Abstract
Understanding carbon dioxide emissions variability in volcanic regions is vital for detecting instabilities in the subvolcanic plumbing system, crucial for managing both volcanic and environmental risks. While changes in magmatic sources drive these variations, non-magmatic processes can complicate signal interpretation, especially in caldera environments. Here, geothermal systems can sequester CO2 within the bedrock through hydrothermal calcite precipitation, significantly impacting surface-level CO2 emissions. Unfortunately, few studies have explored this phenomenon, examining hydrothermal calcite origins and their effects on carbon balances and temporal gaseous patterns in active volcanic settings. Our study developed a specialized methodology for quantifying CO2 sequestered in hydrothermal calcites within alkaline caldera systems. We focused on analyzing hydrothermal calcite in lithics from volcanic deposits of eruptions of varying ages, Volcanic Explosivity Index (VEI), and eruptive vent locations to enhance the representativeness of the entire caldera bedrock. Unlike core samples from geothermal wells, which are infrequent and limited to specific depths, lithics can be easily collected, offering a comprehensive understanding of CO2 sequestration. Through extensive 3D textural characterization and isotopic investigations on hydrothermal calcite within lithic fragments from selected alkaline volcanic deposits in the Campi Flegrei caldera, our findings emphasized the significant influence of calcite sinks on the overall CO2 budget released by volcanoes throughout their evolution.
Similar content being viewed by others
Introduction
Over the past decade, the study of CO2 emissions from volcanic systems has garnered significant attention due to its potential as a critical indicator of changes in the subvolcanic plumbing system and its implications for mitigating volcanic and environmental risks1,2,3. Understanding the spatial and temporal variations in CO2 fluxes is essential for assessing volcano dynamics and potential hazards, as well as for developing effective risk management strategies. While the primary source of these CO2 variations is typically attributed to magmatic processes, it is increasingly evident that additional non-magmatic factors can play a significant role in modulating the observed CO2 emissions4,5,6,7,8,9.
This is even more relevant in the case of calderas as they develop extensive geothermal systems where complex interactions between ascending magmatic volatiles and hydrothermal fluids can lead to the sequestration of CO2 through hydrothermal calcite precipitation in the subsurface rocks. This can profoundly influence the evolution of geochemical parameters, making their interpretation challenging and potentially misleading.
A prominent case study representing the complexities of caldera volcanic dynamics is the active Campi Flegrei caldera, situated in the metropolitan area of Naples, Italy10,11,12,13,14. After a long period of subsidence following the last Monte Nuovo eruption, that occurred in the AD 153815,16, the caldera has experienced unrest episodes, such as those observed in 1950–1952, 1970–1972, and 1982–1984, with the most recent ongoing since 200517,18,19,20. These bradyseismic (gradual ground movements) crises were characterized by intense variations in geophysical (ground uplift and seismicity) and geochemical (CO2 emissions and fumarolic compositions) signals, which led the Italian Civil Protection to raise the volcano alert from the base (green) to the attention (yellow) level in the 2012. The proximity of more than half a million people residing in the caldera's vicinity further emphasizes the urgency to gain a comprehensive understanding of its subvolcanic processes and their potential impact on the evolution of precursory phenomena.
While previous research has provided valuable insights into CO2 emissions from the Campi Flegrei caldera21, limited attention has been given to the process of CO2 fixation in the bedrock through hydrothermal calcite formation and its significance in shaping the overall CO2 budget within the volcanic system. In a pioneer paper22, we conducted a petrological and isotopic (δ13C, δ18O) study on calcite crystals from core samples by geothermal wells drilled into the upper 3 km of the Campi Flegrei caldera. The obtained data allowed us to hypothesize that a large amount of CO2 could be fixed in the rocks of the caldera system over its lifetime. However, the investigated boreholes were essentially localized in limited sectors of the caldera so that a large portion of the caldera volume has remained unexplored.
Addressing the role of CO2 sequestration through calcite formation within calderas requires a comprehensive and representative dataset. In this study, we present a novel approach aimed at accurately quantifying CO2 sequestration in hydrothermal calcites, utilizing lithics from volcanic deposits. These lithic fragments represent portions of the caldera bedrock, randomly entrapped by ascending magma during volcanic eruptions, making them an ideal proxy for investigating the overall carbon sink capacity of the caldera system. We conducted 3D textural and isotopic investigations on hydrothermal calcite entrapped in lithics from representative volcanic deposits from eruptions covering different ages, Volcanic Explosivity Index (VEI), and vent positions.
Here, we present an accurate investigation of CO2 sequestration processes in hydrothermal calcites within the Campi Flegrei caldera. The results contribute to a deeper understanding of the intricate interplay between magmatic and hydrothermal processes in volcanic systems and emphasize the importance of considering calcite sinks for accurate volcano monitoring and risk assessment. Furthermore, the proposed methodology utilizing lithics offers a valuable framework for future investigations in various caldera systems, ultimately advancing our knowledge of volcanic carbon cycling and its implications for volcanic and environmental dynamics.
Material and methods
Sampled eruptions and recognized lithic fragments
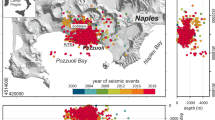
In order to collect lithic samples as representative as possible of the Campi Flegrei caldera bedrocks, we have selected eruptions: (i) of different ages, from the most recent Monte Nuovo eruption, occurred in 1538 AD, up to the oldest one of the Campanian Ignimbrite, recently re-dated at 41.7 ± 1.8 ka23,24; (ii) with an eruptive vent located in different sectors of the caldera; (iii) of different Volcanic Explosivity Index (VEI), between 2 (Strombolian eruptions) up to 7 (super-eruptions), Fig. 1 and Table 1.
Campi Flegrei caldera: location map (digital terrain model of the area has been created by ArcGIS© ESRI, rel. 10.6 - available at www.esri.com - from the Laboratorio di Geomatica e Cartografia of the Istituto Nazionale di Geofisica e Vulcanologia, Osservatorio Vesuviano, Italy) and stratigraphic sequence. (a) Location map of the Campi Flegrei caldera. The caldera rims, reconstructed for the Campanian Ignimbrite eruption (~ 40 ka) and the Neapolitan Yellow Tuff eruption (~ 15 ka) are shown in dashed blue and red lines, respectively, following36. Deep wells location is shown with white circles, whereas sampling location for the selected eruptions with orange circles: (1) Breccia Museo (proximal sequence of Campanian Ignimbrite); (2) Neapolitan Yellow Tuff; (3) Baia; (4) Averno; (5) Monte Nuovo; (6) Cigliano; (7) Solfatara; (8) Astroni; (9) Agnano-Monte Spina. (b) Litho-stratigraphic sequence drilled in the San Vito plain, in the center of the Campi Flegrei caldera (after Rosi and Sbrana, 1987). Depth of the well in meters.
Four types of lithic fragments have been distinguished (see Table 2 and Fig. 2 for details):
-
(a)
Tufaceous fragments of volcanic pyroclastic nature were recognized in all studied eruptions. They have a trachytic composition and pyroclastic textures with a glassy groundmass including phenocrysts, mainly of sanidine. Chlorite is also abundant. Calcite is present both distributed in groundmass and in isolated crystals (Fig. 3).
-
(b)
Lava fragments of volcanic effusive nature were found in all selected eruptions and range in composition from trachybasaltic-latitic to trachytic lava. Porphyritic trachybasaltic-latitic lavas contain predominantly phenocrysts of clinopyroxene, olivine and nepheline; their groundmass is constituted by biotite, feldspar and clinopyroxene. Trachytic lavas have porphyritic texture with phenocrysts consisting mainly of potassium feldspar, clinopyroxene and biotite. Calcite is rarely present in both.
-
(c)
Arenaceous fragments of sedimentary nature were found in the deposit of the Breccia Museo and Monte Nuovo eruptions, and consist of equigranular crystals of quartz, feldspar and biotite. They show moderate presence of calcite.
-
(d)
Syenitic fragments of intrusive volcanic nature were only recognized in the deposit of the Breccia Museo. They mainly consist of plagioclase (70%), K-feldspar (20%), and in minor amount of clinopyroxene, and biotite with chlorite alteration. Less abundant phases are cancrinite, zircon, titanite, apatite, oxides, garnet, hornblende. Calcite is rare.
3D investigation of a lithic sample. (a–d) Example of lithic tuff (from Cigliano eruption). (a) 3D visualization of the digital rock. (b) Selected 2D slice extracted from the reconstructed 3D volumes. White scale bar 1 mm. (c) 3D rendering of the tomogram reported in the first panel after classification of the pixels into various phases (i.e., segmentation): violet = calcite (CC); light gray = feldspar. (d) Extracted single crystal of calcite.
These four lithologies are in agreement with those identified by deep boreholes drilled inside the caldera22, thus ensuring a broad representativeness of the entire bedrock.
The selected lithic fragments were characterized through 3D microtomographic and stable isotope analyses.
Microtomographic analyses
3D imaging. X-ray computed microtomography (micro-CT) analysis is a non-destructive technique that offers the opportunity to visualize and investigate the internal structure of rock samples by generating three-dimensional digital maps with a very high resolution, up to the submicron. In detail, microtomographic investigations allow to reconstruct three-dimensional maps of the linear X-ray attenuation coefficient of the samples (which for the same energy is a function of the density and atomic number of the crossed material), useful to visualize and quantitatively characterize the phases of interest (e.g., shape, size, distribution and orientation of fractures, pores, crystals, etc). This imaging technique entirely avoids stereological corrections, which are instead needed for 2D measurements through conventional analytical methods. In this study, micro-CT analyses have been performed using a Carl Zeiss Xradia 410 Versa 3D X-ray microscope at the Istituto Nazionale di Geofisica e Vulcanologia-Osservatorio Vesuviano (INGV-OV, Naples). Cylinders with maximum diameters of 1.5–3 cm were cut from the lithic samples and the scan was performed over a 360° rotation using from 1601 to 4001 projections, 80 kV voltage, 7 W power. The resulting nominal voxel (volumetric pixel) size is 5 µm. The reconstruction of the attenuation data was performed through the filtered back-projection algorithm using XRM Reconstructor software, version 11.1.8043, producing a stack of 967 cross-sectional, grayscale images composing the digital 3D scans.
3D image analyses. Given the compositional differences, the various phases present in the rocks have been identified through three main steps: (1) Image segmentation or, in other words, assignment of the volume elements (voxels) in the 3D image to the corresponding phases. The success of this stage depends largely on the contrast in X-ray attenuation between the different phases, on which the grayscale depends. Particularly, calcite crystals have been recognized and segmented with manual thresholding. (2) Grain identification, which consists of checking that groups of voxels forming individual grains are calcite crystals. In some cases, calcite crystals have overlapping grayscale intensity with voids, but they are easily distinguished on the basis of shape and texture (rhombohedral cleavage, prismatic shape). The main barrier to the identification of small crystals is the image noise, due to the heterogeneous nature of the glassy matrix in fine scale. To avoid this problem, it was sufficient to apply filters to remove isolated clusters smaller than a given radius (15 µm, i.e., three times the voxel size), such that clusters smaller than the threshold size are automatically excluded (see also16,25,26,27). (3) Quantitative measurements of calcite crystals. These 3D microstructural analyses were performed using the software Dragonfly Pro (ORS), version 2024.1.0.1601, Figs. 3 and 4.
Stable isotope analyses
Stable isotope compositions for carbon and oxygen of hydrothermal calcite were measured at INGV-OV on powder samples by using a Spectrometer Finnigan Mat Delta plus. All data are reported in δ-notation, where δ = (Rsample/Rstandard − 1) × 1000; R is the measured isotopic ratios, PDB and SMOW are the used standard for carbon and oxygen respectively. The accuracy of the measurements is ± 0.15 ‰ for δ13C, ± 0.1‰ for δ18O.
Results
Hydrothermal calcite: amounts of stored CO2
Our results show that calcite crystals are generally absent in the syenitic and lava lithic fragments of Campi Flegrei caldera, possibly due to their low porosity that avoids replacement and precipitation driven by hydrothermal fluids. In contrast, they are almost always present in tufaceous lithics and occasionally in sedimentary lithics, for which no correlation was observed between the content of the calcite and the age, VEI or vent location of the eruption. The only exception is represented by the lithics of greenish tuff found within the deposit of the Neapolitan Yellow Tuff (NYT) that are characterized by the absence of calcite. This type of greenish tuff is also recognized in the lower part of the succession of the NYT in boreholes drilled in the city of Naples and has been interpreted as due to an eruptive phase occurring short before the NYT eruption from submarine vents28. The short time interval between the two eruptive phases (the most recent corresponding to the formation of the primary deposit of NYT and the previous one to the older deposit of greenish tuff from which the lithic fragments derive) was probably not sufficient for the formation of hydrothermal calcite, and therefore can justify the absence of calcite in these greenish tuff fragments.
Calcite is present both in small crystals generally around 100 – 1000 µm, as well as scattered in the volcanic glass, Figure 3. The content ranges from 0 to 5 vol%, Table 2. Particularly, it has a modal value of 2 vol% in tufaceous fragments. This value is here used to evaluate the amount of CO2 fixed in the bedrocks, as tuffs are the most representative lithologies of the Campi Flegrei caldera substructure (see details below). The measured volume percentage of calcite was converted into mass of CaCO3 per unit mass of rock considering their densities. The concentration of CaCO3 in the rock is computed by equation (1):
where the mean density of the tuff (\({\rho }_{l}\)) is 2400 kg/m3, while the density of calcite (\({\rho }_{c}\)) is 2700 kg/m329.
The concentration of calcite found in lithic samples ranges from 0 to 65 g/kg (modal value of 23 g/kg in tufaceous lithics; Table 2). Interestingly, these values are widely consistent with those measured in core samples from Campi Flegrei exploratory geothermal wells, varying from 0.045 to 140 g/kg22. To better compare these data set, two tuff samples from the San Vito 1 borehole previously analyzed by22 were also examined (SV1-1419 = CaCO3 12.9 g/kg, SV1-1715 = CaCO3 10.9 g/kg), obtaining X-ray microtomographic data (18 and 17 g/kg, respectively) in good agreement with previous values.
Finally, to properly assess the extension of the region interested by calcite sink we calculated the volume of tuff that constitutes the caldera-filling rocks based on data available in literature. Particularly, the structure of the caldera has been investigated through 1-to-3 km deep boreholes (Fig. 1a), local earthquake seismic tomography, gravity and magnetic surveys, as well as sporadic observations of teleseismic and wide-angle seismic data30,29,30,33. This information coherently indicates that the caldera’s upper bedrock is formed by a sequence of compacted tuffs and tuffs with interbedded lavas, affected by thermometamorphism at about 2–2.5 km depth. In detail, the sequence drilled in the San Vito plain, the only available well data at the center of the caldera, indicates that about 90% of the caldera-filling consists of tufaceous rocks, extended between 0.5 and 3 km30 (Fig. 1b). On this basis, following the approach proposed by Chiodini et al.22, the volume of the region interested by hydrothermal calcite production is estimated as the 90% of the volume of a cylinder with a diameter of 12 km (extension of the caldera) and a height comprises from 2.5 to 3 km (depth to which tuff rocks were actually cut by deep wells) to 4 km (possible limit between caldera-filling tuffs and underlying basement inferred by geophysical data). These volume estimates (mean value 300 km3) were used, together with the modal CaCO3 concentration value (23 g/kg), to calculate the total amount of CO2 fixed in the bedrock. The resulting value is 7 Gt, which normalized to a depositional interval of ~40 k.y. (corresponding to the occurrence of the Campanian Ignimbrite eruption originating the caldera23,24) results in a time-averaged CO2 flux of ~500 t/d. Although these estimates should be considered with caution due to the related uncertainties, they are compatible with those (800 t/d) obtained analyzing core samples from Campi Flegrei exploratory geothermal wells by22. These values closely approximate those of the CO2 released by diffuse degassing processes at Solfatara-Pisciarelli hydrothermal site before the onset of the ongoing unrest (1100 ± 200 t/d in the 1998–2008 period4) confirming that the carbon dioxide stored in the bedrock is a comparable fraction of the total amount of CO2 emitted on average from the geothermal system at Campi Flegrei caldera.
Hydrothermal calcite: constraints on sources
Chiodini et al. (2015) systematically investigated the isotopic composition of hydrothermal calcites from Campi Flegrei caldera boreholes, documenting (i) δ18OH2O positively correlated with depth (as well as with temperature and fluid inclusion salinity), ranging from heavier magmatic to lighter meteoric/marine compositions; (ii) constant δ13CCO2, identical to the current fumarolic values.
δ18O values of calcites from tufaceous lithics (6.25 and 21.53‰) are almost consistent with those (7.50–19.37‰) obtained by22; conversely δ13C (− 18.16 and 2.17‰) has a wider range (− 4.08 to 0.47‰ in22), reaching significantly lighter values only in lithics from deposits of Astroni eruption. Lower δ13C can be due to contamination of minor contents of calcites formed from groundwater that interacted with biogenic sources (with average δ13CCO2: − 19.4±2.1‰34) and infiltrated in the subaerial (often poorly lithified) volcanic deposits. A decrease in δ13C could be also the consequence of thermal interaction between lithic fragments and magmatic fluids during the ascent towards the surface in the eruptive conduit, leading to decarbonatation (an example trend of Rayleigh volatilization is shown in Fig. 5); however the massive consumption of calcite required to reach the observed values of δ13C through this process is inconsistent with the amounts of calcite found in the lithic fragments, Fig. 5.
δ18O and δ13C of calcites hosted in lithic fragments of the Campi Flegrei eruptions. Values obtained for the calcite from Campi Flegrei caldera boreholes (CF boreholes), δ13CCO2 of biogenic source and δ18O of meteoric water are also shown (data from22,34). The path AA’ (with A’ at remaining mole fraction F of the carbon = 0.025) is an example trend of Rayleigh volatilization, assuming normal calc-silicate decarbonation35 at 500 °C. The errors are within the symbols.
Summarizing, except for Astroni eruption, the obtained δ18O and δ13C are quite compatible with those of22 for well cores and cover most of the range reported by these authors, suggesting that the hydrothermal calcites can be representative of large thicknesses of the Campi Flegrei caldera and originated primarily from solutions arising from the mixing between magmatic volatiles and hydrothermal fluid components, consistent with the composition of the present-day fumaroles4.
Discussion
Our 3D textural analyses revealed distinct patterns in the distribution of calcite within various lithic fragments in the studied area. Specifically, calcite is consistently found in the form of small crystals, typically measuring between 100 and 1000 µm, and it is also dispersed within volcanic matrix glass. This pattern is observed primarily in tufaceous lithic fragments, but similar features are also present in some sedimentary lithic fragments. Interestingly, there is no discernible correlation between the presence of calcite in these lithic fragments and factors such as eruption age, Volcanic Explosivity Index, or the location of the volcanic vent.
This uniform occurrence might suggest that the hydrothermal activity responsible for calcite formation has been consistent over time, impacting both older and younger rock units in a similar manner. Such a finding could confirm the presence of a long-lived or recurring hydrothermal system, which has continuously influenced the mineralization across various geological periods.
Conversely, our investigations have highlighted a notable absence of calcite crystals in syenitic and lava lithic fragments. This absence can be attributed to the low porosity of these lithic fragments, which inhibits the replacement and precipitation processes typically driven by circulation of hydrothermal fluids in connected pores of rocks.
Furthermore, our analysis of δ18O values for calcite found in tufaceous lithic fragments has yielded a wide range, spanning from 6.25 to 21.53‰. These values closely align with those obtained by22 on calcite in core samples. Interestingly, in Chiodini et al.'s work, the δ18OH2O values showed a clear correlation with the depth at which the samples were collected. δ18OH2O for shallower calcites exhibited isotopic compositions like that of local meteoric water (approximately − 6.5‰), while δ18OH2O for deeper calcites displayed values more in line with those of magmatic water typically found in subduction zones (ranging between + 8‰ and + 12‰).
It is worth noting that fluid inclusions trapped in K-feldspar, quartz, and calcite in the core samples, as reported by36, indicate the presence of low-salinity solutions at shallower depths, where calcites exhibited lighter δ18OH2O signatures. In contrast they show high-salinity solutions, at deeper levels, where calcite displayed heavier δ18OH2O compositions (ranging from +11.5 to +12.7‰), thus supporting a magmatic origin for the 18O-rich calcite-precipitating solutions.
In a similar manner, the analysis of δ13C values in calcite embedded in lithic fragments exhibited a wider range, from − 18.16 to 2.17‰, due to lighter values in certain samples (mainly from the Astroni eruption). These values suggest an origin resulting from the mixing of magmatic and shallow volatile sources. Chiodini et al. (2015) found that the light δ13CCO2 compositions for calcite from shallow core samples suggested the infiltration of organic-derived carbon from meteoric waters as they passed through soils in the shallow layers of the caldera succession. However, for deeper core samples, the data strongly suggested a consistent source of CO2, closely matching the composition of the current Solfatara-Pisciarelli fumarolic field.
These isotopic features provide additional evidence of a long-standing hydrothermal system, where deep magmatic volatiles interact with Campi Flegrei rocks, influencing the formation of hydrothermal minerals throughout the caldera's history.
The modal value of 2 vol% of calcite content calculated in tufaceous fragments has used to estimate the amount of CO2 sequestered within the bedrock and to evaluate the potential as a carbon sink. The concentration of calcite in lithic samples, ranging from 0 to 65 g/kg, closely aligns with measurements obtained from geothermal wells (0.045–140 g/kg), thus validating our approach. To determine the extent of the carbon sink, we calculated the volume of tufaceous rocks, which make up the caldera-filling material, based on data available in the literature. This calculation, combined with the modal CaCO3 concentration value (23 g/kg), enabled us to estimate a total of 7 gigatons (Gt) of CO2 sequestered in the bedrock over a time period of approximately 40,000 years.
Despite inherent uncertainties, this estimate aligns well with values obtained through other research methods, such as core samples and observations of diffuse degassing4,22. It underscores the significance of carbon dioxide storage within the bedrock as a substantial component of the total CO2 emissions originating from the geothermal system within the Campi Flegrei caldera.
This research holds significance because the process of CO2 sequestration through calcite precipitation can significantly impact the interpretation of geochemical data for volcanic monitoring and risk assessment. Therefore, it is crucial to consider this factor when assessing volcanic activity and its potential hazards.
Recently Buono et al.8, by comparing the temporal variations of fumarolic (CO2–N2–He) composition with those calculated with magmatic degassing simulations, estimated that between 20 and 40% of the fumarolic CO2 emitted in the Campi Flegrei area during the ongoing unrest started in 2005 is released from non-magmatic sources. Notably, the amount of carbon dioxide from these non-magmatic sources has been steadily increasing since 2005, showing similar growth patterns to the rise in temperature within the hydrothermal system. These authors hypothesized that this process can be driven by the conversion of hydrothermal calcite in carbon dioxide following the circulation of hotter and acidic solutions in the rocks that host the hydrothermal system.
Conclusions
We have conducted a comprehensive textural and isotopic study focused on hydrothermal calcite embedded in lithic fragments collected from selected volcanic deposits of Campi Flegrei caldera. These deposits result from eruptions of varying age, Volcanic Explosivity Index, and eruptive vent locations. Our primary objective was to develop a methodology for estimating the extent of carbon dioxide sequestration within the bedrock of the caldera throughout its entire volcanic history and evaluate its impact on the measured surface emissions of CO2. We aimed to create an approach that could be applied to other caldera systems. To validate our methodology, we compared our findings with similar data obtained from hydrothermal calcite collected from core samples retrieved through geothermal wells drilled into the upper 3 km of the caldera. The proposed strategy capitalizes on the fact that lithic fragments represent portions of caldera-filling rocks that were trapped during the ascent of magma. Unlike core samples from geothermal wells, which are limited in number and often taken from specific depths and regions within the caldera, lithic fragments are more easily collected. By utilizing lithic fragments, which are readily available and representative of the entire caldera bedrock, our approach overcomes the limitations of geothermal well core samples, which often provide localized and incomplete data.
Through comprehensive 3D textural characterization and isotopic analysis of hydrothermal calcite within lithic fragments from selected alkaline volcanic deposits in the Campi Flegrei caldera, our results highlight the substantial impact that calcite sinks in a long-lived hydrothermal system can have on the overall CO2 budget released by volcanoes throughout their evolution. Moving forward, we recommend applying this methodology to investigate carbon sink processes in other caldera systems and volcanic environments worldwide, with the aim of achieving a comprehensive understanding of these processes on a global scale.
In conclusion, the study's outcomes shed light on the intricate interplay between magmatic and hydrothermal processes in volcanic systems and emphasize the importance of considering CO2 sequestration through hydrothermal calcite formation. The proposed methodology for quantifying CO2 sink processes in calderas provides a valuable framework for future investigations, contributing to a deeper understanding of volcanic carbon cycling and its impact on volcanic and environmental dynamics. By expanding our knowledge of these processes, we move closer to developing robust strategies for volcanic risk management in volcanic areas.
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
References
Fischer, T.P. & Chiodini, G. Volcanic, magmatic and hydrothermal gases. In The Encyclopedia of Volcanoes 2nd edn (eds Sigurdsson, H., Houghton, B., McNutt, S.R., Rymer, H. & Stix, J.) 779–797 (Academic Press, 2015). https://doi.org/10.1016/B978-0-12-385938-9.00045-6.
Fischer, T. P. et al. The emissions of CO2 and other volatiles from the world’s subaerial volcanoes. Sci. Rep. 9, 18716. https://doi.org/10.1038/s41598-019-54682-1 (2019).
Werner, C., Fischer, T.P., Aiuppa, A., Edmonds, M., Cardellini, C., Carn, S., Chiodini, G., Cottrell, E., Burton, M., Shinohara, H. & Allard, P. Carbon dioxide emissions from subaerial volcanic regions: Two decades in review. In Deep Carbon; Past to Present (eds Orcutt, B.N., Daniel, I. & Dasgupta, R.) 188–236 (Cambridge University Press, 2019). https://doi.org/10.1017/9781108677950.008.
Chiodini, G. et al. Long-term variations of the Campi Flegrei, Italy, volcanic system as revealed by the monitoring of hydro-thermal activity. J. Geophys. Res. 115, B03205. https://doi.org/10.1029/2008JB006258 (2010).
Hurwitz, S. & Lowenstern, J. B. Dynamics of the Yellowstone hydrothermal system. Rev. Geophys. 52, 375–411. https://doi.org/10.1002/2014RG000452 (2014).
Pappalardo, L. et al. The role of CO2 flushing in triggering the ‘Millennium’ eruption and recent unrests at Changbaishan volcano (China/North Korea). Int. Geol. Rev. 65, 706–719. https://doi.org/10.1080/00206814.2022.2065544 (2022).
Buono, G. et al. New insights into the recent magma dynamics under Campi Flegrei caldera (Italy) from petrological and geochemical evidence. J. Geophys. Res. Solid Earth 127, 2021JB023773. https://doi.org/10.1029/2021JB023773 (2022).
Buono, G., Caliro, S., Paonita, A., Pappalardo, L. & Chiodini, G. Discriminating carbon dioxide sources during a volcanic unrest: The case of Campi Flegrei caldera. Geology 51, 397–401. https://doi.org/10.1130/G50624.1 (2023).
Pereira, R. & Gamboa, D. In situ carbon storage potential in a buried volcano. Geology 51, 803–807. https://doi.org/10.1130/G50965.1 (2023).
Mastrolorenzo, G. & Pappalardo, L. Magma degassing and crystallization processes during eruptions of high-risk Neapolitan-volcanoes: Evidence of common equilibrium rising processes in alkaline magmas. Earth Planet. Sci. Lett. 250, 164–181. https://doi.org/10.1016/j.epsl.2006.07.040 (2006).
Mastrolorenzo, G. et al. Volcanic hazard assessment at the Campi Flegrei caldera. Geol. Soc. Spec. Publ. 269, 159–171. https://doi.org/10.1144/GSL.SP.2006.269.01.10 (2006).
Mastrolorenzo, G., Pappalardo, L., Troise, C., Panizza, A. & De Natale, G. Probabilistic tephra hazard maps for the Neapolitan area: Quantitative volcanological study of Campi Flegrei eruptions. J. Geophys. Res. 113, 1–14. https://doi.org/10.1029/2007JB004954 (2008).
Mastrolorenzo, G., Palladino, D. M., Pappalardo, L. & Rossano, S. Probabilistic numerical assessment of pyroclastic current hazard at Campi Flegrei and Naples city: Multi-VEI scenarios as a tool for “full-scale” risk management. PLoS ONE 12, e0185756. https://doi.org/10.1371/journal.pone.0185756 (2017).
Orsi, G, D’Antonio, M. & Civetta, L. Campi Flegrei - A Restless Caldera in a Densely Populated Area 410 (Springer, 2022). https://doi.org/10.1007/978-3-642-37060-1.
Di Vito, M. A. et al. Magma transfer at Campi Flegrei caldera (Italy) before the 1538 AD eruption. Sci. Rep. 6, 32245. https://doi.org/10.1038/srep32245 (2016).
Liedl, A. et al. A 3D imaging textural characterization of pyroclastic products from the 1538 AD Monte Nuovo eruption (Campi Flegrei, Italy). Lithos 340–341, 316–331. https://doi.org/10.1016/j.lithos.2019.05.010 (2019).
Del Gaudio, C., Aquino, I., Ricciardi, G. P., Ricco, C. & Scandone, R. Unrest episodes at Campi Flegrei: A reconstruction of vertical ground movements dur-ing 1905–2009. J. Volcanol. Geotherm. Res. 195, 48–56. https://doi.org/10.1016/j.jvolgeores.2010.05.014 (2010).
D’Auria, L. et al. Repeated fluid-transfer episodes as a mechanism for the recent dynamics of Campi Flegrei Caldera (1989–2010). J. Geophys. Res. 116, B04313. https://doi.org/10.1029/2010JB007837 (2011).
Chiodini, G. et al. Magmas near the critical degassing pressure drive volcanic unrest towards a critical state. Nat. Commun. 7, 13712. https://doi.org/10.1038/ncomms13712 (2016).
Giudicepietro, F. et al. Campi Flegrei Vesuvius and Ischia Seismicity in the Context of the Neapolitan Volcanic Area. Front. Earth Sci. 9, https://doi.org/10.3389/feart.2021.662113 (2021).
Chiodini, G. et al. Hydrothermal pressure-temperature control on CO2 emissions and seismicity at Campi Flegrei (Italy). J. Volcanol. Geotherm. Res. 414, 107245. https://doi.org/10.1016/j.jvolgeores.2021.107245 (2021).
Chiodini, G., Pappalardo, L., Aiuppa, A. & Caliro, S. The geological CO2 degassing history of a long-lived caldera. Geology 43, 767–770. https://doi.org/10.1130/G36905.1 (2015).
Gebauer, S. K., Schmitt, A. K., Pappalardo, L., Stockli, D. F. & Lovera, O. M. Crystallization and eruption ages of Breccia Museo (Campi Flegrei caldera, Italy) plutonic clasts and their relation to the Campanian ignimbrite. Contrib. Mineral. Petrol. 167, 953. https://doi.org/10.1007/s00410-013-0953-7 (2014).
Wu, W. N., Schmitt, A. K. & Pappalardo, L. U-Th baddeleyite geochronology and its significance to date the emplacement of silica undersaturated magmas. Am. Min. 100, 2082–2090. https://doi.org/10.2138/am-2015-5274 (2015).
Pappalardo, L., Buono, G., Fanara, S. & Petrosino, P. Combining textural and geochemical investigations to explore the dynamics of magma ascent during Plinian eruptions: a Somma-Vesuvius volcano (Italy) case study. Contrib. Mineral. Petrol. 173, 61. https://doi.org/10.1007/s00410-018-1486-x (2018).
Buono, G., Pappalardo, L. & Petrosino, P. Magma storage and ascent during the largest eruption of Somma Vesuvius volcano: Pomici di Base (22 ka) plinian event. Boll. Geofis. Teor. Appl. 61, 23–40. https://doi.org/10.4430/bgta0294 (2020).
Pappalardo, L., D'Auria, L., Cavallo, A., Fiore, S. Petrological and seismic precursors of the paroxysmal phase of the last Vesuvius eruption on March 1944. Sci. Rep. 4(1), 10.1038/srep06297 (2014).
Petrosino, P. et al. Multiproxy approach to urban geology of the historical center of Naples, Italy. Quat. Int. 577, 147–165. https://doi.org/10.1016/j.quaint.2020.12.043 (2021).
Deer, W.A., Howie, R.A. & Zussman, J. An Introduction to the Rock-Forming Minerals 2nd edn (Longman Scientific and Technical, 1992). https://doi.org/10.1180/DHZ.
Rosi, M. & Sbrana, A. Phlegrean fields. CNR Quaderni de La Ricerca Scientifica 114, 175 (1987).
Cassano, E. & La Torre, P. Geophysics. In Phlegrean Fields (eds Rosi, M. & Sbrana, A.) 103–131 (CNR Quaderni de la Ricerca Scientifica, 1987).
Aster, R. C. & Meyer, R. P. Three-dimensional velocity structure and hypocenter distribution in the Campi Flegrei caldera, Italy. Tectonophysics 149, 195–218. https://doi.org/10.1016/0040-1951(88)90173-4 (1988).
Ferrucci, F., Hirn, A., De Natale, G., Virieux, J. & Mirabile, L. P-SV conversions at a shallow boundary beneath Campi Flegrei caldera (Italy): Evidence for the magma chamber. J. Geophys. Res. 97, 15351–15359. https://doi.org/10.1029/92JB00888 (1992).
Chiodini, G. et al. Carbon isotopic composition of soil CO2 efflux, a powerful method to discriminate different sources feeding soil CO2 degassing in volcanic-hydrothermal areas. Earth Planet. Sci. Lett. 274, 372–379. https://doi.org/10.1016/j.epsl.2008.07.051 (2008).
Valley, J.W. Stable isotope geochemistry of metamorphic rocks. In Stable Isotopes in High Temperature Geological Processes, Reviews in Mineralogy and Geochemistry vol 16 (eds Valley, J.W., Taylor, H.P. Jr. & O’Neil, J.R.) 445–489 (1986).
De Vivo, B. et al. The Campi Flegrei (Italy) geothermal system: A fluid inclusion study of the Mofete and San Vito fields. J. Volcanol. Geotherm. Res. 36, 303–326. https://doi.org/10.1016/0377-0273(89)90076-0 (1989).
Pappalardo, L. & Mastrolorenzo, G. Rapid differentiation in a sill-like magma reservoir: A case study from the Campi Flegrei caldera. Sci. Rep. 2, 712. https://doi.org/10.1038/srep00712 (2012).
Pappalardo L. & Buono G. Insights into processes and timescales of magma storage and ascent from textural and geochemical investigations: Case studies from high-risk Neapolitan volcanoes (Italy). In Crustal Magmatic System Evolution: Anatomy, Architecture and Physics-Chemical Processes (eds Masotta, M., Beier C. & Mollo, M.) 213–235 (AGU (American Geophysical Union) Monograph, 2021). https://doi.org/10.1002/9781119564485.ch10.
Vitale, S. & Isaia, R. Fractures and faults in volcanic rocks (Campi Flegrei, southern Italy): Insight into volcano-tectonic processes. Int. J. Earth Sci. (Geol. Rundsch) 103, 801–819. https://doi.org/10.1007/s00531-013-0979-0 (2014).
Acknowledgements
This research was performed in the ambit of the INGV Progetto Dipartimentale LOVE-CF (Linking surface Observables to sub-Volcanic plumbing-system: a multidisciplinary approach for Eruption forecasting at Campi Flegrei caldera (Italy) financed by the INGV, Horizon 2020 project EXCITE (Electron and X-ray microscopy Community for structural and chemical Imaging Techniques for Earth materials) and EPOS (European Plate Observing System). The authors are grateful to the two anonymous reviewers for their valuable comments and suggestions on our manuscript.
Author information
Authors and Affiliations
Contributions
G.B. and L.P.: sampling, acquisition and analysis of micro-CT data; S.C.: acquisition and analysis of isotopic data; All authors G.B., S.C., L.P., G.C.: conceptualization and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Buono, G., Caliro, S., Pappalardo, L. et al. Hydrothermal calcite formation in Campi Flegrei caldera, Italy: unraveling carbon sink processes in alkaline volcanic systems. Sci Rep 14, 16839 (2024). https://doi.org/10.1038/s41598-024-67746-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-67746-8
Keywords
This article is cited by
-
3D structure and dynamics of Campi Flegrei enhance multi-hazard assessment
Nature Communications (2025)