Abstract
Diffusion Kurtosis Imaging (DKI)-derived metrics are recognized as indicators of maturation in neonates with low-grade germinal matrix and intraventricular hemorrhage (GMH-IVH). However, it is not yet known if these factors are associated with neurodevelopmental outcomes. The objective of this study was to acquire DKI-derived metrics in neonates with low-grade GMH-IVH, and to demonstrate their association with later neurodevelopmental outcomes. In this prospective study, neonates with low-grade GMH-IVH and control neonates were recruited, and DKI were performed between January 2020 and March 2021. These neonates underwent the Bayley Scales of Infant Development test at 18 months of age. Mean kurtosis (MK), radial kurtosis (RK) and gray matter values were measured. Spearman correlation analyses were conducted for the measured values and neurodevelopmental outcome scores. Forty controls (18 males, average gestational age (GA) 30 weeks ± 1.3, corrected GA at MRI scan 38 weeks ± 1) and thirty neonates with low-grade GMH-IVH (13 males, average GA 30 weeks ± 1.5, corrected GA at MRI scan 38 weeks ± 1). Neonates with low-grade GMH-IVH exhibited lower MK and RK values in the PLIC and the thalamus (P < 0.05). The MK value in the thalamus was associated with Mental Development Index (MDI) (r = 0.810, 95% CI 0.695–0.13; P < 0.001) and Psychomotor Development Index (PDI) (r = 0.852, 95% CI 0.722–0.912; P < 0.001) scores. RK value in the caudate nucleus significantly and positively correlated with MDI (r = 0.496, 95% CI 0.657–0.933; P < 0.001) and PDI (r = 0.545, 95% CI 0.712–0.942; P < 0.001) scores. The area under the curve (AUC) were used to assess diagnostic performance of MK and RK in thalamus (AUC = 0.866, 0.787) and caudate nucleus (AUC = 0.833, 0.671) for predicting neurodevelopmental outcomes. As quantitative neuroimaging markers, MK in thalamus and RK in caudate nucleus may help predict neurodevelopmental outcomes in neonates with low-grade GMH-IVH.
Similar content being viewed by others
Introduction
Germinal Matrix Hemorrhage-Intraventricular Hemorrhage (GMH-IVH) is a prevalent intracranial complication in preterm infants, despite advancements in their care1. Neonates with GMH-IVH continue to pose a significant concern in this vulnerable population. Approximately 25 to 50% of cases involve clinically silent GMH-IVH, detected through routine cranial ultrasound screening2. According to the classification of Volpe3, the term "low-grade" GMH-IVH encompasses Grade I (bleeding confined to the germinal matrix or GMH plus IVH occupying < 10% of the lateral ventricular area.) or Grade II (GMH plus IVH occupying 10 to 50% of the lateral ventricular area). These neonates are at risk of neurodevelopmental impairment4. However, such impairment often takes over a year to manifest in those with low-grade GMH-IVH. Recognizing the benefits of early intervention, including rehabilitative treatment for improved prognosis, researchers are actively seeking early biomarkers in these neonates. MRI has gained prominence among imaging tools for marking these biomarkers, given its noninvasive nature and ability to provide qualitative and quantitative insights into the brains of neonates with low-grade GMH-IVH.
Neonates experiencing low-grade GMH-IVH may exhibit subtle white matter microstructural abnormalities that often go undetected with conventional cranial ultrasonography and routine MRI. Addressing this challenge, diffusion kurtosis imaging (DKI) emerges as a valuable MRI tool capable of quantifying these microstructural abnormalities5. Among the DKI metrics, mean kurtosis (MK) and radial kurtosis (RK) stand out, offering direct physical relevance to the diffusion tensor6. MK reflects average diffusion kurtosis, while RK represents kurtosis along the radial direction, providing insight into the microstructural complexity of imaged tissue7. While conventional diffusion tensor imaging (DTI) sequence has shown potential in detecting mild GMH-IVH, they are limited by their reliance on a Gaussian diffusion model, unable to address the challenge of cross-fiber orientation in brain tissue8. In contrast, DKI, rooted in the non-Gaussian diffusion of water in biological systems, presents a promising technology. Its applications extend to various conditions, including prematurity9, spinal cord imaging10, and acute bilirubin encephalopathy11. Associations with neurodevelopmental outcomes have been explored, revealing adverse motor and language abilities in preterm infants with severe intraventricular hemorrhage12. Notably, these findings suggest a potential relationship between regional DKI-derived metrics and neurodevelopmental outcomes in preterm infants with severe intraventricular hemorrhage, yet few studies have specifically evaluated the correlation between DKI-derived metrics and neurodevelopmental outcomes in neonates with low-grade GMH-IVH.
This study aimed to investigate whether early DKI metrics in neonates with low-grade GMH-IVH were associated with subsequent neurodevelopmental outcomes. To test this hypothesis, we prospectively enrolled neonates, measuring regional fractional anisotropy (FA), MK, and RK derived from DKI. Subsequently, we correlated these values with neurodevelopmental outcomes assessed by the Bayley Scales of Infant Development at 18 months of age.
Materials and methods
Participants
This is a retrospective study conducted at our institution, and written informed consent was obtained from each patient's guardian. The study design received approval from the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University, and the protocol of the project conformed to the ethical standards of the Helsinki Declaration of 1975, revised in 2008. All data were anonymized before processing the MRI data.
Preterm neonates admitted to our hospital between January 2020 and March 2021 were enrolled. The inclusion criteria for the case group were (a) neonates diagnosed with low-grade GMH-IVH through transcranial ultrasound (Supplemental 1), (b) gestational age (GA) less than 34 weeks, (c) birth weight less than 1500 g, and (d) completion of the BSID-III scale assessment at 18 months. The inclusion criteria for the control group were (a) GA less than 34 weeks, (c) birth weight less than 1500 g, and (d) completion of the BSID-III scale assessment at 18 months. Exclusion criteria for both case and control groups included neonates with (a) congenital malformation, infection, or metabolic diseases, (b) brain lesions other than low-grade GMH-IVH, (c) any structural abnormality on prior ultrasounds, (d) incomplete DKI imaging, and (e) inadequate coverage of the whole brain or motion artifacts on the MRI. These excluded conditions have been linked to poor neurodevelopmental outcomes13 and may obscure or confound our evaluation of the significance of quantified MRI values for outcome prediction. Initially, there were a total of 70 neonates recruited (low-grade GMH-IVH group (n = 30) and control group (n = 40)) that met the inclusion and exclusion criteria.
MRI data acquisition
Neonates were scanned at a corrected GA of 38 weeks ± 1 using a 3 T MRI scanner (Pioneer, GE Healthcare, Milwaukee, WI) (total n = 70), equipped with a dedicated 8-channel phased-array head coil. To minimize movement, neonates were positioned on an immobilization pillow. Mini muffs (Natus Medical Inc., San Carlos, CA) were utilized to reduce noise from the MRI scanner. Throughout the procedure, all neonates were monitored using pulse oximetry and electrocardiographic monitoring. Detailed MRI acquisition protocols are listed in Table S1.
An advanced diffusion imaging technique, DKI, corrects Gaussian model defects and quantifies the deviation of each voxel from free diffusion. DKI sequence parameters included TR = 2000 ms, TE = 103 ms, employing 30 diffusion directions for b-values of 0, 1000, and 2000 mm2/s, respectively. Slice thickness was set at 4.0 mm without a gap, the field of view at 256 mm × 256 mm, and the acquisition time at 7.5 min.
MRI data analysis
The regular MRI data were transmitted to the hospital's PACS for incidental findings screening. Three radiologists, with 30, 10, and 5 years of experience in neuroradiology, respectively, and blinded to clinical data apart from basic participant information displayed on PACS, independently reviewed each scan soon after its completion. This review aimed to ensure the absence of clinically diagnosable incidental findings, and no variability measurement was conducted for the screening process.
All MR imaging data preprocessing and analysis were performed using the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl). Skull stripping was performed using the brain extraction tool (BET) function. Eddy current and head motion artifact were corrected in FSL by aligning diffusion-weighted images to the first b0 image with an affine DKI transformation with 12 degrees of freedom. The three commonly used DKI measures, including FA, MK, and RK were calculated using standard methods14. Additionally, we utilized ITK-SNAP 4.0 (http://www.itksnap.org/pmwiki/pmwiki.php) for region of interest (ROI) delineation15. ITK-SNAP offers semi-automatic segmentation via active contour methods, manual delineation, and image navigation, along with various supporting utilities. ROIs were drawn for the thalamus, caudate nucleus, posterior limbs of the internal capsule (PLIC), genu of the corpus callosum (GCC), and occipital lobe. Because these ROIs all have been related to the long-term outcome3,16,17,18. We found that DTI measurement comparison shows that there is no statistical difference between the two hemispheres of the brain, so the average value of both sides is taken as the result of ROI. Each regional value was obtained for both right and left hemispheres, and their mean values were utilized for the final analysis19. Two neuroradiologists, with 12 and 15 years of experience, independently measured all ROIs on T1WI. They reached a consensus on the image outline before measurement, and the final data used for analysis represented the average of their measurements. Subsequently, a third neuroradiologist, possessing 15 years of experience, examined the results.
Neurodevelopmental outcomes at 18 months
Neurodevelopmental outcomes were evaluated in neonates at 18 months of corrected age using the 2nd edition of the Bayley Scales of Infant and Toddler Development. A trained examiner conducted the examination, assessing the Mental Development Index (MDI) and the Psychomotor Development Index (PDI). The MDI measures cognition, evaluating environmental responsiveness, sensory and perceptual abilities, learning, memory, and early language and communication skills. The PDI assesses motor skills, covering both gross and fine motor skills. The Bayley Scales of Infant and Toddler Development is a widely used test, with higher scores indicating better outcomes. Additional details are available in S2.
Statistical analysis
To compare clinical characteristics and MRI findings between the two groups classified according to the neurodevelopmental outcome, we conducted the t test, Mann–Whitney U test, or χ2 analysis. Group differences in DKI measures were adjusted for confounding parameters (sex, gestational age and weight) by using a general linear regression model20. Due to the large number of ROIs and MRI metrics in this study, we employed Storey False Discovery Rate (FDR) correction for multiple comparisons. The FDR P Value < 0.1 was deemed statistically significant. Additionally, Spearman correlations were utilized to assess the relationship between quantitative MRI metrics and neurodevelopmental outcome scores. Receiver operating characteristic (ROC) analysis was performed to recognize the diagnostic performance of DKI metrics in different brain regions for predicting adverse neurodevelopmental. Intraclass correlation coefficients (ICC) were computed to analyze interobserver agreement; further details are available in Table S2. SPSS software, version 28.0 (IBM SPSS Statistics), was utilized for the analysis. A p value < 0.05 was deemed statistically significant.
Results
Participants
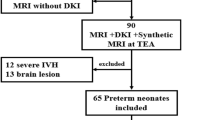
A retrospective evaluation was conducted on brain MRI studies of 245 neonates. DKI and synthetic MRI sequences were performed in 145 of these neonates. Exclusions were made for 35 neonates (24.1%): 16 had other brain lesions (including 13 punctate WM lesions, two arteriovenous malformations, and one callosal dysgenesis), 4 had severe GMH-IVH, 2 had incomplete DKI, 3 had brain areas not covered by the scans, and 10 had DKI sequences affected by motion artifacts or other artifacts. Consequently, the MRI studies of 110 out of 145 (75.9%) neonates were included in the imaging cohort. Forty neonates with lost follow-up using Bayley—II Scales were excluded. The study ultimately included 70 neonates, with 30 cases showing the presence of low-grade GMH-IVH (illustrated in Fig. 1). Among these, 40 (57.1%, 40/70) were controls (17 females, average GA 30 weeks ± 1.3, corrected GA at MRI scan 38 weeks ± 1), while the remaining 30 (42.9%, 30/70) were neonates with low-grade GMH-IVH (22 females, average GA 30 weeks ± 1.5, corrected GA at MRI scan 38 weeks ± 1). Detailed demographic information for the neonates and their mothers is presented in Table 1. Analysis of this matched cohort revealed no differences in perinatal, maternal, and other factors.
MRI characteristics
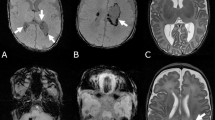
Representative examples from two distinct neurodevelopmental outcome groups underwent DKI scans. An infant without encephalopathy is illustrated in Fig. 2A–E. This male neonate, born at 31 weeks with a weight of 1640 g, had a corrected gestational age of 38 weeks at the MRI scan. The Bayley-II scores for MDI and PDI were 125 and 121, respectively. Another neonate with low-grade GMH-IVH is depicted in Fig. 2F–J. This male neonate, born at 30 weeks with a weight of 1693 g, had a corrected gestational age of 38 weeks at the MRI scan. The Bayley-II scores for MDI and PDI were 93 and 100, respectively.
Representative neonatal images (T1WI, T2WI, FA, MK, and RK) without (top row) and with (bottom row) low-grade GMH-IVH. (A-E) showcases a male neonate with a gestational age of 31 weeks, corrected gestational age at MRI scan is 38 weeks, with Bayley—II scores of MDI and PDI at 125 and 121. (F-J) features a male neonate with a gestational age of 30 weeks, corrected gestational age at MRI scan is 38 weeks, and Bayley—II scores of MDI and PDI at 93 and 100. DKI Diffusion kurtosis imaging, FA Fractional anisotropy, MK mean kurtosis, RK radial kurtosis, low-grade GMH-IVH low-grade Germinal Matrix Hemorrhage-Intraventricular Hemorrhage, Bayley—II Bayley Scales of Infant and Toddler Development 2nd Edition, MDI Mental Development Index, PDI Psychomotor Development Index.
Neonates with low-grade GMH-IVH exhibited lower MK values in the PLIC compared to controls (0.541 ± 0.134 and 0.623 ± 0.189, respectively; P < 0.05 and FDR P < 0.1) and lower RK values (0.704 ± 0.178 and 0.930 ± 0.241, respectively; P < 0.001 and FDR P < 0.1). Additionally, these neonates demonstrated significantly lower MK (0.327 ± 0.073 and 0.431 ± 0.087, respectively; P < 0.05 and FDR P < 0.1) and RK (0.411 ± 0.135 and 0.574 ± 0.106, respectively; P < 0.001 and FDR P < 0.1) values in the thalamus. Similarly, neonates with low-grade GMH-IVH presented significantly lower RK than controls (0.384 ± 0.088 and 0.416 ± 0.131, respectively; P < 0.05 and FDR P < 0.1) in the caudate nucleus. Therefore, MK and RK in the PLIC, MK and RK in the thalamus and RK in the caudate nucleus exhibited significantly differences between the two groups. No regions of interest (ROIs) exhibited significantly lower FA values in low-grade GMH-IVH neonates compared to controls (P > 0.05 and FDR P > 0.1), and no differences in GCC were observed (P > 0.05 and FDR P > 0.1) (Table 2). Additionally, we selected the occipital lobe as the control ROI, and there were no differences between the two groups. The ICCs for DKI-derived metric measurements in each brain region were generally good or excellent (range, 0.756–0.985) (Supplementary Table 1).
Neurodevelopmental outcomes
Lower neurodevelopmental outcomes were observed in infants with low-grade GMH-IVH. Out of the 70 neonates subjected to neurodevelopmental assessment using Bayley-II scales of infant development at 18 months of age, significant differences emerged between neonates with low-grade GMH-IVH and controls across various parameters, as outlined in Table 3. Specifically, there were notable disparities in MDI scores (91.533 ± 8.775 and 120.344 ± 6.409 for low-grade GMH-IVH and controls, respectively; P < 0.001). Similarly, significant differences were noted in PDI scores between neonates with low-grade GMH-IVH and controls (91.533 ± 10.007 and 121.922 ± 7.912, respectively; P < 0.001).
MRI metrics are associated with neurodevelopmental outcomes
Notably, the MK value in the thalamus of neonates with low-grade GMH-IVH exhibited a significant association with MDI (r = 0.810, 95% CI, 0.695–0.13; P < 0.001) and PDI (r = 0.852, 95% CI, 0.722–0.912; P < 0.001) scores (see Fig. 3A,B). Concurrently, the RK value in the caudate nucleus demonstrated a significant positive correlation with MDI (r = 0.496, 95% CI, 0.657–0.933; P < 0.001) and PDI (r = 0.545, 95% CI, 0.712–0.942; P < 0.001) scores for neonates with low-grade GMH-IVH (see Fig. 3I,J). Notably, no significant correlations were observed between other DKI-derived metrics and neurodevelopmental outcomes (see Fig. 3C–H).
Correlation between DKI-derived metrics or volume and MDI and PDI scores at 18 months for neonates with low-grade GMH-IVH. (A–D) Pearson correlation between MK or RK in the thalamus and MDI or PDI scores. (E–H) The relationship between MK or RK in the PLIC and MDI or PDI scores. (I,J) The correlation between RK in the caudate nucleus and MDI or PDI scores. DKI Diffusion kurtosis imaging, MDI Mental Development Index, PDI Psychomotor Development Index, MK mean kurtosis, RK radial kurtosis, low-grade GMH-IVH low-grade Germinal Matrix Hemorrhage-Intraventricular Hemorrhage, PLIC Posterior Limbs of the Internal Capsule.
Diagnostic performance of DKI metrics in different brain regions for predicting adverse neurodevelopmental outcomes
The ROC curve and AUC were used to assess diagnostic performance of MK and RK in thalamus and caudate nucleus for predicting MDI and PDI. The differential diagnosis ability of MK and RK was found to be good in thalamus (AUC = 0.866 and 0.787) and caudate nucleus (AUC = 0.833 and 0.671) (see Table 4).
Discussion
This case–control study reveals abnormal microstructural alterations in the brains of neonates with low-grade GMH-IVH, suggesting a potential link between these abnormalities and neurodevelopmental outcomes at 18 months within this subgroup.
Specifically, our findings highlight altered DKI metrics in early- myelinated supratentorial white matter regions, including the PLIC, GCC, thalamus, and caudate nucleus, in neonates with low-grade GMH-IVH compared to controls. We observed reduced MK and RK, with no significant differences in FA values. MK is generally proportional to the heterogeneity and complexity of brain microstructure. An increase in MK may indicate more densely packed cells or higher cellular complexity, while a decrease may suggest loss of cellular structure21. Low-grade GMH-IVH can trigger inflammation in adjacent in adjacent white matter through activated microglia, red blood cell passage, and red blood cell degradation22. Such conditions can impair both white and gray matter in this toxic environment23. Additionally, RK evaluation may serve as a novel method for assessing the integrity of cell membranes and surrounding myelin sheaths7. Intriguingly, simultaneous decreases in MK and RK have been observed in other regions of brain injury resulting from axonal loss, followed by a decrease in the extracellular matrix and glial cells12,24,25. On the other hand, the lack of significant changes in FA values suggests that MK and RK metrics can reflect subtle differences, likely indicating milder microstructural alterations, such as minimal fiber loss without gross tissue damage7. So MK and RK values can be more sensitive to detect damage to microstructures.
Our investigation identified significant deviations in MK within the thalamus and RK within the caudate nucleus, all of which emerged as pivotal factors associated with adverse outcomes in both MDI and PDI. This aligns seamlessly with existing literature that underscores the impact of low-grade GMH-IVH on macroscopic thalamus and caudate nucleus development, thereby contributing to compromised neurodevelopmental outcomes12,26,27. Supporting our findings, Han Y et al. highlighted a correlation between neurodevelopmental outcomes and RK values in the caudate nucleus among neonates with encephalopathy21. Additionally, Jeanie L et al.'s prior work demonstrated a connection between thalamus abnormalities and MDI / PDI scores at 18 months of age28. The noteworthy impact of low-grade GMH-IVH on thalamus and caudate nucleus development stems from the germinal matrix's extension along the lateral ventricles until approximately 28 weeks' gestational age, influencing the crucial development of these structures29. The thalamus, responsible for processing all sensory information (except smell)30, and the caudate nucleus, playing a critical role in various higher neurological functions31, are particularly susceptible to impairment when brain damaged. Our study revealed a clear association between alterations in white matter microstructure, and subsequent neurodevelopmental impairments at 18 months of age in a subset of the neonates under investigation. To better discern infants at risk of adverse neurological outcomes due to low-grade GMH-IVH, we conducted an ROC analysis to assess the diagnostic efficacy of DKI metrics across various ROIs. Our findings revealed that MK and RK exhibited strong predictive capabilities, particularly in the thalamus and caudate nucleus regions, for forecasting MDI and PDI outcomes. The maturation of the thalamus and caudate nucleus is closely linked to both cognitive and physical functionalities32,33. MK and RK are sensitive indicators for detecting the development level of nerve fiber tracts34. Therefore, we posit that the identification of abnormal fiber tract development in these specific ROIs by MK and RK serves as a predictive marker for alterations in both MDI and PDI parameters.
Several limitations characterize our study. The relatively small sample size, although justified by the low incidence rate of low-grade GMH-IVH, necessitates cautious interpretation of the findings. Additionally, the neurodevelopmental follow-up was relatively short, limited to a subset of neonates at 18 months of age during the study period. Another constraint is the reliance on a single system for data collection. Future investigations should aim to gather data from diverse systems and time points to enhance both volume and variety of information.
Conclusions
Our study underscores the significant associations between MK in the thalamus, and RK in the caudate nucleus alterations with cognitive and motor outcome scores at 18 months. These findings emphasize the potential relevance of these specific metrics as early indicators of cognitive and motor development in neonates with low-grade GMH-IVH. Precise anticipation of outcomes based on early DKI metrics holds the potential to enable earlier interventions for infants at risk and could greatly enhance the efficacy of intervention strategies. We hope to leverage this information to enhance clinical outcomes for neonates affected by low-grade GMH-IVH.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- DKI:
-
Diffusion kurtosis imaging
- MK:
-
Mean kurtosis
- RK:
-
Radial kurtosis
- DTI:
-
Diffusion tensor imaging
- GMH-IVH:
-
Germinal matrix hemorrhage-intraventricular hemorrhage
- GA:
-
Gestational age
- MDI:
-
Mental development index
- PDI:
-
Psychomotor development index
- ROI:
-
Region of interest
- PLIC:
-
Posterior limbs of the internal capsule
- GCC:
-
Genu of corpus callosum
References
Parodi, A., Govaert, P., Horsch, S., Bravo, M. C. & Ramenghi, L. A. Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr. Res. 87(Suppl 1), 13–24 (2020).
Parodi, A. et al. Low-grade intraventricular hemorrhage: Is ultrasound good enough?. J. Maternal-Fetal Neonatal Med. 28(Suppl 1), 2261–2264 (2015).
Volpe, J. J. Impaired neurodevelopmental outcome after mild germinal matrix-intraventricular hemorrhage. Pediatrics 136(6), 1185–1187 (2015).
Brouwer, A. J., Groenendaal, F., Benders, M. J. & de Vries, L. S. Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: What is new?. Neonatology 106(4), 296–303 (2014).
Jensen, J. H., Helpern, J. A., Ramani, A., Lu, H. & Kaczynski, K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 53(6), 1432–1440 (2005).
Wu, E. X. & Cheung, M. M. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 23(7), 836–848 (2010).
Steven, A. J., Zhuo, J. & Melhem, E. R. Diffusion kurtosis imaging: An emerging technique for evaluating the microstructural environment of the brain. AJR Am. J. Roentgenol. 202(1), W26-33 (2014).
Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66(1), 259–267 (1994).
Zhao, X. et al. The value of diffusion kurtosis imaging in detecting delayed brain development of premature infants. Front. Neurol. 12, 789254 (2021).
Trò, R. et al. Diffusion kurtosis imaging of neonatal spinal cord in clinical routine. Front. Radiol. 2, 794981 (2022).
Zheng, H., Lin, J., Lin, Q. & Zheng, W. Magnetic resonance image of neonatal acute bilirubin encephalopathy: A diffusion kurtosis imaging study. Front. Neurol. 12, 645534 (2021).
Guo, L. M. et al. Microstructural changes of white matter assessed with diffusional kurtosis imaging in extremely preterm infants with severe intraventricular hemorrhage. Front. Pediatr. 10, 1054443 (2022).
Arthur, R. Magnetic resonance imaging in preterm infants. Pediatr. Radiol. 36(7), 593–607 (2006).
Wang, M. L., Wei, X. E., Yu, M. M. & Li, W. B. Cognitive impairment in mild traumatic brain injury: A diffusion kurtosis imaging and volumetric study. Acta Radiologica (Stockholm, Sweden: 1987) 63(4), 504–512 (2022).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31(3), 1116–1128 (2006).
Reubsaet, P. et al. The impact of low-grade germinal matrix-intraventricular hemorrhage on neurodevelopmental outcome of very preterm infants. Neonatology 112(3), 203–210 (2017).
Xi, G., Keep, R. F. & Hoff, J. T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 5(1), 53–63 (2006).
Sharma, D. R., Agyemang, A. & Ballabh, P. Cerebral gray matter injuries in infants with intraventricular hemorrhage. Semin. Perinatol. 46(5), 151595 (2022).
Zhang, C. et al. Evaluation of white matter microstructural alterations in premature infants with necrotizing enterocolitis. Quant. Imaging Med. Surg. 13(10), 6412–6423 (2023).
Tortora, D. et al. The effects of mild germinal matrix-intraventricular haemorrhage on the developmental white matter microstructure of preterm neonates: A DTI study. Eur. Radiol. 28(3), 1157–1166 (2018).
Han, Y., Wu, P., Tian, J., Chen, H. & Yang, C. Diffusion kurtosis imaging and diffusion weighted imaging comparison in diagnosis of early hypoxic-ischemic brain edema. Eur. J. Med. Res. 28(1), 159 (2023).
Atienza-Navarro, I., Alves-Martinez, P., Lubian-Lopez, S. & Garcia-Alloza, M. Germinal matrix-intraventricular hemorrhage of the preterm newborn and preclinical models: Inflammatory considerations. Int. J. Mol. Sci. 21(21), 8343 (2020).
Gilard, V., Tebani, A., Bekri, S. & Marret, S. Intraventricular hemorrhage in very preterm infants: A comprehensive review. J. Clin. Med. 9(8), 2447 (2020).
Ouyang, M. et al. Differential cortical microstructural maturation in the preterm human brain with diffusion kurtosis and tensor imaging. Proc. Natl. Acad. Sci. U. S. A. 116(10), 4681–4688 (2019).
Gullion, L. et al. The impact of early neuroimaging and developmental assessment in a preterm infant diagnosed with cerebral palsy. Case Rep. Pediatr. 2019, 9612507 (2019).
Argyropoulou, M. I. et al. Is low-grade intraventricular hemorrhage in very preterm infants an innocent condition? structural and functional evaluation of the brain reveals regional neurodevelopmental abnormalities. AJNR Am. J. Neuroradiol. 41(3), 542–547 (2020).
Vohr, B. R. Neurodevelopmental outcomes of premature infants with intraventricular hemorrhage across a lifespan. Semin. Perinatol. 46(5), 151594 (2022).
Cheong, J. L. et al. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 121(6), e1534-1540 (2008).
Tortora, D. et al. Regional impairment of cortical and deep gray matter perfusion in preterm neonates with low-grade germinal matrix-intraventricular hemorrhage: An ASL study. Neuroradiology 62(12), 1689–1699 (2020).
Als, H. et al. Early experience alters brain function and structure. Pediatrics 113(4), 846–857 (2004).
Gonzalez-Moreira, E. et al. Prevention of neurological sequelae in preterm infants. Brain Sci. 13(5), 753 (2023).
Herrero, M. T., Barcia, C. & Navarro, J. M. Functional anatomy of thalamus and basal ganglia. Child’s Nerv. Syst. ChNS 18(8), 386–404 (2002).
Driscoll ME, Bollu PC, Tadi P: Neuroanatomy, Nucleus Caudate. In: StatPearls. edn. Treasure Island (FL) ineligible companies. Disclosure: Pradeep Bollu declares no relevant financial relationships with ineligible companies. Disclosure: Prasanna Tadi declares no relevant financial relationships with ineligible companies. (StatPearls Publishing).
DiPiero, M., Rodrigues, P. G., Gromala, A. & Dean, D. C. 3rd. Applications of advanced diffusion MRI in early brain development: A comprehensive review. Brain Struct. Funct. 228(2), 367–392 (2023).
Funding
This research was funded by The National Natural Science Foundation of China, Grant No. 82371929.
Author information
Authors and Affiliations
Contributions
Guarantors of integrity of entire study, Chunxiang Zhang, Xiaoan Zhang, Xin Zhao; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, Zihe Wang, Chi Qin, Jinze Yang, Yu Lu,; clinical studies, Lin Lu, Meiying Cheng, Honglei Shang; statistical analysis, Zitao Zhu; and manuscript editing, Chunxiang Zhang, Kaiyu Wang, Brianna F. Moon, Sheng Shen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, C., Cheng, M., Zhu, Z. et al. Associations between diffusion kurtosis imaging metrics and neurodevelopmental outcomes in neonates with low-grade germinal matrix and intraventricular hemorrhage. Sci Rep 14, 16455 (2024). https://doi.org/10.1038/s41598-024-67517-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67517-5