Abstract
Soil contamination with heavy metals presents a substantial environmental peril, necessitating the exploration of innovative remediation approaches. This research aimed to investigate the efficiency of nano-silica in stabilizing heavy metals in a calcareous heavy metal-contaminated soil. The soil was treated with five nano-silica levels of 0, 100, 200, 500, and 1000 mg/kg and incubated for two months. The results showed that nano-silica had a specific surface area of 179.68 \({\text{m}}^{2}/\text{g}\). At 1000 mg/kg, the DTPA-extractable concentrations of Pb, Zn, Cu, Ni, and Cr decreased by 12%, 11%, 11.6%, 10%, and 9.5% compared to the controls, respectively. Additionally, as the nano-silica application rate increased, both soil pH and specific surface area increased. The augmentation of nano-silica adsorbent in the soil led to a decline in the exchangeable (EX) and carbonate-bound fractions of Pb, Cu, Zn, Ni, and Cr, while the distribution of heavy metals in fractions bonded with Fe–Mn oxides, organic matter, and residue increased. The use of 1000 mg/kg nano-silica resulted in an 8.0% reduction in EX Pb, 4.5% in EX Cu, 7.3% in EX Zn, 7.1% in EX Ni, and 7.9% in EX Cr compared to the control treatment. Overall, our study highlights the potential of nano silica as a promising remediation strategy for addressing heavy metal pollution in contaminated soils, offering sustainable solutions for environmental restoration and ecosystem protection.
Similar content being viewed by others
Introduction
Soil, a critical component of the ecosystem, significantly affects the health of plants, animals, and humans. In the ongoing battle against environmental pollution, soil contamination with heavy metals presents a significant challenge, threatening both ecosystems and human health. The behavior of heavy metals in soils varies with soil type, and composition, and over time, with chemical reactions often reducing their bioavailability and solubility. Unlike organic pollutants, heavy metals remain in the soil for long periods, resisting chemical and microbial decomposition, necessitating their relocation, removal, or impact reduction1. Traditional remediation methods often fail to effectively address the complex nature of heavy metal contamination in soils. However, emerging technologies are offering innovative and sustainable solutions to remediate heavy metal-contaminated soils.
A cost-effective method for mitigating heavy metal contamination in soil is immobilization2. This environmentally friendly approach aims to prevent toxic compounds from entering biological cycles by reducing their solubility or toxicity3. Immobilization involves mixing contaminated soil with suitable compounds, which induces changes in pH, specific surface area (SSA), ion exchange, adsorption, and stabilization processes, thereby reducing the mobility and toxicity of pollutants4. Various soil components, such as silicate minerals, organic matter, clay minerals, and iron and manganese oxides, can trap heavy metals in their lattice structures or form bonds with them. The strength of these bonds affects the retention or release of heavy metals from the soil5.
Heavy metals in soils exist in different geochemical forms. The distribution of these geochemical forms in soils varies based on pH, cation exchange capacity, soil texture, redox state, organic matter, lime, and Fe–Mn oxide contents6. An understanding of the distribution of heavy metals among these various geochemical forms is crucial in determining their solubility, availability, and toxicity in the soil7. The sequential extraction method5 serves as a valuable tool for identifying the chemical forms of heavy metals and assessing their bioavailability8.
The traditional stabilizers for the immobilization of heavy metals include lime, hydroxyapatite, zeolite, phosphates9, bentonite10, fly ash and red mud, and so on11. Furthermore, new materials, such as nano-materials12, biochar13,14, polymer15 and modified material16 are also used as stabilizer to remediate heavy metal contaminated soils. These stabilizers can reduce the activity of heavy metals in soils to a certain extent, but their specificity and long-term stability are not enough, and their influence on soil properties has not been detected, which limits their large-scale application. Therefore, it is necessary to develop a new stabilizer with strong specificity, long-term stability and few adverse effects on soil environment.
Silica or functionalized silica can be used as an adsorbent to remove heavy metals from aqueous systems and to immobilize them in soil. Silica is an inorganic solid that is made of a three-dimensional network structure and a porous structure with a very large surface area. Silica is mainly composed of siloxane groups (Si–O–Si) inside and silanol groups (Si–OH) on the particle surface. Silanol groups can be separated into three types (single classified silanol), geminal (binary), and adjacent silanol. Silanol groups located on the surface of silica easily react with various agents. Many properties such as adsorption, adhesion, catalytic, and chemical properties of silica depend on the chemistry of these surface groups17. In addition to the surface, silanol groups may also be found inside the silica skeleton. Silanol groups are hydrophilic and siloxanes are hydrophobic18.
Nanoparticles are used in many sciences. Many nanoparticles have very different properties from micro and macro materials. The main reason for using nanoparticles is their very small size and large specific surface area, which play an important role in chemical reactions. Nanoparticles are widely used in the stabilization of heavy metals in soils due to their high adsorption capacity, high reactivity, and unsaturated surfaces. Silicon dioxide modified nanoparticles have been used in some studies as a sorbent to remediate heavy metals in soil and alleviate the stress of heavy metals on plants. For example, Zhang19 reported that the application of modified nano-silica transformed Cu, Pb, and Zn to a more stable fraction in soil. Lian20 indicated that the nano-silica decreased the DTPA-extractable Cd in soil effectively. However, the application rates of nano-silica in their research were much higher than an acceptable rate for large-scale applications (the maximum application rate is 6%). In recent years, research on the application of nano-silica for the removal or stabilization of heavy metals in aquatic environments21,22,23,24,25,26 and functionalized nano-silica for the stabilization of heavy metals in soils27,28,29,30 at high rates economically unfeasible has shown promising results. However, the use of un-functionalized silica nanoparticles for the remediation of heavy metals in complex soil environments, especially at economically feasible rates, has been relatively overlooked. Building on this foundation, a new study has been conducted to explore using un-functionalized silica nanoparticles to stabilize heavy metals in soil. This innovative approach not only aims to improve the efficiency of metal stabilization but also seeks to understand the impact of nano-silica on soil properties and the re-distribution of metals within different soil solid phases.
The goals of this research include (i) investigating the influences of un-functionalized nano-silica on specific surface area and pH of calcareous soil as two important soil factors affecting the sorption and availability of heavy metals, (ii) assessing the effectiveness of un-functionalized nano-silica in stabilizing heavy metals in calcareous soil, and (iii) unraveling the mechanisms underlying the stabilization of heavy metals by un-functionalized nano-silica. By shedding light on these aspects, the study provides valuable insights into the potential of un-functionalized nano-silica for the remediation of heavy metal-contaminated soils.
Materials and methods
Soil analysis
A composite soil sample at a depth of 0–15 cm was collected from an urban park located in Tehran, Iran. The sample was air-dried at room temperature, passed through a 2 mm sieve, analyzed for physico-chemical properties, and used for this study. Soil texture was determined by the hydrometer method31. The pH at a 1:5 soil-to-water ratio and the electrical conductivity of saturated paste extract (ECe) were measured using a pH meter and an EC meter, respectively32,33. Organic carbon and calcium carbonate contents in the soil were measured by using Walkley–Black34 and the Calcimetry methods35. Cation exchange capacity (CEC) was determined using the sodium acetate method36, and specific surface area (SSA) was measured by BET. The available fractions of heavy metals in the soil were extracted by DTPA37, and their concentrations were determined using ICP-MS. Total concentration of heavy metals in the soil was measured by ICP-MS after aqua regia digestion38.
Soil amendment
Nano-silica with a chemical formula \({\text{SiO}}_{2}\) and a purity of 99.5% was prepared by Pasargad Novin Chemical Company. Some characteristics of the prepared nano-silica were determined using XRF, XRD, SEM, FTIR, and BET techniques.
Pot experiment
To investigate the effect of nano-silica on the immobilization of Pb, Zn, Cu, Ni, and Cr in the soil, a pot experiment was conducted under greenhouse conditions using a completely randomized design and three replications. The nano-silica was mixed with three kg of urban soil at five rates of 0, 100, 200, 500, and 1000 mg nano-silica per kg soil. Treated and untreated (control) soil samples were incubated for two mouths at the moisture of field capacity. At the end of the incubation period, treated and untreated soil samples were air-dried at room temperature and used to evaluate the impacts of different levels of nano-silica on available and chemical fractions of Pb, Zn, Cu, Ni, and Cr in the soil.
Sequential extraction
Chemical fractions of heavy metals were determined in treated and untreated soil samples by the sequential extraction method5. This procedure partitions the total content of heavy metal into five fractions: exchangeable (EX), bound to carbonates (CAR), bound to Fe–Mn oxides (OX), bound to organic matter (OM), and residual (RES). Each fraction was extracted by a special extractant at a given time and temperature presented in Table 1.
Statistical analysis
The experimental data were analyzed using the SPSS 21.0 statistical software package and Microsoft Excel 2016. The experiment was conducted based on a completely randomized design (CRD) with three replicates for each treatment. The treatments included five nano-silica levels of 0, 100, 200, 500, and 1000 mg/kg. A one-way ANOVA was performed to determine the effect of different levels of nano-silica on each response variable. The means for each treatment group were compared using Duncan’s multiple range test at a significance level of P < 0.05.
Results and discussion
Soil characteristics
Some physico-chemical properties of the soil used in this study are summarized in Table 2. The studied soil was non-saline, calcareous, poor in OC content, and with a basic pH and a silty loam texture.
Nano-silica characteristics
XRF analysis
The results of the chemical analysis of nano-silica by XRF are shown in Table 3. Silica nanoparticles have more than 99% silicon dioxide, and the impurities include Fe and Na, with amounts less than 20 and 50 mg/kg, respectively. Calcium and Ti are also present, with values less than 70 and 120 mg/kg, respectively.
XRD analysis
The XRD pattern of silica nano adsorbent is shown in Fig. 1. Intense peaks at 22.15 and 44.3 angles indicate the presence of \({\text{SiO}}_{2}\) crystal structure in the tetragonal crystal system. Parameters a, b, and c are determined as 4.7, 4.7, and 7.4, respectively. Among other crystallographic parameters of this material, we can mention alpha, beta, and gamma, all of which are 90° (Fig. 1).
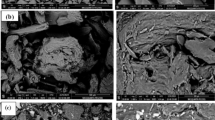
The SEM image
The surface morphology of silica particles determined by a scanning electron microscope (SEM) is shown in Fig. 2. Silica nanoparticles have a spherical shape.
The FTIR spectrum
FTIR analysis was used to determine surface functional groups affecting adsorption. The FTIR spectrum of silica nanoparticles is shown in Fig. 3. The strong peaks in the regions of 471.04, 812.12, and 1138.25 \({\text{cm}}^{-1}\) are related to the asymmetric stretching vibrations of siloxane groups (Si–O–Si). The peak in the region of 3427.6 \({\text{cm}}^{-1}\) corresponds to the vibrational stretching of the O–H group, which overlaps with the silanol (Si–OH) group39,40.
BET specific surface area
Based on the results of the BET technique, the adsorption and desorption curve of nano-silica is type IV, indicating the mesoporous structure of silica. Silica nanoparticles have a specific surface area of 179.68 \({\text{m}}^{2}/\text{g}\) and the percentage of porosity is 93.95% (Fig. 4a). The mesopore volume and diameter are obtained from the BJH curve. According to the BJH curve, the total volume and diameter of the nano-silica pores were 0.397 \({\text{cm}}^{3}/\text{g}\) and 2.42 nm, respectively (Fig. 4b).
The nano-silica effect on soil pH
The results of The nano-silica effects on soil pH are presented in Fig. 5. The results showed that application of nano-silica caused an increase in soil pH compared to the control treatment. The pH value increased from 7.43 in the control to 7.87 and 7.88 with the application of 500 and 1000 mg of silica per kg of soil, respectively. pH is one of the important factors in controlling the balance between heavy metal solution in soil41. Previous reports indicated that the addition of Si-based materials changed the pH of the soil42, which was observed in the present research.
The nano-silica effect on the DTPA-extractable concentrations of metals
The mean comparison results of the effects of different nano-silica levels on the DTPA extractable concentration of heavy metals showed that the highest available concentration of metals was in the control treatment, while the lowest available concentration of metals was in the 1000 mg/kg Nano-silica treatment (Fig. 6).
The highest and lowest concentrations of DTPA extractable Pb were 7.54 and 6.63 mg/kg, with a 12% decrease observed when applying 1000 mg/kg of nano-silica compared to the control treatment. For Zn and Cu, the highest concentrations found in the control treatment were 27.12 and 7.75 mg/kg, respectively, while the lowest concentrations observed in the 1000 mg/kg treatment were 24.08 and 6.85 mg/kg, resulting in an 11% decrease for Zn and an 11.6% decrease for Cu compared to the control. Similarly, for Ni and Cr, the highest concentrations found in the control treatment were 6.24 and 9.42 mg/kg, respectively, and the lowest concentrations observed in the 1000 mg/kg treatment were 5.61 and 8.52 mg/kg, corresponding to a 10% decrease for Ni and a 9.5% decrease for Cr compared to the control treatment. Overall, the most significant reduction in the available concentration of heavy metals in the tested soil was observed for Pb, followed by Cu, Zn, Ni, and Cr (4).
Unique properties exist between different heavy metal ions, such as ionic radius, electronegativity, and hydration radius43. Previous studies have shown that these intrinsic properties are inseparable from the adsorption properties of heavy metal ions, and the adsorption stability and adsorption energy are also affected by them43. In a study conducted by Pan43, they stated the modified biomass-based adsorption technique has attracted much attention in heavy metal ions removal, a carboxylated biogas residue (BR–COOH) was prepared to remove the \({\text{Cu}}^{2+}\) and \({\text{Zn}}^{2+}\) from single/binary heavy metal ions solution and explore selective adsorption mechanism. The results exhibited that the adsorption capacities of BR–COOH for \({\text{Cu}}^{2+}\) was higher than that for \({\text{Zn}}^{2+}\) obviously, whether in the single or binary heavy metal ions solution. Meanwhile, the inconsistency in the change of adsorption capacity for \({\text{Cu}}^{2+}\) and \({\text{Zn}}^{2+}\) also confirmed that differences in affinity exist between BR–COOH and different heavy metal ions, and \({\text{Cu}}^{2+}\) seems to be more readily captured. The maximum adsorption capability of \({\text{Cu}}^{2+}\) was visibly higher than that of \({\text{Zn}}^{2+}\), indicating that the \({\text{Cu}}^{2+}\) preferentially adsorbed to the carboxyl functional groups and occupied the active sites at the same time. The adsorbed \({\text{Cu}}^{2+}\) was unable to be exchanged into solution by \({\text{Zn}}^{2+}\). They also reported the adsorbed quantities of these metal ions followed the order of \({\text{Hg}}^{2+}\) > \({\text{Cu}}^{2+}\) >\({\text{Pb}}^{2+}\) > \({\text{Fe}}^{2+}\) > \({\text{Cd}}^{2+}\) > \({\text{Zn}}^{2+}\) > \({\text{Mn}}^{2+}\) > \({\text{Mg}}^{2+}\). The difference in the amounts of Pb, Cu and Zn adsorbed may be due to the acid–base theory. Pb belongs to hard acids and tends to complex with hydroxy (hard base) groups on the surface of silica more than Cu and Zn. Thus, Pb is more prone to immobilization than Cu and Cd44. The amount of specific adsorption of ions on solid surfaces depends largely on the electric charge, the hydration radius of the ions, the sealing energy, and the electronegativity of the ions. Reducing the hydrated radius and energy, and increasing the electronegativity increases the tendency to ion-specific adsorption45. The reason for the higher adsorption of Pb and Cu ions than Zn is likely due to the lower hydrated radius of the Pb (0.401 nm), and Cu (0.419 nm) compared to the Zn (0.43 nm) and their higher electronegativity46,47. Pan43 stated that \({\text{Cu}}^{2+}\) was more easily adsorbed onto carboxylated biosorbent than \({\text{Zn}}^{2+}\).
In the present study, it was observed that soil pH increased with increasing the application level of nano-silica, but the concentration of DTPA-extractable Cr decreased from 9.4 mg/kg in the control treatment to 8.5 mg/kg at the highest nano-silica application level. A negative correlation between soil pH and heavy metal mobility in soil and bioavailability to plants has been well documented in the literature48. However, the effect of soil pH on Cr sorption/desorption in soil varies with its chemical form and oxidation state. Chromium in soil exists in two common oxidation states: Cr(III), and Cr (VI). In the Cr(III) valence state, Cr is a metal cation (as the free \({\text{Cr}}^{3+}\) species or as a hydrolysis product: \({\text{CrOH}}^{2+}\) or \({\text{Cr}(\text{OH})}_{2}^{+}\) depending on solution pH). In the Cr (VI) state, Cr occurs in the chromate species: \({\text{HCrO}}_{4}^{-}\) and \({\text{CrO}}_{4}^{2-}\)49. Thus, increasing soil pH has a contrary effect on the sorption of Cr(III) and Cr (VI) species. The sorption of Cr(III) on soil solids increases with an increase in pH, while Cr (VI) sorption on soil particles decreases with an increase in pH48. Nano-silica induced coordination, co-precipitation, and other geochemical behaviors with Cr, which inhibit the increased electrostatic repulsion of Cr (VI) with soil colloids resulted from an increase in soil pH, could be reasons for this decreased DTPA-extractable concentration of Cr observed in the present study50,51.
It seems that the application of 1000 mg/kg nano-silica provided sufficient surfaces for the adsorption of heavy metals thus decreasing their concentration in the soil solution. The surfaces of nano-silica have hydroxyl active groups that have high adsorption capacity and are in the forms of free silanol (Si–OH) groups, free silanol diol groups (\({\text{Si}-(\text{OH})}_{2}\)) and atomic bridges with oxygen ions (Si–O–Si) in surface17. Silanol groups on the silica surface react easily with a variety of agents. The adsorption capacity of the silica depends on the charge and electronegativity of the metals; the metal cations in the solution form a chemical bond with the siloxane oxygen attached to the surface of the silica. Both silanol and siloxane groups in the nano-silica surface play a very important role in the adsorption capacity of metals52. Lian20 reported that \({\text{SiO}}_{2}-\text{SH}\) can significantly (P \(<\) 0.05) decrease the heavy metal concentration in the plants, which indicates that the \({\text{SiO}}_{2}-\text{SH}\) can immobilize the heavy metals in the contaminated soil and reduce their phytoavailability. Some Si-based materials have been used to remediate heavy metals in soil and alleviate the stress of heavy metals on plants13,53.
Zhang27 reported that the application of modified nano-silica transformed Cu, Pb, and Zn to a more stable fraction in soil. Lian20 indicated that the nano-silica decreased the DTPA-extractable Cd in soil effectively. Silica nanoparticles (\({\text{NSiO}}_{2}\)) are very efficient in removing metal ions due to the surface characteristics of silica54. Investigations showed that among various organic and inorganic modifiers, silica nanoparticles are widely used due to their large surface area and suitable places for metal adsorption. Studies on the adsorption of heavy metals Ni, Cd, and Pb by porous silica nanoparticles in aqueous environments have been also carried out. Rezvani-Barojni55 stated that nano-silica has a high adsorption capacity for Hg and this adsorbent had inhomogeneous adsorption sites that had different adsorption potentials.
The soil pH effect on the DTPA-extractable concentrations of metals
The results showed by increasing the soil pH, the DTPA extractable concentrations of heavy metals decreased (Fig. 7). The surface charge of silica increases with increasing pH, and at higher pH, the negative charge of the silica surface causes more metal cations to be adsorbed56. An increase in pH causes a decrease in metals in the available fractions of heavy metals and a reduction in their bioavailability in the soil10,57. Heidari57 also reported that by increasing the pH of the solution, the adsorption capacity of silica for Ni, Cd, and Pb increased.
In the adsorption process, solution pH is crucial; it influences both the contaminants’ ionization level and the adsorbent’s surface charge58. Meky26 in a study about the pH effect on removing Pb by nano-silica from aqua medium reported when pH falls below 3, the synthesized nano \({\text{SiO}}_{2}\)’s zeta potential data shows that it has reached the isoelectric point, which is the point at which the positive and negative charges produced by the silanol groups on the surface of the silica particles are equal. The silanol groups with the Si–OH structure are stable at that moment. The zeta potential grows in negative proportion as the pH value rises over 3.5, signifying a rise in the amount of negative charges on the particle surface. The equilibrium of the SiOH/SiO—acid/base dissociation means that an increase in negative charges will lead to an increase in \({\text{SiO}}^{-}\) species and the surface energy59. Subsequently, the surface of \({\text{SiO}}_{2}\) is positively charged at any pH value below the pzc and negatively charged at any pH value over the pzc. For Pb (II), when the pH of the solution increases (pH > 3), the surface of the synthesized nano-\({\text{SiO}}_{2}\) becomes negatively charged, and as a result, the adsorption of positively charged Pb (II) is enhanced due to the electrostatic attraction. Ahmad60 also investigated the effect of silica nanoparticles on Cu adsorption in the aqueous medium and found that by increasing the pH of the solution from 4 to 6.5, the amount of Cu adsorption increased. This shows that at lower pH, the concentration of \({\text{H}}^{+}\) ions is high and these ions are competing with other metal ions to form chelate and complexation in the exchange sites of the silica surface. At higher pH, hydroxyl ions in the reaction medium increase, and metal ions tend to form hydroxide or react with surface hydroxyls.
The nano-silica effect on soil SSA and the relationship between soil SSA and DTPA-extractable concentrations of metals
The application of nano-silica to soil increased its SSA. The soil SSA increased from 19.63 \({\text{m}}^{2}/\text{g}\) in the control treatment to 21.23, 22.83, 24.43, and 26.03 \({\text{m}}^{2}/\text{g}\) with the application of 100, 200, 500, and 1000 mg/kg nano-silica, respectively. Increasing the soil SSA by applying nano-silica reduced the DTPA-extractable concentrations of heavy metals (Fig. 8). There was a good correlation coefficient \({\text{R}}^{2}\) between the soil SSA and DTPA extractable concentrations of heavy metals. The R2s obtained were obtained were 87% for Pb and Cu > 86% for Zn and Cr > 83% for Ni. The inverse correlation between soil SSA and DTPA extractable concentrations of heavy metals suggests that the increased surface area of the soil due to the application of nano-silica leads to increased adsorption of heavy metals, resulting in a reduction of their concentrations in the solution phase. The higher the amount of nano-silica present, the greater the available surface area provided, leading to increased metal adsorption. Specific surface area is the most effective property in the soil treated with silica Si nanoparticles, leading to many changes in physico-chemical properties61,62. Bayat63 reported the positive effects of different nanomaterials on soil surface area using magnesium oxide (MgO).
A material with a higher SSA can adsorb more heavy metal ions per unit mass compared to a material with a lower surface area. This is because a larger surface area provides more sites for the heavy metal ions to attach to the adsorbent material. Specific surface area is often correlated with the pore structure of the adsorbent material. Materials with higher surface areas tend to have a greater proportion of mesopores and micropores, which can provide additional surface area for adsorption and offer diffusion pathways for heavy metal ions into the material. The SSA influences the kinetics and thermodynamics of the adsorption process. A higher surface area can accelerate the adsorption kinetics by providing more sites for heavy metal ions to interact with the adsorbent material. Additionally, it can enhance the thermodynamic driving force for adsorption, leading to higher adsorption capacities. The high surface area of the silica compared to soil, provided high reactive sites, which allowed metal ions to be adsorbed on them64. Silica nanoparticles (\({\text{SiO}}_{2}\)) are very efficient in removing metal ions due to the surface characteristics of silica54. Investigations showed that among various organic and inorganic modifiers, silica nanoparticles are widely used due to their large surface area and suitable places for metal absorption.
In a study by Al-Saeed65 on the contribution of nano-silica in affecting some of the physico-chemical properties of cultivated soil, it was noted that varying rates of nano-silica have a significant effect on the percentage of clay particles, cation exchange capacity (CEC), sodium adsorption ratio (SAR), porosity, saturation percentage, SSA, and the concentrations of total nitrogen (N) and silicon (\({\text{Si}}^{4+}\)).
The effect of different silica nano levels on the fractionation of heavy metals in soil
The distribution of different geochemical forms of heavy metals Pb, Zn, Cu, Ni and Cr in untreated soil (control treatment) was as follow: CAR (43.79%) > OX (16.98%) > OM (14.49%) > RES (12.78%) > EX (11.1%) for Pb; CAR (45%) > OX (17%) > RES (15.5%) > OM (13.2%) > EX (9.3%) for Zn; CAR \(=\) OX (28.13%) > RES (23.49%) > OM \(=\) EX (10.19%) for Cu; OX (44.8%) > CAR (20.78%) > RES (14.48%) > EX (11.9%) > OM (8.04%) for N; and CAR (40.8%) > OX (18.52%) > RES (15.19%) > OM (14.19%) > EX (11.3%) for Cr (Table 5 and Fig. 9).
Mean comparisons showed that with increasing the nano-silica application level in the soil, the exchangeable and carbonate-bounded fractions of Pb, Cu, Zn, Ni, and Cr decreased but the Fe–Mn OX, OM, and RES fractions increased (Table 5 and Fig. 9).
At the nano-silica application level of 1000 mg/kg, the concentration of the EX and CAR bound fractions of Pb decreased from 6.73 and 26.03 mg/kg in the control treatment to 6.19 and 25.42 mg/kg, accounting for 8.02% and 2.34%, respectively. However, when applying nano-silica at a level of 1000 mg/kg, the concentration of the OX fraction of Pb increased from 10.28 mg/kg in the control treatment to 10.59 mg/kg. Similarly, the concentration of the OM fraction of Pb rose from 8.75 to 8.92 mg/kg, and the concentration of the RES fraction of Pb increased from 8.03 to 8.33 mg/kg. The increase in the concentration of different chemical forms of Pb using silica nanoparticles follows the order: RES (3.73%) > OX (3.01%) > OM (1.94%).
For Cu, by an increase in the amount of nano-silica in the soil, the concentration of EX and CAR fractions of Cu decreased by 4.46% and 2.26%, respectively, compared to the control treatment, so that the concentration of EX fraction of Cu was 6.27 mg/kg in the control treatment reached to 5.99 mg/kg in the 1000 mg/kg nano-silica treatment. The CAR fraction of Cu decreased from 17.22 mg/kg in the control treatment to 16.83 mg/kg in the 1000 mg/kg of nano-silica. In 1000 mg/kg nano-silica, the amount of OX fraction of Cu increased from 17.24 mg/kg in the control treatment to 17.39 mg/kg. The OM fraction of Cu also increased from 6.27 mg/kg in the control treatment to 6.63 mg/kg with the application of nano-silica. Similarly, the Res fraction of Cu increased from 14.44 mg/kg in the control treatment to 14.57 mg/kg. The augmentation of Cu chemical forms through the application of silica nanoparticles followed this order: OM (5.74%) > RES (0.9%) > OX (0.87%).
The concentration of the EX and CAR bound fractions of Pb decreased from 6.27 and 17.22 mg/kg in the control treatment to 5.99 and 16.83 mg/kg at the nano-silica application level of 1000 mg/kg, representing 4.46% and 2.26% reductions, respectively. Conversely, in the 1000 mg/kg nano-silica treatment, the concentration of the OX fraction of Cu increased from 17.24 mg/kg in the control treatment to 17.39 mg/kg.
The concentration of the EX and CAR bound fractions of Zn decreased from 18.68 and 90.42 mg/kg in the control treatment to 17.32 and 88.7 mg/kg at the nano-silica application level of 1000 mg/kg, representing 7.28% and 1.9% reductions, respectively. Conversely, in the 1000 mg/kg nano-silica treatment, the concentration of the OX and Res fractions of Zn increased from 26.52 and 31.14 mg/kg in the control treatment to 26.88 and 31.25 mg/kg, in 1000 mg/kg nano-silica treatment, respectively. The effect of silica nanoparticles on changing the distribution of Zn chemical forms followed as OX (6.76%) > OM (1.35%) > RES (0.35%).
For Ni, the concentration of the EX and CAR bound fractions of Ni decreased from 4.76 and 8.31 mg/kg in the control treatment to 4.42 and 7.97 mg/kg at the nano-silica application level of 1000 mg/kg, corresponding to 7.14% and 4.09% reductions, respectively. Conversely, in the 1000 mg/kg nano-silica treatment, the concentration of the OX and Res fractions of Ni increased from 17.93 and 5.76 mg/kg in the control treatment to 18.26 and 5.96 mg/kg, in 1000 mg/kg nano-silica treatment, respectively. The effect of silica nanoparticles on changing the distribution of Ni chemical forms followed the order OM (6.54%) > RES (3.47%) > OX (1.88%).
For Cr, the concentration of the EX and CAR bound fractions of Cr decreased from 8.87 and 32.03 mg/kg in the control treatment to 8.17 and 31.77 mg/kg at the nano-silica application level of 1000 mg/kg, accounting for 7.9% and 0.8%, respectively. Conversely, in the 1000 mg/kg nano-silica treatment, the concentration of the OX, OM, and Res fractions of Cr increased from 14.53, 11.14, and 12.32 mg/kg in the control treatment to 14.77, 11.22, and 12.47 mg/kg, in 1000 mg/kg nano-silica treatment, respectively. The effect of silica nanoparticles on changing the distribution of Cr chemical forms followed the order OX (1.72%) > RES (1.21%) > OM (0.71%).
In the present study, the exchangeable and carbonate-bounded fractions of heavy metals decreased by using nano-silica. Previous research indicated that the exchangeable and carbonate-bounded fractions of heavy metals usually determine the real environmental risk14, which means the addition of nano-silica reduced the risk of heavy metals in the contaminated soil. Similar results have also been reported by Lian20 and Wang53.
Several mechanisms, such as adsorption, complexation, co-precipitation, and changes in soil properties like pH and surface area, have been reported in the literature for the redistribution and immobilization of heavy metals in soils induced by the nanosilica application. Nano-silica has a high specific surface area and can provide numerous active sites for adsorption of heavy metals such as Pb, Cu, Zn, Ni, and Cr through inner-sphere surface complexation20,66,67. The sorption of heavy metals onto \({\text{SiO}}_{2}\) surfaces can reduce their concentrations in the more easily extractable fractions (e.g., exchangeable, and carbonated bound), effectively sequestering them and preventing their leaching or mobility20. Heavy metals that are strongly adsorbed to the nano-silica surface will also be associated with the residual fraction, as they become more resistant to extraction20. \({\text{SiO}}_{2}\) can also co-precipitate with trace metals, incorporating them into the \({\text{SiO}}_{2}\) mineral structure66. The co-precipitation of heavy metals with \({\text{SiO}}_{2}\) can reduce their concentrations in the carbonated bound and Fe–Mn oxide fractions, as the co-precipitated metals will be associated with the residual fraction, which represents the most recalcitrant and structurally incorporated forms of the metals5. Nano-silica can alter the soil pH, which can influence the solubility and mobility of heavy metals. A higher pH can lead to the precipitation of metal hydroxides, which can then become associated with Fe/Mn oxyhydroxides30. This transformation can result in a decrease in the exchangeable and carbonate bounded fractions as metals are immobilized in less bioavailable forms30. The presence of nano-silica can also enhance the binding of heavy metals to organic matter in the soil, as it can act as a bridge between the metal ions and organic functional groups68. This results in an increase in the organic matter bounded fraction of heavy metals53.
Conclusions
Applying the nano-silica increased the soil pH and SSA but decreased the DTPA-extractable concentrations of heavy metals in the studied calcareous soil. The nano-silica caused a significant decrease in the concentrations of Pb, Cu, Zn, Ni, and Cr in the exchangeable and carbonate-bound fractions, while the distribution of heavy metals in fractions bonded with Fe–Mn oxides, organic matter, and residue increased. The highest effect of silica nano sorbent in reducing the available concentration of heavy metals in the studied soil was Pb > Cu > Zn > Ni > Cr. There was a good correlation coefficient (\({\text{R}}^{2}\)) between the soil SSA and DTPA-extractable concentrations of heavy metals. Nano-silica could be used to remediate heavy metal-contaminated agricultural soils. The stabilization mechanism of heavy metals could be attributed to the -SH and -OH bonds of Nano-silica. This study suggests that nano-silica has advantages and potential in the remediation of heavy metal-contaminated agricultural soils.
While nano-silica-mediated stabilization of heavy metals in contaminated soils holds great promise, further research is warranted to address certain aspects and optimize its application. The following future directions could guide ongoing investigations: Understanding the interactions at the nanoscale is essential for tailoring nano-silica to target different contaminants. Transitioning from laboratory experiments to field-scale trials is critical for validating the efficacy of nano-silica under real-world conditions. This step will provide insights into its performance in diverse soil types, climates, and contaminant scenarios. Also, a comprehensive assessment of the ecological impact of nano-silica on non-target organisms, plant growth, and overall soil biodiversity is necessary. This will ensure that the technology does not inadvertently lead to unintended environmental consequences.
Evaluating the economic feasibility of large-scale nano-silica applications is crucial for its practical implementation. Assessing the cost-effectiveness of the technology will contribute to its adoption by regulatory bodies and industries involved in environmental remediation.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Adriano, D. C. et al. Role of assisted natural remediation in environmental cleanup. Geoderma 122(2–4), 121–142 (2004).
Paff, S. W. & Bosilovich, B. E. Use of lead reclamation in secondary lead smelters for the remediation of lead contaminated sites. J. Hazard. Mater. 40(2), 139–164 (1995).
Malone, P., Jones, L. and Larson, R. Guide to the disposal of chemically stabilized and solidified waste, SW-872. 1982, Office of Water and Waste Management, USEPA. Washington DC, USA.
Stegmann, R., et al., Treatment of contaminated soil: Fundamentals, analysis, applications. Springer Science & Business Media, (2013).
Tessier, A., Campbell, P. G. & Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51(7), 844–851 (1979).
Zheng, H. et al. Sorption isotherm and kinetic modeling of aniline on Cr-bentonite. J. Hazard. Mater. 167(1–3), 141–147 (2009).
Adu-Gyamfi, J. et al. Geochemical assessment and pollution evaluation of stream sediments’ quality impacted by industrial activities at Suame Magazine area, Kumasi, Ghana. Arab. J. Geosci. 16(4), 256 (2023).
Saffari, M. et al. Effect of calcium carbonate removal on the chemical forms of zinc in calcareous soils by three sequential extraction methods. Res. J. Biol. Sci. 4(7), 858–865 (2009).
Xenidis, A., Stouraiti, C. & Papassiopi, N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J. Hazard. Mater. 177(1–3), 929–937 (2010).
Sun, Y. et al. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 105, 200–206 (2015).
Wang, Y. et al. Stabilization of Cd-, Pb-, Cu-and Zn-contaminated calcareous agricultural soil using red mud: A field experiment. Environ. Geochem. Health 40, 2143–2153 (2018).
Cui, H. et al. Fractions of Cu, Cd, and enzyme activities in a contaminated soil as affected by applications of micro-and nanohydroxyapatite. J. Soils Sedim. 13, 742–752 (2013).
Lu, H. et al. Influences of calcium silicate on chemical forms and subcellular distribution of cadmium in Amaranthus hypochondriacus L.. Sci. Rep. 7(1), 40583 (2017).
Hamzenejad Taghlidabad, R. & Sepehr, E. Heavy metals immobilization in contaminated soil by grape-pruning-residue biochar. Arch. Agron. Soil Sci. 64(8), 1041–1052 (2018).
Yuan, Y.-N. et al. Application of polymeric aluminum salts in remediation of soil contaminated by Pb, Cd, Cu, and Zn. J. Central South Univ. 20(6), 1638–1644 (2013).
Chen, W.-F. et al. Investigation of heavy metal (Cu, Pb, Cd, and Cr) stabilization in river sediment by nano-zero-valent iron/activated carbon composite. Environ. Sci. Pollut. Res. 23, 1460–1470 (2016).
Zhuravlev, L. The surface chemistry of amorphous silica Zhuravlev model. Colloids Surf. A Phys. Eng. Asp. 173(1–3), 1–38 (2000).
Wagh, P. & Ingale, S. Comparison of some physico-chemical properties of hydrophilic and hydrophobic silica aerogels. Ceram. Int. 28(1), 43–50 (2002).
Zhang, L.-Y., et al., Effects of heavy metals pollution on paddy soil aggregates composition and heavy metals distribution. Yingyong Shengtai Xuebao, 20(11), (2009).
Lian, M. et al. Highly effective immobilization of Pb and Cd in severely contaminated soils by environment-compatible, mercapto-functionalized reactive nanosilica. J. Clean. Prod. 235, 583–589 (2019).
Kumar, R., Rauwel, P. & Rauwel, E. Nanoadsorbants for the removal of heavy metals from contaminated water: Current scenario and future directions. Processes 9(8), 1379 (2021).
Bai, L. et al. Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J. Hazard. Mater. 195, 261–275 (2011).
Salmani, M. H. et al. Synthesis, characterization and application of mesoporous silica in removal of cobalt ions from contaminated water. Groundw. Sustain. Dev. 11, 100425 (2020).
Al-Saida, B. et al. Synthesis of nanosilica for the removal of multicomponent Cd2+ and Cu2+ from synthetic water: An experimental and theoretical study. Molecules 27(21), 7536 (2022).
Al-Wasidi, A. S. et al. Modification of silica nanoparticles with 1-hydroxy-2-acetonaphthone as a novel composite for the efficient removal of Ni (II), Cu (II), Zn (II), and Hg (II) ions from aqueous media. Arab. J. Chem. 15(8), 104010 (2022).
Meky, N. et al. Synthesis of nano-silica oxide for heavy metal decontamination from aqueous solutions. Water, Air, Soil Pollut. 235(2), 154 (2024).
Zhang, L. et al. Speciation analysis and speciation transformation of heavy metal ions in passivation process with thiol-functionalized nano-silica. Chem. Eng. J. 369, 979–987 (2019).
Wang, Y. et al. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 197, 110600 (2020).
Wang, Y. et al. Effect of surface-modified nano-silica on the mobility and fraction of Cd in contaminated agricultural soils. Soil Sedim. Contam. An Int. J. 29(1), 96–106 (2020).
Lei, C. et al. Immobilization of Pb and Zn in contaminated soil using alumina-silica nano-amendments synthesized from coal fly ash. Int. J. Environ. Res. Public Health 19(23), 16204 (2022).
Gee, G. W. & Bauder, J. W. Particle-size analysis. Methods Soil Anal. Part 1 Phys. Mineral. Methods 5, 383–411 (1986).
Thomas, G. W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 5, 475–490 (1996).
Rhoades, J., Salinity: Electrical conductivity and total dissolved solids. Methods Soil Anal. Part 3—Chem. Methods, 417–435 (1996).
Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37(1), 29–38 (1934).
Helmke, P. A. & Sparks, D. L. Lithium, sodium, potassium, rubidium, and cesium. Methods Soil Anal. Part 3 Chem. Methods 5, 551–574 (1996).
Bower, C. & Hatcher, J. Simultaneous determination of surface area and cation-exchange capacity. Soil Sci. Soc. Am. J. 30(4), 525–527 (1966).
Lindsay, W. L. & Norvell, W. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 42(3), 421–428 (1978).
Soon, Y. and Abboud, S. Cadmium, chromium, lead and nickel. 1993, Chap.
Lin, J. et al. Immobilization of cadmium in polluted soils by phytogenic iron oxide nanoparticles. Sci. Total Environ. 659, 491–498 (2019).
Ramezani, M., Vaezi, M. R. & Kazemzadeh, A. Preparation of silane-functionalized silica films via two-step dip coating sol–gel and evaluation of their superhydrophobic properties. Appl. Surf. Sci. 317, 147–153 (2014).
Malandrino, M. et al. Accumulation of heavy metals from contaminated soil to plants and evaluation of soil remediation by vermiculite. Chemosphere 82(2), 169–178 (2011).
Ji, X. et al. Effect of silicon fertilizers on cadmium in rice (Oryza sativa) tissue at tillering stage. Environ. Sci. Pollut. Res. 24, 10740–10748 (2017).
Pan, J. et al. Insights into selective adsorption mechanism of copper and zinc ions onto biogas residue-based adsorbent: Theoretical calculation and electronegativity difference. Sci. Total Environ. 805, 150413 (2022).
Chaturvedi, P. K., Seth, C. S. & Misra, V. Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 64(7), 1109–1114 (2006).
Vico, L. Acid–base behaviour and Cu2+ and Zn2+ complexation properties of the sepiolite/water interface. Chem. Geol. 198(3–4), 213–222 (2003).
Nightingale, E. Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 63(9), 1381–1387 (1959).
Abd-Elfattah, A. & Wada, K. Adsorption of lead, copper, zinc, cobalt, and cadmium by soils that differ in cation-exchange materials. J. Soil Sci. 32(2), 271–283 (1981).
Shahid, M. et al. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 178, 513–533 (2017).
Essington, M. E., Soil and water chemistry: an integrative approach. CRC press (2015).
Lyu, P. et al. Ternary Ca–Mg–Al layered double-hydroxides for synergistic remediation of As, Cd, and Pb from both contaminated soil and groundwater: Characteristics, effectiveness, and immobilization mechanisms. J. Hazard. Mater. 442, 130030 (2023).
Lyu, P. et al. Magnetic biochar-supported layered double hydroxide for simultaneous remediation of As and Cd in soil: Effectiveness, retention durability, and insight into a new immobilization mechanism. J. Clean. Prod. 434, 140136 (2024).
Flores-Cano, J. V. et al. Adsorption of heavy metals on diatomite: Mechanism and effect of operating variables. Adsorpt. Sci. Technol. 31(2–3), 275–291 (2013).
Wang, D. et al. Stabilization of Cd-contaminated agricultural soils by modified nano-silica. Environ. Chem 38(5), 1106–1112 (2019).
Huang, H. et al. Preparation and characterization of octyl and octadecyl-modified mesoporous SBA-15 silica molecular sieves for adsorption of dimethyl phthalate and diethyl phthalate. Microporous Mesoporous Mater. 111(1–3), 254–259 (2008).
Rezvani-Boroujeni, A. et al. Adsorption properties of thiol-functionalized silica nanoparticles prepared for application in poly (ether sulfone) nanocomposite membranes. J. Text. Polym. 5(1), 37–47 (2017).
Barisik, M. et al. Size dependent surface charge properties of silica nanoparticles. J. Phys. Chem. C 118, 1836 (2014).
Heidari, A., Younesi, H. & Mehraban, Z. Removal of Ni (II), Cd (II), and Pb (II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem. Eng. J. 153(1–3), 70–79 (2009).
Kuang, Y., Zhang, X. & Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 12(2), 587 (2020).
Xu, P. et al. Preparation and morphology of SiO 2/PMMA nanohybrids by microemulsion polymerization. Colloid Polym. Sci. 284, 755–762 (2006).
Ahmad, P. et al. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 13(1), 64–72 (2018).
Ghormade, V., Deshpande, M. V. & Paknikar, K. M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 29(6), 792–803 (2011).
Pérez-Hernández, H. et al. Effect of engineered nanoparticles on soil biota: Do they improve the soil quality and crop production or jeopardize them?. Land Degrad. Dev. 31(16), 2213–2230 (2020).
Bayat, H. et al. Iron and magnesium nano-oxide effects on some physical and mechanical properties of a loamy Hypocalcic Cambisol. Geoderma 335, 57–68 (2019).
Little, D. N. and Nair, S. Recommended practice for stabilization of subgrade soils and base materials. (2009).
Al-Saeedi, A. H. Contribution of nano-silica in affecting some of the physico-chemical properties of cultivated soil with the common bean (Phaseolus vulgaris). J. Adv. Agric. Res. 25(4), 389–400 (2020).
Yadav, M., George, N. and Dwibedi, V. Emergence of toxic trace elements in plant environments: Insights into potential of silica nanoparticles for mitigation of metal toxicity in plants. Environ. Pollut., 122112 (2023).
Morsy, H. Y. et al. Nano-silica synthesis, characterization and their effects on rice production in the presence of calcium humate under salinity soil stress. World J. Agric. Sci. 19(5), 189–203 (2023).
Liang, L., Luo, L. & Zhang, S. Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano-and micro-scales. Colloids Surf A: Physicochem Eng Asp. 384(1–3), 126–130 (2011).
Author information
Authors and Affiliations
Contributions
M.S., A.G., and H.A.A.; Writing-original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. A.F.G., and Y.K.A, A.M, U.A, R.P, D.S.M, M.A, P.T, S.M.; Writing-review & editing, Methodology, Investigation, Data curation, Conceptualization. A.G., and H.A.A.; Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Samani, M., Ahlawat, Y.K., Golchin, A. et al. Nano silica-mediated stabilization of heavy metals in contaminated soils. Sci Rep 14, 20496 (2024). https://doi.org/10.1038/s41598-024-69182-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69182-0
Keywords
This article is cited by
-
Stabilization of Leachate-Contaminated Clay with Micro and Nano Silica: An Experimental Study
Indian Geotechnical Journal (2026)
-
Silica-Based Encapsulation for Immobilizing Heavy Metal Mobility in Agricultural Soil
Water, Air, & Soil Pollution (2026)
-
Green remediation of lead (pb) from Pb-toxic soil by combined use of silicon nanomaterials and leguminous Lens culinaris L. plants
Scientific Reports (2025)
-
Experimental and Numerical Study on Nano-Silica-Stabilized Clayey Subgrades with Geogrid Support
Transportation Infrastructure Geotechnology (2025)
-
Microbially Induced Calcite Precipitation: A Critical Review on a Sustainable Method for Improving Expansive Soil in the Era of Climate Change
Arabian Journal for Science and Engineering (2025)