Abstract
Dyes are one of the common contaminants in industrial wastewater. Adsorption is the most widely method which used to treat dye-contaminated water due to their easy use, cost-effectiveness, and their efficiency was high. The aim of this study is the investigating of the utilization of the activated carbon which prepared from Raphanus seeds solid residual (ACRS) as a low cost adsorbent for removing of cationic Methylene Blue dye (MB)from wastewater. measuring the surface area using BET methods and SEM. The FT‒IR and XRD was measured. Different variables (e.g.: initial concentration of the dye, pH, contact time, and dosage) have been studied. Process has been systematically investigated experimentally at (25 ± 1 °C). The % removal of MB reached 99.4% after 90-min MB adsorption (40 mg/L) was observed within 5 min of contact time for the Raphanus seeds solid residual (ACRS) dosage of 4 g/L. MB initial concentration (10 ppm) Raphanus seeds solid residual (ACRS) effectively adsorbed MB (> 99%) over a widely range of pH (from pH 2 to pH 8). However, a swift decline in removal was observed when the pH was set at 7. The results of the adsorption kinetics analysis indicate a strong correlation with the pseudo-second-order model, as evidenced by the high regression coefficients. However, the adsorption capacity diminished with a rise in temperature. Thermodynamic calculations of (MB) onto Raphanus seeds solid residual (ACRS) is an exothermic reaction. The results have been indicated that the effectiveness of MB removal by activated carbon prepared from Raphanus seeds solid residual is favorable under neutral conditions, Raphanus seeds solid residual (ACRS) can be considered an efficient, environmentally friendly, readily available, and economical adsorbent that could treat industrial wastewater contaminated with cationic textile dyes. The objective of the experiments was to investigate the impact of various factors on the response of a process or formulation. To accomplish this goal, response surface methodology (RSM) has employed as a statistical model. RSM is an efficient and effective method for optimizing processes through the use of a quadratic polynomial model. The utilization of RSM allows for a reduction in the number of experiments needed, thus minimizing the associated costs of extensive analysis. This method has been done using Box–Behnken Design (BBD) to optimize % removal of MB. The optimal conditions as obtained from the RSM is pH 7,contact time 120 min, initial concentration 10 ppm, ACRS dosage 1 g, adsorption temperature 45 °C.
Similar content being viewed by others
Introduction
The mention of the need for effective methods to remove dyes from wastewater in line 7 is intricately linked to the broader context of dye contamination discussed in the preceding sentences. In recent times, water pollution has emerged from various sources, both natural and anthropogenic, including industrial discharges, agricultural runoff, sewage discharges, and oil spills. The comprehensive management of this issue necessitates a multifaceted approach, involving strategies such as wastewater treatment, the enforcement of regulations and policies, and public awareness and education programs1.
The persistence of pollutants, highlighted in the context of dye contamination, emphasizes their enduring presence in the environment and the potential for bioaccumulation in organisms. This persistence underscores the urgency for the development and implementation of efficient methods to remove dyes from wastewater. The significance of this endeavor is underscored by its role in safeguarding aquatic life and preserving the overall environmental integrity. Various approaches, spanning physical, chemical, and biological methods, exist for the removal of dyes. However, achieving an optimal and sustainable solution requires a comprehensive understanding of dye removal mechanisms, careful consideration of potential trade-offs, and an assessment of economic, social, and environmental factors2. This holistic approach ensures that the chosen methods not only effectively remove dyes but also align with broader goals of sustainability and environmental protection.
Furthermore, the release of these dyes into the environment carries health risks for humans. Contaminating drinking water sources and adversely affecting crops and livestock that consume the contaminated water, the presence of these dyes poses a direct threat to public health. Hence, the call for effective methods to remove dyes from wastewater is intricately connected to the broader narrative of addressing water pollution and safeguarding both the environment and human well-being.
The large-scale production of textiles and clothing leads to significant amounts of dye-containing wastewater being generated and released into the environment, potentially contaminating local water sources and having adverse impacts on the surrounding ecosystems and human health. To mitigate these negative effects, various treatment methods developed as physical, chemical, and biological methods3,4,5.
MB is cationic dye which has used in textile industry, but it is harmful to the environment and human health. It has been linked to toxicity, mutagenicity, and potential cancer-causing effects. Therefore, it is important for removing MB from textile effluent in order to create a cleaner and safer environment6.
MB has been chosen in this study because it is an adsorbent with strong adsorption onto solids. Its molecular weight is 373.9. The structure of MB was given below7 in Fig. 1.
Multiples of chemical-physical methods which are the flocculation, the coagulation, the membrane, the filtration and the adsorption processes are available and used for the treatment of the textile wastewater. Figure 2 shows the different methods for wastewater treatment.
The existing research on the development of low-cost adsorbents is not thoroughly expounded upon. It is imperative to delve into this area, considering its significance in the context of wastewater treatment. Adsorption stands out as one of the most economical and effective methods for dye removal from wastewater. The choice of adsorbent material, whether natural (e.g., activated carbon) or synthetic (e.g., zeolites, clay minerals, or metal oxides), depends on specific application needs, considering factors like pollutant type and concentration, as well as the desired level of decontamination7.
The efficacy of the adsorption method, particularly utilizing activated carbon, is widely acknowledged due to its extensive surface area, impressive adsorption capacity, and varied pore size distribution. However, the high cost associated with commercial activated carbon poses a constraint on its widespread utilization. Consequently, there is an urgent demand for exploring low-cost alternative adsorbents that can efficiently remove dyes from wastewater. The ongoing research in this field is focusing on the development of low-cost alternative adsorbents derived from agricultural wastes, industrial waste, and by-products, garnering increased attention in recent years8,9. This research direction not only addresses the economic challenges associated with conventional adsorbents but also aligns with sustainability goals by repurposing waste materials for environmental benefit.
Figure 3 illustrates some low-cost adsorbent10. Activated carbon stands out as a potent adsorbent material for removing dyes from wastewater.9 Fig. 4 shows the carbon used as adsorption material in MB removal from 2008 to 202110.
Sources of activated carbon for MB elimination10.
The industrial processes of squeezing oils produce residuals from the squeezing process in large quantities cause serious environmental damage. The use of these residuals from oil extraction as a sorbent for removing the dyes from wastewater of the textile industry provides a solution to both the disposal of the residuals and the removing dyes from the environment. This process can be done through adsorption, where the residuals act as a sponge to absorb the dyes from the wastewater. The use of these residuals not only provides a solution to environmental problems but also adds economic value to what was previously considered waste.9.
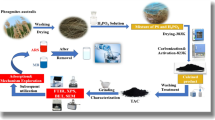
The aim of this study is the investigating of the utilization of the activated carbon which prepared from Raphanus seeds solid residual (ACRS) as a low cost adsorbent for removing of cationic methylene blue dye (MB) from wastewater.
Experimental study
Materials
The chemicals utilized in this investigation procured from the Research-Lab of Fine Chemical Industries. Preparation of solutions was conducted depending on established protocols, and distilled water was utilized throughout the experiment.
Adsorbent material preparation
Raphanus seeds were washed and then dried in the oven at 110 °C to eliminate any moisture content. The squeezing process for the seeds was executed to extract any oil content. The solid residual from this process underwent a drying process in the oven for 5 h at 100 °C and was subsequently incinerated in a muffle furnace at 400 °C for 3 h.
MB stock solution preparation
A 1000 mg/L stock solution of methylene blue (MB) was prepared by dissolving 1 g of MB in 1 l of distilled water. Dilutions of the MB stock solution were achieved by adding distilled water following the principles of dilution. The pH of the solutions was adjusted to the desired values by the addition of (0.1 N) NaOH or 0.1 N H2SO4, maintaining a constant temperature of 25 °C.
Adsorption experiment
The experiments were conducted in batches, stirring Raphanus seeds solid residual (ACRS) from Raphanus seeds with a digital magnetic stirrer MS-H-Pro using a Teflon bar (2 cm length) with a temperature sensor PT 1000 and 250 ml of an aqueous solution of MB at a stirring speed of 300 rpm. Varying pH values from 2 to 8 were employed to understand the pH impact on MB adsorption. The experiments were executed at different temperatures (25, 30, 35, 40, and 45 °C), various initial concentrations (10, 20, 30, 40, and 50 ppm) for MB, and different weights of Raphanus seeds solid residual (ACRS) = (0.1, 0.3, 0.5, 0.7, and 1 g/250 ml). Five milliliters of aliquots were taken from the reaction solution at different time intervals for MB analysis over a contact time of 90 min. The samples were taken at regular time intervals. The UV–Visible spectrophotometer (Varian Flame AA240 Fs; Graphite GTA 120) was used to record the MB concentration at the wavelength of 664 nm. Figure 5 depicts the results.
The % Removal (% adsorption) has been calculated using Eq. (1)
Co and Ct are the initial concentration and the concentration at the equilibrium of MB solution at (t) time (ppm) respectively.
The adsorption parameters consist pH, time(min), concentration of MB (ppm), and Dose (mg/250 ml). The response surface approach i.e. (Box-Behnken's design) has been used for optimization of % removal percent of MB using activated carbon from Raphanus seeds residual11.
Box–Behnken design
Box–Behnken design with four factors were examined at three levels (−1, 0 and 1)12,13. Table 1 illustrates the elements and the levels, taking into four different variables, using the Box–Behnken design of the experiments (BBD), For the statistical analysis, there were a certain number of experiments needed (twenty-seven experimental runs which were carried out to predict the result of five independent factors pH, A (2–7), time(min), B (0–90), concentration of MB (ppm),C (10–50), and Dose (mg/100 ml), D (0.1–0.5) on % removal of the MB from in solution. Experimental conditions and the results are shown in Table 1. A regression analysis was performed to generate a model for the response, and its efficacy was evaluated through the use of ANOVA and F-tests14. the evaluation and improvement of the data process were carried out utilizing the Statistica software, and the interaction effects were represented through the application of a quadratic equation (Eq. 2)
The variables in the equation are defined as follows: Y represents the predicted response, β0 is the intercept term, βi represents the linear effect, βii represents the square effect, and βij represents the interaction effect.
Surface study
The sample's surface area was calculated from N2 adsorption which measured at -196 oC using the BELSORP equipment, Japan. The sample was initially out-gassed under vacuum (10–4 Torr) at 300 °C. The BET surface area (SBET) was calculated by the BET equation15.
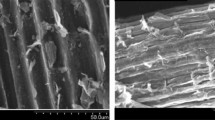
The samples' surface morphology was analyzed using a Scanning Electron Microscope (SEM) model (Quanta 250 FEG, manufactured by FEI Company in the Netherlands). The instrument was employed at an acceleration voltage of 30 kV to increase the resolution of the images. To reduce sample charging due to the electron beam, a 3.5 nm layer of gold was deposited onto the sample surface, effectively minimizing the charging effects.
The FT‒IR and XRD measurement
The Fourier transform infrared (FT-IR) spectrum of the sample was obtained using a PerkinElmer Spectrum 2 instrument. Additionally, the powder X-ray diffraction (XRD) analysis of the pattern was conducted using a PW3040/60 P analytical diffractometer.
Results and discussion
The effect of pH
Evaluate the pH at the point of zero charge (pzc) for the utilized adsorbent. The pH of the solution plays a crucial role in the adsorption process, particularly in the extraction of methylene blue (MB) from an aqueous solution. It influences surface charges, ionization degree, and Adsorbate specification of the adsorbent. Initial parameters for MB adsorption, including a concentration of 10 ppm and a contact time of 90 min, were established to achieve equilibrium. Equilibrium studies, conducted at varying pH values from 2 to 8, aimed to comprehend the pH impact on MB adsorption. The pH of solutions was measured using the HANNA pH 211-Romania pH-meter which clear in Fig. 6.
The observed increase in pH correlated with a higher % removal16), attributed to the acidic state of the bioadsorbent. As the pH increases, the adsorbent's surface becomes negatively charged, leading to an increased adsorption capacity17, reaching its peak at pH 7. Results demonstrate a significant rise in the percentage removal of methylene blue (MB) from 82.7% to 99.4% as the solution's pH increases from 2 to 7. Beyond pH 7, the % removal of MB decreases with an increase in the solution's pH, indicating a reduction in adsorption capacity. The maximum removal obtained at pH 7 was 99.4%, as shown in Fig. 7.
Effect of initial concentration
An investigation into the effect of initial methylene blue MB concentration on its removal onto Raphanus seeds solid residual (ACRS) was performed under controlled conditions. The conditions were maintained at a temperature of 25 °C, a pH of 7, a contact time of 90 min, and an agitation speed of 300 rpm. adsorbent dose = 0.5 g/250 ml for different MB initial concentrations (10,20,30,40and 50 ppm), As depicted in Fig. 8, the percentage removal of methylene blue (MB) decreases with an increase in the initial MB concentration. At low concentrations, MB is adsorbed on the vacant sites of the adsorbent surface and these places are saturated and filled by increasing the concentration. When methylene blue's initial concentration is low, an abundance of active spots on the adsorbent's surface become available for dye adsorption. However, if the initial concentration of MB dye is increased, the number of moles of the MB dye is higher than the number of vacant sites. Therefore, the available sites are quickly saturated and the dye removal rate decreases4.
The result reveals that as the initial concentration of methylene blue (MB) increased from 10 to 50 ppm, the percentage removal of MB decreased from 99.4 to 50.9%, as demonstrated in Fig. 918.
The effect of the adsorbent dose
The impact of the adsorbent dose on the removal of methylene blue (MB) was evaluated at an initial MB concentration of 10 ppm, 90 min contact, and temperature of 25 °C. Different adsorbent doses (ACRS = 0.1, 0.3, 0.5, 0.7, and 1 g/250 ml). According to Fig. 10, the percentage removal of methylene blue (MB) increases with an increase in the amount of adsorbent used. This result is expected because a rise in the amount of adsorbent results in increased surface area with available adsorptive sites. at a fixed initial concentration of sorbate i.e. the increased accessibility increases the number of exchangeable sites or the surface area insured which enhanced the uptake of MB18,19,20.
The outcomes revealed that the uptake or percentage removal of methylene blue (MB) increased from 63.6 to 99.4% as the adsorbent dose increased from 0.1 g/250 ml to 1 g/250 ml, as illustrated in Fig. 11
The effect of temperature
Figure 12 demonstrates the impact of temperature on the percentage removal of methylene blue (MB) from wastewater using (ACRS). Different temperatures (25, 30, 35, 40, and 45 °C) with an initial concentration of 10 ppm of MB were used in this study in the presence of (1 g/250 ml) for (ACRS). The figure indicated that within 90 min, the removal efficiency increased as the temperature increased because Higher temperatures facilitated elimination of methylene blue (MB) by promoting the adsorption at the coordination sites of the adsorbent. This was due to the speeding up of some previously slow steps and the creation of additional activation sites on the adsorbent surface21,22,23.
The result reveals that the uptake of a Fig. 13 demonstrates that the percentage removal of methylene blue (MB) increased from 97.22 to 99.64% with increasing the temperature from 25 to 45 °C.
RSM analysis for optimization of % removal of the methylene blue from aqueous solution
The study of the removal of methylene blue was performed using a Box–Behnken factorial design-based response surface methodology (BBD). The initial step involved investigating the influence of individual variables through single parameter experiments. The levels of selected parameters were then used in a series of experiments, the results of which were recorded and analyzed using statistical plans and response procedures. The details of the selected the parameters' levels are presented in Table 1, while the results of the statistical analysis are outlined in Table 2
The results demonstrated that the % of removal of methylene blue in varying degrees of the four variables were different, due to the impact of various variable levels on the % of removal of MB. As indicated by the results in Table 2, the highest MB removal rate was achieved in test experiment 14, reaching a value of 79%. Additionally, the small discrepancy between the actual and predicted MB removal percentages reflects the high model accuracies24,25. As a result, the study employed a quadratic model of analysis of polynomial equations and the second order to establish the connection between the key elements and the response variable, which is the percentage removal of Methylene Blue, as the optimal model. The validation of the model was performed using ANOVA analysis and the results of variance analysis are presented in Table 3. Multivariate Regression Analysis of the experimental results resulted following second-order polynomial equation, represented as Equation (Eq. 3). The equation gotten through analysis of the experiment's outcomes using multiple regression (Eq. 3) is a second-order polynomial equation that can explain the % removal of Methylene Blue for the significant variables. The linear parameters in the equation are represented by the variables A, B, C, and D.; second-order parameters of the model were A2, B2, C2, and D2, along with the interaction parameters AB, AC, AD, BC, BD, and CD:
where the response variable is represented by "Y", which is the amount of Methylene Blue removed, and "A", "B", "C", and "D" are coding values for the significant test variables, including pH, time (min), the concentration of Methylene Blue (in parts per million), and the adsorbent dose (in grams per 250 ml), respectively.
The importance of the model was assessed using the F-value and p-value parameters. a significant F-value and a small p-value (less than 0.05) suggest that the model accurately predicts the experimental results. In this case, the F-value was 16.21027 and the p-value was 0.000003, indicating a completely significant model. Table 3 revealed that pH, contact time (minutes), initial methylene blue concentration (ppm), and adsorbent dose (g/250 ml) were significant factors affecting the % removal of methylene blue, with initial concentration being the most effective factor.
The predictor variables have been plotted between the X and Y axes, with the response variable displayed on the Z axis, in order to identify the important impact of interactions among the various predictors (Fig. 14A–F) on % removal of MB using activated carbon from Raphanus seeds residual. The response surface's 2D contour plot have been generated in three dimensions for the pairwise combinations of the four factors (AB, AC, AD, BC, BD, CD), with the third factor kept at its central point level (0). As illustrated in Fig. 2E, the interactive effect of the dose and concentration resulted in the highest response.
Response surface methodology of % removal of MB using activated carbon from Raphanus seeds residual: (A) time (min)/pH, (B) effect of concentration of MB (ppm)/pH, (C) adsorbent dose (g/250 ml)/ pH, (D) effect of concentration of MB (ppm)/time(min), (E) adsorbent dose (g/250 ml)/time (min), (F) adsorbent dose (g/250 ml)/concentration of MB (ppm).
Figure 14A, D, E demonstrates some variables (effect of contact time, pH, Conc. of MB, and Adsorbent dose on % removal of MB using activated carbon from Raphanus seeds residual which increased with lengthening the time from 0 to 90 min due to increasing the time, networks of the adsorbent where fast spread at the early stage and then reach to equilibrium after 90 min. So, it was much easier for MB molecules at the first time to penetrate inside the activated carbon from Raphanus seeds residual and include the adsorption sites showed an initial increase with time, however, with further progression, the adsorbent's surface-active groups began to decrease and the percentage removal of MB stabilized26.
Characterization
The raw seeds, known as the residual solid from Raphanus seeds (ACRS), serve as a crucial component in the study. The characterization of these raw seeds is imperative for understanding their properties and potential applications.
The physical and chemical properties of ACRS were analyzed to provide comprehensive insights into its composition. Commonly assessed characteristics include morphology, particle size, surface area, elemental composition, and structural features. Techniques such as scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), and elemental analysis are commonly employed for characterization. Morphological studies using SEM reveal the surface topography, size, and shape of the ACRS particles. XRD provides information about the crystalline structure, while FTIR helps identify functional groups present in the material. Elemental analysis assists in determining the elemental composition, providing data on the presence of key elements.
This characterization is essential for establishing a baseline understanding of the raw seeds' properties, which, in turn, contributes to the interpretation of the adsorption process and the effectiveness of ACRS as an adsorbent.
Surface study
The surface area calculated by BET equation of the sample was 9.25 m2/g. The surface morphology studied by SEM (Fig. 15) shows that the sample is nonporous. It obvious, the structure of the sample has a small surface area. This is attributed to the simple and inexpensive preparation method that matches the environmental conditions, which is the direct burning of the sample at 400 degrees. The observed surface area is likely due to the small voids formed by the aggregation of small pieces of solid matter.
X-ray diffraction analysis (XRD)
Figure 16 presents the X-ray diffraction (XRD) pattern obtained from the radish seeds residual sample, which was exposed to Cu Kα radiation (λ = 1.54 Å) with a 2θ scanning range of 5 to 80 degrees. The broad peak observed at 2θ = 21.10 suggests that the radish seeds residual powder is amorphous in nature27. Additionally, the XRD pattern reveals that the crystalline structure of the radish seeds residual sample is disordered.
Fourier transform infrared spectra
The FTIR spectrum of the sample has been recorded between 4000 and 400 cm−1 at resolution (0.1 cm−1)28. Figure 17 displays the FT-IR spectra of the natural radish seeds residual, with the vibrational assignments presented in Table 1. The broad band with intense intensity at 3414 cm−1 is a result of the hydroxyl (OH) group's stretching vibrations. The bands for the aliphatic C–H stretching occur between 2851 and 2920 cm−1. The stretching of the alkyne group is observed at 2207 cm−1, and the stretching of the carbonyl group is seen at 1615 cm−1. The stretching mode of the C=O group can be found at 1112 cm−1. FTIR spectral data indicate the presence of flavonoids and polyphenols in Radish Seed extract (Table 4). All of the aforementioned groups in the FT-IR analysis facilitate MB adsorption due to the formation of a physical bond.
Adsorption of kinetics
Pseudo-first order model
The pseudo-first-order equation explains adsorption rate. Lagergren (1898) proposed a model of pseudo–first order kinetic which was given by Eq. (2):
the form in Eq. (4) with boundary conditions of t = 0, qt = 0 and t = t
The plot of time (t) versus the natural logarithm of (qe − qt) in Fig. 18 displays a linear relationship. The parameters qe and k1 can be calculated from the intercept and slope of the graph, respectively29.
Pseudo-second order model
The pseudo-second order kinetic rate can be determined using the following Eq. (7):
where k2 indicates the rate constant for the pseudo second order adsorption.(mg g−1 min−1). The Eq. (8) can be derived from Eq. (7) variables first be separated, then integrated, under the conditions (qt = 0 at t = 0 and qt = qe at t = t) yielding a linear expression that describes the adsorption kinetics in a linearized integral form20:
The integral form represented by Eq. (8) that indicated the ratio of time over the adsorbed amount of MB (t/qt) should be a linear function of time, as demonstrated in Fig. 19. Corresponding correlation coefficient values (R2) indicate that the pseudo-first-order model is better obeyed than the pseudo-second-order model. This is because the R2 value for the pseudo-first-order model is slightly higher than the R2 value for the pseudo-second-order model.
Weber and Morris model
The Weber-Morris model, which is also known as the model of intra-particle diffusion, is of significance in the field of liquid systems as it determines the rate of adsorption. Equation (9) provides a general representation of the kinetics involved in this model, where the intercept is a direct function of mass transfer across the boundary layer and the exponent is expected to have a value of 0.5
where: km is the intra- particle diffusion rate constant (mg/g min1/2).
The plot of qt versus t1/2 in Fig. 20 demonstrates a straight line with a slope (km) and an intercept (C). The value of C represents an approximation of the boundary layer thickness, with a greater value indicating a thicker boundary layer.
In conclusion, the kinetic modeling of methylene blue (MB) adsorption onto Raphanus seeds solid residual (ACRS) was investigated using various kinetic models, namely the pseudo-first order, pseudo-second order, and Weber-Morris models.
For the pseudo-first-order model, Lagergren's kinetic equation was applied, revealing a linear relationship between the natural logarithm of (qe − qt) and time (t). The calculated parameters qe and k1 were obtained from the intercept and slope, respectively. However, this model may not be the most accurate representation, as indicated by the correlation coefficient (R2) values.
The pseudo-second-order model, described by the equation dq_t/dt = k2(qe − qt)2, was employed to assess the kinetic rate. The linearized integral form demonstrated that the pseudo-first-order model better adheres to the experimental data, with higher R2 values compared to the pseudo-second-order model.
Furthermore, the Weber-Morris model, focusing on intra-particle diffusion, was utilized. The linear plot of qt versus t1/2 revealed valuable insights into the mass transfer mechanism. The slope (km) and intercept (C) provided information about the intra-particle diffusion rate constant and boundary layer thickness, respectively.
Overall, the comparison of these kinetic models helps elucidate the dominant mechanisms governing the adsorption process. The pseudo-first-order model demonstrated a closer fit to the experimental data, suggesting its appropriateness for describing the kinetics of MB adsorption onto ACRS.
The adsorption of MB onto ACRS was studied under conditions of initial concentration of 10 ppm, contact time of 90 min, agitation rate of 300 rpm, and temperature of 25 °C. The results of the kinetics models and related parameters were calculated and presented in Table 5.
Thermodynamic parameters
The adsorption equilibrium data obtained at different temperatures were used to evaluate the important thermodynamic properties, including the standard Gibbs free energy (ΔG°), standard enthalpy change (ΔH°), and standard entropy change (ΔS°). The standard Gibbs free energy of the MB adsorption process was calculated using Eq. (8).30
The adsorption equilibrium constant (Ke) can be determined for any temperature using Eq. (11).
where Ce (mg/L) represents the equilibrium concentration of MB in the solution, R stands for the gas constant (8.314 J/mol·K), T denotes the absolute temperature in Kelvin, and qe (mg/g) represents the amount of MB adsorbed from the solution at equilibrium.
Equation (12) displays ΔS° and ΔH°, which were obtained from the intercept and slope, respectively, of the Van't Hoff plot of 1/T versus ln(keq) for MB, as depicted in Fig. 21.
Table 6 presents the values of ΔG°, ΔH°, and ΔS°, which illustrate the energetic properties of the divalent MB exchange. The positive ΔH° value suggests a high energy demand for the process, while the positive ΔS° value indicates a favorable interaction between the adsorbate and adsorbent. On the other hand, a negative ΔG° value implies that the adsorption process is feasible and spontaneous31,32,33,34.
Isothermal models
Analysis of equilibrium data is a fundamental aspect in the evaluation of the maximum adsorption capacity of adsorbents. This analysis plays a crucial role in the determination of this capacity. Furthermore, it is critical to formulate an equation that accurately captures the experimental results, as this equation can then be utilized for design purposes. The Freundlich and Langmuir equations are the most commonly used models for the representation of adsorption equilibrium in an adsorption system29,35.
Langmuir adsorption isotherm
The assumption of homogeneous surface adsorption for the solute molecule MB was made, implying that the process occurs via monolayer adsorption without any interaction between the adsorbed species. The Langmuir equation is mathematically represented as Eq. (13)30,36:
In the context of adsorption, The term "qe" represents the equilibrium concentration of the adsorbate (methylene blue, MB) on the adsorbent, expressed in parts per million (ppm). "Ce" represents the equilibrium concentration of MB in the solution, while "qmax" represents the maximum achievable amount of MB that can adsorb onto the surface of the adsorbent, forming a monolayer. "b" is known as the Langmuir constant. The relationship between Ce and qe can be visualized through a plot, which is shown in Fig. 22. This plot demonstrates that the adsorption of MB adheres to the Langmuir isotherm model, as evidenced by the linear relationship between Ce/qe and Ce. The slope of this plot corresponds to the reciprocal of qmax (1/qmax), while the intercept corresponds to the reciprocal of qmax times b (1/qmax.b). The Langmuir isotherm can be characterized by a dimensionless constant referred to as the separation factor or equilibrium parameter, RL.
Freundlich adsorption isotherm
The Freundlich adsorption isotherm is a commonly employed mathematical model for fitting experimental data over a wide range of concentrations. This isotherm accounts for both surface heterogeneity and the exponential distribution of active sites and their energies. The Freundlich model is represented in a nonlinear fashion as follows37:
The linear from of Freundlich model is expressed as follows:
where "Kf" is the Freundlich constant representing the adsorption capacity and "n" is a constant related to the sorption intensity, which varies based on the heterogeneity of the adsorbent. A plot of log qe versus log Ce results in a linear relationship with a slope (1/n) and an intercept (log Kf), as depicted in Fig. 2338,39.
Table 7 provides a comparison between the Langmuir and Freundlich models. In the Langmuir model, the maximum monolayer sorption capacity qmax decreased with increasing doses of (ACRS), however in the Freundlich model.
Conclusion
This study was conducted to examine the adsorption of methylene blue (MB) from an aqueous solution using natural activated carbon derived from Raphanus seeds solid residual (ACRS) as the adsorbent a novel good adsorbent used. The batch equilibrium method was utilized to analyze the adsorption of MB. The results showed that the adsorption capacity of ACRS is 99.4% and it was observed that the adsorption of MB was dependent on the initial concentration of the solution, the dosage of the adsorbent, and the initial pH of the solution. The relationship between the apparent surface area and weight of the sample was analyzed in relation to the amount of adsorbed pollutants, leading to the observation that natural activated carbon from Raphanus seeds solid residual (ACRS) exhibits a high adsorption capacity. The FT-IR analysis revealed that the MB adsorption was due to the formation of a physical bond between the MB and the ACRS. The results of the experiments were found to be in agreement with both the Langmuir and Freundlich isotherm models, with the Langmuir model being more favorable. The results of this study demonstrate that natural activated carbon derived from Raphanus seeds solid residual (ACRS) is a viable option for the removal of methylene blue (MB) from aqueous solutions. The optimization carried out using the Response Surface Methodology (RSM) confirms the conformity between the predicted and measured MB removal, indicating that RSM is a valuable method for optimizing and designing parameters in addition to its uses as an experimental design and statistical analysis tool. The application of RSM serves to streamline the experimental process by reducing the number of necessary trials, thereby minimizing the associated costs of extensive analyses. The Box–Behnken Design (BBD) was utilized within the RSM framework to optimize the percentage removal of methylene blue (%MB). The optimal conditions, as determined by RSM, include a pH of 7, a contact time of 120 min, an initial concentration of 10 ppm, ACRS dosage of 1 gm, and an adsorption temperature of 45 °C. These findings highlight the potential of ACRS under the specified conditions for efficient removal of cationic textile dyes from industrial wastewater.
Permission to collect Raphanus seeds
There is no permission to collect Raphanus seeds because we not work on the seeds, we work on the residual of squeezing oils which is waste get rid of it. the plant seeds were bought from a shop called (Hagg Day for oil seeds). All procedures were conducted in accordance to the guidelines.
Data availability
All data available in the paper and with the corresponding author.
References
Abdus-Salam, N., Ikudayisi-Ugbe, A. V. & Ugbe, F. A. Adsorptive removal of methylene blue from synthetic wastewater using date palm seeds, goethite, and their composite. Acta Sci. Malaysia 5(1), 27–35. https://doi.org/10.26480/asm.01.2021.27.35 (2021).
Gago, D., Chagas, R., Ferreira, L. M., Velizarov, S. & Coelhoso, I. A novel cellulose-based polymer for efficient removal of methylene blue. Membranes 10, 13. https://doi.org/10.3390/membranes10010013 (2020).
Salimi, F., Rahimi, H. & Karamib, C. Removal of methylene blue from water solution by modified nano goethite by Cu. Desalin. Water Treat. 137, 334–344. https://doi.org/10.5004/dwt.2019.22922 (2019).
Akpanudo, N. W. & Chibuzo, O. U. Musanga cecropioides sawdust as an adsorbent for the removal of methylene blue from aqueous solution. Commun. Phys. Sci. 5(3), 362–370 (2020).
Mahmouda, E., Hammad, W. & Hakami, O. Effect of water treatment residuals and cement kiln dust on COD adsorption and heavy metals from textile wastewater. Desalin. Water Treat. 205, 336–342. https://doi.org/10.5004/dwt.2020.26404 (2020).
Kara, H. T., Anshebo, S. T., Sabir, F. K. & Workineh, G. A. Removal of methylene blue dye from wastewater using periodiated modified nanocellulose. Int. J. Chem. Eng. https://doi.org/10.1155/2021/9965452 (2021).
Mandake, M. B., Shingare, S. P. & Swamy, A. Removal of methylene blue from aqueous solution using peanut hull as adsorbent. Glob. Res. J. Chem. 4(2), 53–64 (2021).
Mulushewa, Z., Dinbore, W. T. & Ayele, Y. Removal of methylene blue from textile wastewater using kaolin and zeolite-x synthesized from Ethiopian kaolin. Environ. Anal. Health Toxicol. 36(1), 13. https://doi.org/10.5620/eaht.2021007 (2021).
Lelifajri, R., Supriatno, R. & Susilawati,. Preparation of activated carbon from Gnetum gnemon shell waste by furnace-NaCl activation for methylene blue adsorption. J. Phys. 1940, 012040. https://doi.org/10.1088/1742-6596/1940/1/012040 (2021).
Hamad, H. N. & Idrus, S. Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers 14, 783. https://doi.org/10.3390/polym14040783 (2022).
Masoud, M. et al. Response surface methodology (RSM) and its application for optimization of ammonium ions removal from aqueous solutions by pumice as a natural and low-cost adsorbent. Arch. Environ. Protect. 42(2), 33–43 (2016).
Noha, A. E. E. et al. Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol. Environ. Saf. 145, 57–68 (2017).
Box, G. & Behnken, D. W. Some new three-level designs for the study of quantitative variables. Technometrics 2, 455–475 (1960).
Sarani, S. & Priyabrata, S. Modeling of growth kinetics of isolated Pseudomonas sp. and optimization of parameters for enhancement of xanthine oxidoreductase production by statistical design of experiments. J. Environ. Sci. Health Part A 54(1), 65–78 (2019).
Wang, Z. et al. Comparative analysis of pore structure parameters of coal by using low-pressure argon and nitrogen adsorption. Fuel 309, 122120 (2022).
Abdus-Salam, N., Ugbe, F. A. & Ikudayisi-Ugbe, V. A. Application of natural and synthetic goethite for the removal of Methylene Blue from aqueous solutions. Songklanakarin J. Sci. Technol. 44(2), 332–338 (2022).
Aminuddin, A. F. & Baharim, N. H. Removal of MB in aqueous solution by using bioadsorbent from banana pseudo stem. Asian J. Life Sci. 1(2), 46–50 (2021).
Rana, J., Goindi, G. & Kaur, N. The potential of cellulose acetate for the removal of MB dye from aqueous streams. Int. J. Innov. Technol. Explor. Eng. 8(10), 2278–3075 (2019).
Darweesh, M. A. et al. Adsorption isotherm, kinetic, and optimization studies for copper (II) removal from aqueous solutions by banana leaves and derived activated carbon. S. Afr. J. Chem. Eng. 40, 10–20. https://doi.org/10.1016/j.sajce.2022.01.002 (2022).
El-Sawy, A. M., Abdo, M. H., Darweesh, M. A. & Salahuddin, N. A. Synthesis of modified PANI/CQDs nanocomposite by dimethylgyloxime for removal of Ni (II) from aqueous solution. Surf. Interfaces 26, 101392. https://doi.org/10.1016/j.surfin.2021.101392 (2021).
Ahmed, A. M., Ayed, M. I., Aledkawy, M. A., Darweesh, M. A. & Elmelegy, E. M. Removal of iron, zinc, and nickel ions using nano bentonite and its applications on power station wastewater. Heliyon 7, e063015 (2021).
Ahmed, A. M., Darweesh, M., Mahgoub, F. M. & Hammad, W. A. Adsorption kinetic study for removal of heavy metal from aqueous solution by a natural adsorbent. DJ Eng. Chem. Fuel 4(1), 12–26. https://doi.org/10.18831/djchem.org/2019011002 (2019).
Ahmed, A. M., Aggor, Y. A., Darweesh, M. A. & Noureldeen, M. I. Removal of ferrous ions from city water by activated carbon prepared from palm fronds. DJ J. Eng. Chem. Fuel 3(1), 46–63. https://doi.org/10.18831/djchem.org/2018011005 (2018).
Reza, S., Mohammad, T. S., Mojtaba, A. & Yousef, P. Modeling and optimization of removal of cefalexin from aquatic solutions by enzymatic oxidation using experimental design. Braz. J. Chem. Eng. 35(03), 943–956 (2018).
Mai, S., Basant, Y., Ahmed, Z. & Nahla, A. T. Optimization of chitosan surface response methodology (natural and commercial) used for chromium ion removal from wastewater across different parameters. Sustainability 13, 13494. https://doi.org/10.3390/su132313494 (2021).
El Mohamed, S. M. et al. Carboxylated alginate hydrogel beads for methylene blue removal: Formulation, kinetic and isothermal studies. Desalin. Water Treat. 168, 308–323 (2019).
Rowe, M. C., & Brewer, B. J. AMOPRH: A Statistical Program for Characterizing Amorphous Materials by X-Ray Diffraction. arXiv:1709.04556 (2017).
Jeyaram, S., Sivanukkalai, & Geethakrishnan, T. Vibrational spectroscopic, linear, and nonlinear optical characteristics of anthocyanin extracted from blueberry. Results Opt. 1, 100010. https://doi.org/10.1016/j.rio.2020.100010 (2020).
Abaza, S. F., Ahmed, A. M. & Darweesh, M. A. Effect of magnetic field on rate of production of nickel powder from nickel solution by cementation of zinc. Asian J. Chem. 27(2), 4317–4319 (2015).
Darweesh, M. & Ahmed, A. M. Removal of toxic lead ions in presence of cationic surfactants. Asian J. Chem. 25(11), 5907–5911 (2013).
Darweesh, M. A. et al. Adsorption isotherm, kinetic, and optimization studies for copper (II) removal from aqueous solutions by banana leaves and derived activated carbon. S. Afr. J. Chem. Eng. 40, 10–20. https://doi.org/10.1016/j.sajce.2022.01.002 (2022).
El-Sawy, A. M., Abdo, M. H., Darweesh, M. A. & Salahuddin, N. A. Synthesis of modified PANI/CQDs nanocomposite by dimethylglyoxime for removal of Ni (II) from aqueous solution. Surf. Interfaces 26, 101392. https://doi.org/10.1016/j.surfin.2021.101392 (2021).
Ahmed, A. M., Ayed, M. I., Aledkawy, M. A., Darweesh, M. A. & Elmelegy, E. M. Removal of iron, zinc, and nickel-ions using nano bentonite and its applications on power station wastewater. Heliyon 7, e063015 (2021).
Ahmed, A. M., Darweesh, M., Mahgoub, F. M. & Hammad, W. A. Adsorption kinetic study for removal of heavy metal from aqueous solution by natural adsorbent. DJ Eng. Chem. Fuel 4(1), 12–26. https://doi.org/10.18831/djchem.org/2019011002 (2019).
Ahmed, A. M., Aggor, Y. A., Darweesh, M. A. & Noureldeen, M. I. Removal of ferrous ions from city water by activated carbon prepared from palm fronds. DJ J. Eng. Chem. Fuel 3(1), 46–63. https://doi.org/10.18831/djchem.org/2018011005 (2018).
Darweesh, M. A. & Salem, D. M. S. A. Removal of Cr+6 ions from wastewater in presence of quaternary ammonium salts. Int. J. Basic Appl. Chem. Sci. 2(1), 46–49 (2012).
Darweesh, M. A. Reduction of Cr+6 ions in presence of non-toxic acid. Life Sci. J. 9(2), 2 (2012).
Darweesh, M. A. et al. A unique, inexpensive, and abundantly available adsorbent: composite of synthesized silver nanoparticles (AgNPs) and banana leaves powder (BLP). Heliyon 8, e09279. https://doi.org/10.1016/j.heliyon.2022.e09279 (2022).
Zhou, M. et al. Scalable fabrication of metallopolymeric superstructures for highly efficient removal of methylene blue. Nanomaterials 9, 1001. https://doi.org/10.3390/nano9071001 (2019).
Acknowledgements
The author thanks the Laboratory, Faculty of Engineering, Tanta University, Engineering, Egypt, for their assistance during this work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors extend their appreciation to the Deputyship for research & innovation Ministry of Education in Saudi Arabia for funding the research work through project number ISP22-4.
Author information
Authors and Affiliations
Contributions
Wafaa .A.Hammad . Work the experimental work and wrote the final version of the paper Mona. A. Darweesh Wrote the idea and revised the final version of the article. Nasser Zouli wrote the first version of the paper. M. H. A.Amr revised some experimental data and shared it in the paper's first version writing. Basant Eweida made the experimental data on ANOVA and made mathematical modeling for the article. Samir M.Osman make the revised manuscript after first version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hammad, W.A., Darweesh, M.A., Zouli, N. et al. Adsorption of cationic dye onto Raphanus seeds: optimization, adsorption kinetics, thermodynamic studies. Sci Rep 14, 17827 (2024). https://doi.org/10.1038/s41598-024-66761-z
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-66761-z
Keywords
This article is cited by
-
Preparation and Selective Adsorption Performance for Norfloxacin of Molecularly Imprinted Polymer Co-MOF/CNT@MIPs
Korean Journal of Chemical Engineering (2026)
-
Exploring the efficiency of chemically activated palm frond carbon in heavy metal adsorption a modeling approach
Scientific Reports (2025)
-
High efficiency adsorption of hexavalent chromium using bioderived activated carbon kinetics, isotherms, and thermodynamics
Scientific Reports (2025)
-
Natural cellulose fibers from Agave Americana L. ASPARAGACEAE as an effective adsorbent for mercury in aqueous solutions
Adsorption (2025)
-
Hybrid adsorption–photocatalysis composites: a sustainable route for efficient water purification
Materials for Renewable and Sustainable Energy (2025)