. & Cavaglieri, L. R. Antifungal and aflatoxin-reducing activity of extracellular compounds produced by soil Bacillus strains with potential application in agriculture. Food Control 85, 392–399 (2018).
Rao, K., Vipin, A. V., Hariprasad, P., Appaiah, K. A. & Venkateswaran, G. Biological detoxification of Aflatoxin B1 by Bacillus licheniformis CFR1. Food Control 71, 234–241 (2017).
Meyer, B. N. et al. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 45(1), 31–34 (1982).
Manilal, A., Sujith, S., Kiran, G. S., Selvin, J. & Shakir, C. Cytotoxic potentials of red alga, Laurencia brandenii collected from the indian coast. Glob. J. Pharmacol. 3(2), 90–94 (2009).
Haneen, A. I., Neihaya, H. Z. & Alhammar, A. Antibacterial, antibiofilm and anticancer activities of ethyl acetate extract of Bacillus spp. Int. J. Drug Deliv. Technol. 11(4), 1262–1268 (2021).
Ganguly, R. K., Midya, S. & Chakraborty, S. K. Antioxidant and anticancer roles of a novel strain of Bacillus anthracis isolated from vermicompost prepared from paper mill sludge. Biomed. Res. Int. 2018, 1–7 (2018).
Abdelghani, Z., Hourani, N., Zaidan, Z., Dbaibo, G. & Mrad, M. Therapeutic applications and biological activities of bacterial bioactive extracts. Arch. Microbiol. 203, 4755–4776 (2021).
Mehmood, M. A., Verma, P., Shah, M. P. & Betenbaugh, M. J. Pharmaceutical and Nutraceutical Potential of Cyanobacteria (Springer, 2024).
Abdel-Nasser, A. Maximizing the Benefits of Bacterial Metabolites as Biocontrol Agents Against Mycotoxigenic Fungi (Caio University, 2022).
Abdel-Nasser, A., Fathy, H. M., Badr, A. N., Hathout, A. S. & Barakat, O. S. M. Prevalence of aflatoxigenic fungi in cereal grains and their related chemical metabolites. Egypt. J. Chem. 65(10), 455–470 (2022).
Kumar, V., Jain, L., Jain, S. K., Chaturvedi, S. & Kaushal, P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. S. Afr. J. Bot. 134, 50–63 (2020).
Juodeikiene, G. et al. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxin reduction in malting wheat grains. LWT Food Sci. Technol. 89(2017), 307–314 (2018).
Sandle, T. Gram’s stain : History and explanation of the fundamental technique of determinative bacteriology. IST Sci. Technol. J. 54(2004), 3–4 (2004).
Dharmappa, D. C., Anokhe, A. & Kalia, V. Oxidase test: A biochemical methods in bacterial identification dharmappa. AgriCose Newsl. 3(1), 31–33 (2022).
Ashfaq, M. Y. et al. Isolation, identification and biodiversity of antiscalant degrading seawater bacteria using MALDI-TOF-MS and multivariate analysis. Sci. Total Environ. 656, 910–920 (2019).
Nisa, K., Rosyida, V. T., Nurhayati, S., Indrianingsih, A. W., Darsih, C. & Apriyana, W. Total phenolic contents and antioxidant activity of rice bran fermented with lactic acid bacteria. In IOP Conference Series: Earth and Environmental Science, vol. 251, no. 1 (2019).
Salman, M. et al. In vitro antimicrobial and antioxidant activities of lactobacillus coryniformis BCH-4 bioactive compounds and determination of their bioprotective effects on nutritional components of maize (Zea mays L.). Molecules 25, 4685 (2020).
Mims, C. W. Using electron microscopy to study plant pathogenic fungi. Mycologia 83(1), 1 (1991).
Gong, A. D. et al. Inhibitory effect of volatiles emitted from alcaligenes faecalis N1-4 on Aspergillus flavus and aflatoxins in storage. Front. Microbiol. 10, 1419 (2019).
Ghareeb, M. A. et al. Cytotoxic screening of three Egyptian plants using brine shrimp lethality test. Int. J. Pharm. Pharm. Sci. 7(9), 507–509 (2015).
Abd El-Rahman, A. A. A., Abd El-Aleem, I. M., Refahy, L. A. & El-Shazly, M. A. Total phenolic content, cytotoxic and antioxidant activities of Quisqualis indica (Linn.) growing in Egypt. Der Pharma Chem. 8(3), 53–59 (2016).
Ghareeb, M. A., Saad, A. M., Abdel-Aleem, A. H., Abdel-Aziz, M. S. & Hamed, M. M. Antioxidant, antimicrobial, cytotoxic activities and biosynthesis of silver & gold nanoparticles using Syzygium jambos leaves growing in Egypt. Der Pharma Chem. 8, 277–286 (2016).
Saad, A. M. et al. In vitro antioxidant, antimicrobial and cytotoxic activities and green biosynthesis of silver & gold nanoparticles using Callistemon citrinus leaf extract. J. Appl. Pharm. Sci. 7(6), 141–149 (2017).
Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Immunol. Methods 65, 55–63 (1983).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
A.A., A.H., A.B., and M.G. carried out the work; A.A. wrote the original draft of the manuscript; O.B., H.F., A.B., and A.H. jointly supervised the work; O.B. and A.H. reviewed, and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Nasser, A., Badr, A.N., Fathy, H.M. et al. Antifungal, antiaflatoxigenic, and cytotoxic properties of bioactive secondary metabolites derived from Bacillus species. Sci Rep 14, 16590 (2024). https://doi.org/10.1038/s41598-024-66700-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66700-y
Keywords
This article is cited by
-
Tropical fruit-derived Lactiplantibacillus as potential probiotic and antifungal agents against Fusarium oxysporum
Scientific Reports (2025)
-
Effects of Bacillus thuringiensis KA36 as an environmentally sustainable fungicide alternative on the morphological, physicochemical, and qualitative attributes of Alternaria solani-infected tomatoes
Journal of Plant Pathology (2025)
-
Indigenous aseel chicken-derived probiotics as biofactories of antifungal metabolites to control mycotoxin contamination in poultry feed
Molecular Biology Reports (2025)
-
Biological Profiling of Postbiotics of Lactobacillus plantarum: Antibacterial, Antioxidant, and Cytotoxic Properties Under In Vitro and Food Circumstances
Probiotics and Antimicrobial Proteins (2025)
-
Characterization of soil-derived Bacillus subtilis metabolites against breast cancer: In vitro and in silico studies
Saudi Pharmaceutical Journal (2025)