Abstract
The role of neoadjuvant chemotherapy and its benefits in patients with triple-negative breast cancer (TNBC) and small tumors are unclear. This study aims to compare survival differences between clinical T1 TNBC receiving neoadjuvant chemotherapy (NAC) and adjuvant chemotherapy (AC). Data for patients with clinical T1 TNBC were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Patients were categorized according to whether they received chemotherapy before or after surgery. Propensity Score Matching (PSM) was used to minimize the influence of confounding factors. OS and BCSS were compared between the two treatment sequences using Kaplan–Meier and univariate and multivariable Cox proportional hazards regression analyses. The study included 6249 women with T1 TNBC. In multivariate analysis, compared with that in the AC group, the hazard ratio for death in the NAC group was 1.54 (95% confidence interval 1.26–1.89, p < 0.001). NAC offers no additional benefits in any age group or T, N subgroups. Our findings suggest that NAC does not provide additional benefit to patients with clinical T1 TNBC, even in the presence of lymph node metastasis, or T1c.
Similar content being viewed by others
Introduction
Triple-negative breast cancer (TNBC), defined by the absence of immunostaining for estrogen and progesterone receptors and lack of overexpression or amplification of human epidermal growth factor receptor 2, accounts for approximately 15–20% of all breast cancers1,2. Compared with hormone receptor-positive breast cancers, TNBC has a worse prognosis, with most events occurring relatively early. More than 50% of patients experience relapse in the first 3–5 years after diagnosis3. Studies have demonstrated that TNBC is more responsive to systemic chemotherapy than are other molecular subtypes, and taxane-containing and anthracycline-containing chemotherapy is currently the main systemic treatment option for this type of breast cancer4,5. Adjuvant chemotherapy (AC) and neoadjuvant chemotherapy (NAC) yield similar disease-free survival and overall survival (OS) rates6,7,8.
Multiagent chemotherapy regimens have shown benefit in terms of improving both disease-specific survival and OS in patients with early TNBC whether administered before or after surgery and are widely recommended to reduce the risk of relapse9,10. The international guidelines list sequential use of a taxane and anthracycline plus cyclophosphamide as one of the preferred regimens for early TNBC11. However, despite treatment with anthracycline-taxane-based therapy and adjuvant capecitabine in patients with residual disease at surgery, 5-year metastasis-free survival remains at approximately 70%, with approximately 30–40% of patients developing metastatic disease and dying of their cancer12,13,14. Therefore, improvements in long-term treatment outcomes are needed for patients with early-stage TNBC.
NAC also provides an opportunity to collect biological and prognostic information during the response to guide further treatment. Although AC remains more common globally, NAC is now considered standard practice and its use is becoming more frequent15. NAC is the current standard of care for patients with early disease10,13,16,17. The short-term goal of NAC in patients with TNBC is a pathological complete response because of its association with prolonged event-free survival and OS7,18. The agents in the chemotherapy regimens do not differ between NAC and AC other than use of pembrolizumab in the neoadjuvant setting for high-risk patients. However, NAC allows pathological response-guided adjuvant treatment that can improve survival. Furthermore, NAC provides the opportunity to downstage the primary tumor and axillary nodes, allows for de-escalation of locoregional interventions, and the tumor response can help guide the need for subsequent AC and foster drug discovery19. Studies have shown that NAC with atezolizumab in combination with nab-paclitaxel and anthracycline-based chemotherapy significantly improves the pathological complete response rate20. However, in a series of 194 patients with TNBC, similar locoregional and distant recurrence-free survival rates were found for those with T1mic/T1a or T1b tumors (94.5% vs 95.5%), with no differences in outcomes according to whether or not chemotherapy was administered (95.9% vs 94.5%)21. Patients with extensive residual invasive cancer after NAC are at high risk of recurrent metastatic disease but whether survival is better in patients with small T1 TNBC who receive NAC than in those who receive AC after upfront surgery is uncertain. NAC is the standard for TNBC higher than T1 stage while data on the efficacy of NAC in T1 TNBC are unclear. The primary objective of this study was to compare OS and BCSS in patients with clinical T1 TNBC who have undergone upfront surgery followed by AC with that in those who have undergone NAC followed by surgery.
Results
Baseline characteristics
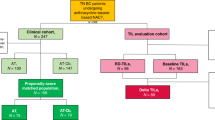
Application of the inclusion criteria identified 6249 eligible women with cT1 TNBC between 2010 and 2015, including 5404 (86.5%) who underwent surgery followed by AC (the AC group) and 845 (13.5%) who received NAC followed by surgery (the NAC group). The patient selection process is shown in Fig. 1. The disease characteristics at baseline are compared between the NAC group and the AC group in Table 1. Significant differences in the distributions of age, T stage, N stage, and surgery were observed between the study groups. Most of the patients were aged over 50 years, of white ethnicity, and married, had grade 3 T1c N0 stage tumors, and had undergone breast-conserving surgery (BCS). More than half of the patients had received radiotherapy. Compared with patients in the AC group, those in the NAC group tended to be younger than 50 years and had a higher lymph node-positive rate. After PSM to balance confounding factors, the χ2 test suggested balance and comparability between the two groups.
Overall survival outcomes
Kaplan–Meier curves were generated for the NAC and AC groups. Before and after PSM (Fig. 2A,C), OS was poorer in the NAC group. Univariate Cox analyses revealed significant between-group differences in age, marital status, disease grade and stage, type of surgery, and type of adjuvant therapy received. In view of the potential for bias stemming from mutual associations between these variables, the subsequent Cox proportional hazards regression analysis was performed, and the results are shown in Table 2. The risk of death was significantly higher in the NAC group than in the AC group (HR 1.46, 95% CI 1.21–1.76; p < 0.001). OS was poorer in patients who were aged 50 years or older than in patients who were younger (HR 1.27, 95% CI 1.07–1.50; p = 0.006) and poorer in patients who were single, divorced, separated, or widowed than in patients who were married (HR 1.28, 95% CI 1.10–1.49; p = 0.002). As expected, a higher tumor grade was associated with a worse prognosis. The HR was 2.17 (95% CI 1.28–3.69; p = 0.004) for T1c disease in comparison with T1a disease; however, there was no significant difference between T1b and T1a disease (HR 1.47, 95% CI 0.84–2.57; p = 0.179). The prognosis was significantly worse in patients with positive lymph nodes than in those with negative lymph nodes (HR 4.79, 95% CI 3.81–6.00; p < 0.001). Furthermore, OS was worse in patients who underwent radical mastectomy than in those who underwent BCS (HR 1.27, 95% CI 1.06–1.52; p = 0.008). However, there was no significant survival benefit from radiotherapy.
Breast cancer-specific survival outcomes
The Kaplan–Meier curves show that the prognosis of BCSS with better in the AC group than in the NAC group (Fig. 2B,D). Table 3, which summarizes the results of the multivariable analysis, shows that BCSS was significantly poorer in the NAC group than in the AC group (HR 1.54, 95% CI 1.26–1.89; p < 0.001), which was consistent with the findings for OS. However, unlike with the OS results, age and marital status was not identified as an independent risk factor for BCSS, and no significant difference in BCSS was found between the grade and surgery subgroups. BCSS was poorer in patients with T1c BC than in those with T1a BC (HR 1.93, 95% CI 1.09–3.43; p = 0.029) but no significant difference was found between patients with T1b and those with T1a breast cancer (HR 1.34, 95% CI 0.73–2.46; p = 0.349). As expected, node-positive disease was associated with worse BCSS (HR 6.40, 95% CI 5.01–8.18; p < 0.001). Furthermore, radiotherapy was not associated with improved BCSS.
Subgroup analysis stratified by age, T stage, and N stage
To further investigate the effect of NAC on survival, the population was divided into T1a, T1b, T1c, N0, N1 and N2-3 subgroups according to T stage and N stage, and baseline statistics and PSM were performed for each subgroup (Supplementary Tables 1–6). In different T1 stages, both before and after PSM, the OS and BCSS of the NAC group were worse than those of the AC group (Fig. 3A–L). In the T1N0 group, there were no statistically significant differences in OS and BCSS between the AC and NAC groups before and after PSM (Fig. 4A,D,G,J). However, in the lymph node-positive subgroup, both before and after PSM, the OS and BCSS of the NAC group were inferior to those of the AC group (Fig. 4B,C,E,F,H,I,K,L).
Comparison of OS for patients with cT1 triple-negative breast cancer between receiving NAC and upfront surgery + AC based on clinical T category. (A) Before PSM, OS of T1a; (B) Before PSM, OS of T1b; (C) Before PSM, OS of T1c; (D) Before PSM, BCSS of T1a; (E) Before PSM, BCSS of T1b; (F) Before PSM, BCSS of T1c; (G) After PSM, OS of T1a; (H) After PSM, OS of T1b; (I) After PSM, OS of T1c; (J) After PSM, BCSS of T1a; (K) After PSM, BCSS of T1b; (L) After PSM, BCSS of T1c.
Comparison of OS for patients with cT1 triple-negative breast cancer between receiving NAC and upfront surgery AC based on clinical N category. (A) Before PSM, OS of N0; (B) Before PSM, OS of N1; (C) Before PSM, OS of N2-3; (D) Before PSM, BCSS of N0, (E) Before PSM, BCSS of N1; (F) Before PSM, BCSS of N2-3; (G) After PSM, OS of N0; (H) After PSM, OS of N1; (I) After PSM, OS of N2-3; (J) After PSM, BCSS of N0, (K) After PSM, BCSS of N1; (L) After PSM, BCSS of N2-3.
We stratified all cases according to age, T stage, and N stage and performed multivariate analyses to evaluate the prognostic association of NAC in selected demographic and pathological subgroups (Table 4). Receiving NAC was associated with worse OS and BCSS across age groups. Similarly, NAC was associated with worse OS and BCSS across the various T stages than in patients who received AC. NAC was also associated with poorer OS and BCSS across the N stages in patients with positive lymph nodes. In patients with negative lymph nodes, there was no significant difference in OS or BCSS between the NAC group and the AC group.
Discussion
In this retrospective analysis of data for patients with T1 TNBC identified in a large nationwide US database, 86.5% (n = 5404) received AC and 13.5% (n = 845) received NAC. OS and BCSS were worse in the patients who received NAC than in those who received AC. We compared the prognostic impact of the two treatments in various demographic and clinical subgroups and found that, for patients with triple-negative breast cancer at T1N0 stage, there was no significant difference in OS and BCSS between NAC and AC. However, NAC was associated with worse OS and BCSS in patients with T1 TNBC who were clinically node-positive. This suggests that even in cases of lymph node positivity, the indications for neoadjuvant chemotherapy should be carefully considered for TNBC patients with T ≤ 2 cm.
AC as the traditional treatment of T1 TNBC, the main purpose is to eradicate residual microscopic cancer cells after surgery, further reducing the risk of recurrence and metastasis22. AC can significantly improve survival rates in patients with T1 TNBC and lymph node involvement or other strong risk factors23. Moreover, evidence is now emerging from clinical trials suggesting that surgery followed by chemotherapy might not be the optimal strategy to maximize the chances of survival in patients with TNBC. Therefore, it is necessary to explore the effectiveness of NAC. The core goal of NAC is to effectively reduce the tumor volume preoperatively, thereby downstaging the tumor, increasing eligibility for BCS, and decreasing the need for axillary dissection. Furthermore, NAC can effectively remove small metastases, thereby significantly reducing the risk of postoperative recurrence and metastasis and creating more favorable conditions for patient rehabilitation and follow-up treatment. Patients presenting with node-negative tumors measuring < 2 cm do not usually require downstaging because most are already candidates for BCS22,23. However, T1 TNBC, although relatively small, is usually aggressive and prone to recurrence, and NAC may be a suitable option if the tumor is large and lymph node metastasis or other risk factors are present. Consistent with these observations, 79.1% of the women with T1 TNBC who received NAC in our study had T1c disease and 41.3% were lymph node-positive.
NAC provides an opportunity to directly observe the efficacy of a particular chemotherapy regimen, however there are currently no biomarkers to reliably predict response to systemic therapy for TNBC, and patients who have extensive residual invasive cancer after NAC are at high risk of recurrent metastatic disease. TNBCs often achieve pCR by neoadjuvant chemotherapy. With modern anthracycline and taxane containing regimens, pCR rates are reported in 30–40% of the cases24,25. Several strategies have been pursued to increase the pCR rate in TNBC. The addition of platinoids increases the probability of pCR by about 45%26. This moderate increase is associated with more complexity and side effects and, thus far, has not yet resulted in an advantage in long-term outcomes25,27,28. Therefore, there is some controversy regarding the choice of NAC or AC for T1 TNBC, and the choice may need to be individualized after a detailed assessment of the patient. Our data argues that although NAC allows for a tailored approach to systemic therapy after breast surgery, providing more potentially toxic neoadjuvant systemic therapy may not be in the patient’s best interest in small TNBC. Compared with NAC, patients in the AC group were associated with better OS and BCSS, while avoiding the risk of PD from NAC, and adjuvant therapy was tailored to accurate pathological staging.
However, it is important to note that NAC and AC are not mutually exclusive. In some cases, patients may need both NAC and AC to maximize the effectiveness of treatment, AC may be necessary for patients with lymph node metastasis or other risk factors identified in the postoperative pathology report, and patients failing to achieve pCR after a full course of neoadjuvant chemotherapy can have their prognosis improved with additional treatments9. The NAC group in our study included patients who received chemotherapy both before and after surgery. Comparison of OS and BCSS in patients with stage T1 TNBC according to whether they received NAC or AC showed that in most patients, NAC was associated with poorer survival, suggesting that NAC may not be suitable for patients with T1 TNBC.
Through univariate and multivariate Cox regression analyses, we identified age, tumor size, lymph node as robustly associated with survival outcomes. The size of the tumor is an important prognostic indicator for triple-negative breast cancer, as it directly affects the degree of lymph node involvement and the risk of recurrence and metastasis. Axillary lymph node metastasis also significantly impacts the patient's prognosis23,29,30. Our data showed no benefit from neoadjuvant chemotherapy in T1N0 TNBC, while in node-positive T1 TNBC, neoadjuvant chemotherapy increased the risk of death compared to adjuvant chemotherapy. We further analyzed the difference in survival between the two treatment modalities in tumors of different sizes, and the results showed that even in patients with T1c, neoadjuvant chemotherapy significantly increased the risk of death. In addition, neoadjuvant chemotherapy was associated with poorer OS and BCSS in either age group. In summary, for T1 TNBC, systemic responses and side effects should be weighed even if the tumor is larger than 10 mm or combined with lymph node metastasis, especially in the absence of other risk factors, NAC should be carefully considered.
Limitations
This study has several limitations. First, despite our efforts to adjust for multiple confounders, the inherent bias of retrospective studies is difficult to eliminate by existing analytic instruments. Second, the variables used to determine the treatment sequence did not include information on all treatments that were administered before or after surgery, only that “systemic therapy” was given. Systemic therapy is not limited to chemotherapy and does not indicate the specific chemotherapy regimens provided. Third, there is a time delay in SEER data, which may not reflect the latest treatment methods. Finally, the clinical characteristics and therapeutic interventions used were limited to the US population and might be different in other countries.
Methods
Data source and study population
The study data were obtained from the SEER database. The SEER program is sponsored by the National Cancer Institute, contains data collected from 18 population-based cancer registries between 1975 and 2016, and provides deidentified cancer statistics for the US population31. This study was deemed to be exempt from Institutional Board Review at the participating research institutions.
Data for 15,195 patients identified to have T1 TNBC between 2010 and 2015 were extracted for evaluation of the association of NAC with survival. Patient staging was confirmed as per the seventh edition of the American Joint Committee on Cancer (AJCC) staging system (2010). Tumors with a largest diameter of ≤ 2 cm in the gross pathological specimen were defined as stage T1 and classified as T1a (1–5 mm), T1b (6–10 mm), or T1c (11–20 mm). Data for patients with T1mic and Tis were excluded. We selected patients with one primary malignant neoplasm only and retrieved their demographic and clinical data, including age, sex, race/ethnicity, marital status, tumor grade, Seventh AJCC T stage, surgery, chemotherapy, and radiotherapy. Patients with incomplete information were excluded, as were male patients.
Statistical analysis
Frequencies and proportions were calculated to describe the baseline characteristics of eligible patients, and categorical variables were compared between the different stages using Pearson’s chi-squared test. OS and BCSS were investigated in the total study population. To improve the comparability between groups, 1:1 propensity score matching (PSM, method = "nearest", caliper = 0.02) was used to reduce the influence of confounding factors. Kaplan–Meier survival was generated and log-rank tests applied to determine the influence of NAC on the prognosis. In further univariate and multivariable Cox proportional analyses, hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated to evaluate the association of NAC with OS and BCSS. Next, to verify the National Comprehensive Cancer Network guidelines for TNBC, we calculated adjusted HRs and corresponding 95% CIs for patients who received NAC in comparison with those who received AC when stratified by age and T and N stage to handle potential biases and evaluate the prognostic association with NAC. All statistical analyses and generation of figures were performed using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value of < 0.05 was considered statistically significant.
Ethics declarations
The SEER database is publicly accessible and does not provide personal identifying information. Therefore, institutional review board approval and informed patient consent were not required.
Data availability
All the data analyzed in this study are available online: https://seer.cancer.gov.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49. https://doi.org/10.3322/caac.21820 (2024).
Harbeck, N. & Gnant, M. Breast cancer. Lancet 389, 1134–1150. https://doi.org/10.1016/s0140-6736(16)31891-8 (2017).
Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 23(Suppl 6), vi7-12. https://doi.org/10.1093/annonc/mds187 (2012).
Rouzier, R. et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 11, 5678–5685. https://doi.org/10.1158/1078-0432.Ccr-04-2421 (2005).
Carey, L. A. et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 13, 2329–2334. https://doi.org/10.1158/1078-0432.Ccr-06-1109 (2007).
Li, Y. et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 15, 121. https://doi.org/10.1186/s13045-022-01341-0 (2022).
Liedtke, C. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 41, 1809–1815. https://doi.org/10.1200/jco.22.02572 (2023).
Curigliano, G. et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 34, 970–986. https://doi.org/10.1016/j.annonc.2023.08.017 (2023).
Gradishar, W. J. et al. NCCN guidelines® insights: Breast cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. 21, 594–608. https://doi.org/10.6004/jnccn.2023.0031 (2023).
Loibl, S. et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 35, 159–182. https://doi.org/10.1016/j.annonc.2023.11.016 (2024).
Dent, R. et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434. https://doi.org/10.1158/1078-0432.Ccr-06-3045 (2007).
Cardoso, F. et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 30, 1194–1220. https://doi.org/10.1093/annonc/mdz173 (2019).
Pusztai, L., Foldi, J., Dhawan, A., DiGiovanna, M. P. & Mamounas, E. P. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol. 20, e390–e396. https://doi.org/10.1016/s1470-2045(19)30158-5 (2019).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 30, 1181. https://doi.org/10.1093/annonc/mdy537 (2019).
Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Primers. 5, 66. https://doi.org/10.1038/s41572-019-0111-2 (2019).
Curigliano, G. et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 28, 1700–1712. https://doi.org/10.1093/annonc/mdx308 (2017).
Burstein, H. J. et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 30, 1541–1557. https://doi.org/10.1093/annonc/mdz235 (2019).
Shepherd, J. H. et al. CALGB 40603 (Alliance): Long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J. Clin. Oncol. 40, 1323–1334. https://doi.org/10.1200/jco.21.01506 (2022).
Leon-Ferre, R. A. & Goetz, M. P. Advances in systemic therapies for triple negative breast cancer. BMJ 381, e071674. https://doi.org/10.1136/bmj-2022-071674 (2023).
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100. https://doi.org/10.1016/s0140-6736(20)31953-x (2020).
Ho, A. Y. et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer 118, 4944–4952. https://doi.org/10.1002/cncr.27480 (2012).
Korde, L. A., Somerfield, M. R. & Hershman, D. L. Use of immune checkpoint inhibitor pembrolizumab in the treatment of high-risk, early-stage triple-negative breast cancer: ASCO guideline rapid recommendation update. J. Clin. Oncol. 40, 1696–1698. https://doi.org/10.1200/jco.22.00503 (2022).
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 39, 1485–1505. https://doi.org/10.1200/jco.20.03399 (2021).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804. https://doi.org/10.1200/jco.2011.38.8595 (2012).
Sikov, W. M. et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 33, 13–21. https://doi.org/10.1200/jco.2014.57.0572 (2015).
Dieci, M. V. et al. Inclusion of platinum agents in neoadjuvant chemotherapy regimens for triple-negative breast cancer patients: Development of GRADE (grades of recommendation, assessment, development and evaluation) recommendation by the Italian Association of Medical Oncology (AIOM). Cancers (Basel). https://doi.org/10.3390/cancers11081137 (2019).
von Minckwitz, G. et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 15, 747–756. https://doi.org/10.1016/s1470-2045(14)70160-3 (2014).
Schneeweiss, A. et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): A randomised phase III trial. Eur. J. Cancer 106, 181–192. https://doi.org/10.1016/j.ejca.2018.10.015 (2019).
Donegan, W. L. Tumor-related prognostic factors for breast cancer. CA Cancer J. Clin. 47, 28–51. https://doi.org/10.3322/canjclin.47.1.28 (1997).
Gajdos, C., Tartter, P. I. & Bleiweiss, I. J. Lymphatic invasion, tumor size, and age are independent predictors of axillary lymph node metastases in women with T1 breast cancers. Ann. Surg. 230, 692–696. https://doi.org/10.1097/00000658-199911000-00012 (1999).
Cronin, K. A., Ries, L. A. & Edwards, B. K. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer 120(Suppl 23), 3755–3757. https://doi.org/10.1002/cncr.29049 (2014).
Acknowledgements
We acknowledge the data support of the SEER program, as well as the Instrument Analysis Center of Xi’an Jiaotong University.
Funding
This study was supported by the National Natural Science Foundation of China (No.52203186), the Basic Research Program of Natural Science Foundation of Shaanxi Province (No.2021JQ-422), and the Natural Science Foundation of Shaanxi Provincial Department of Education (No. 2022KW-01 and 2022JM-101).
Author information
Authors and Affiliations
Contributions
H.K., X.M., and H.W. designed this study and directed the specific study concepts, Q.H. and L.D. analyzed the data and wrote the main text of the manuscript. L.C., D.S. and D.L. collected the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hao, Q., Dai, L., Chang, L. et al. Evaluation of neoadjuvant chemotherapy for clinical T1 triple-negative breast cancer. Sci Rep 14, 18055 (2024). https://doi.org/10.1038/s41598-024-68719-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68719-7