Abstract
The primary aim of this study was to evaluate computed tomography (CT)-based bone density analysis at the level of thoracic vertebra 12 (Th12) as a screening method for decreased bone density in patients admitted to the intensive care unit (ICU). Interobserver variability was analyzed. Secondary aims were to assess the prevalence of CT-based low bone density upon ICU admission in a cohort of COVID-19 patients and to assess the potential effect of long-term ICU stay on bone density in these patients. Retrospective single-center cohort study. ICU of the Leiden University Medical Center (LUMC), the Netherlands. Patients admitted to the ICU of the LUMC between March 1st, 2020 and February 1st, 2022 with a diagnosis of COVID-19, and a length of ICU stay of ≥ 21 days. In the included patients both baseline chest CT scans (obtained upon ICU admission) and follow-up chest CT scans (obtained ≥ 21 days after ICU admission) were available for analysis. A total of 118 CT scans in 38 patients were analyzed. There was a good interobserver variability, with an overall mean absolute difference (between measurements of three observers) of 9.7 Hounsfield Units (HU) and an intraclass correlation coefficient (ICC) of 0.93 (95% CI 0.88–0.96). The effect of intravenous contrast administration on bone density measurements was small (+ 7.5 HU (95% CI 3.4–11.5 HU)) higher in contrast enhanced CT images compared to non contrast enhanced CT images). Thirty-seven percent of patients had a bone density < 140 HU, suggestive of osteoporosis. No significant difference was found between bone density upon ICU admission and bone density at follow-up (≥ 21 days after ICU admission). Vertebral CT-based bone density analysis using routine CT scans is an easily applicable method to identify ICU patients with decreased bone density, which could enable enrollment in osteoporosis prevention programs. A high prevalence of low bone density was found in our cohort of ICU patients. There were no changes observed in bone density between baseline and follow-up measurements.
Similar content being viewed by others
Introduction
Despite increasing awareness of long term health consequences associated with intensive care unit (ICU) admission, bone loss and increased risk of fractures after ICU admission is still an underexposed topic. Little is known about the prevalence of osteoporosis in this population, although factors such as advanced age and comorbidity are likely to predispose a significant proportion of ICU patients to low bone density1. Furthermore, ICU stay in itself, often characterized by prolonged immobilization, inflammation, endocrine dysfunction and low circulating levels of vitamin D may further exacerbate the risk of accelerated bone loss2,3,4,5. Also, patients are often treated with corticosteroids and continuous veno-venous hemofiltration (CVVH), which are both associated with increased bone loss6,7. Indeed, a significant decrease in bone mass density was found in critically ill patients in the year after ICU admission with an increase in 10-year fracture risk for major fractures8.
Screening ICU patients for decreased bone mass would facilitate the identification of patients at risk who can be referred to osteoporosis prevention programs, which are shown to significantly reduce osteoporotic fractures and mortality9,10. To date, dual-energy X-ray absorptiometry (DXA) serves as the gold standard for quantifying bone mineral density (BMD) and detecting osteoporosis11. Due to practical constraints including costs and logistical challenges, performing DXA scans of all ICU patients would not be feasible. As an alternative, bone density of vertebral bodies can be measured using computed tomography (CT) scans already obtained for other indications. It has been shown that CT density values, as represented by Hounsfield Units (HU), of trabecular bone of the vertebral body of the 12th thoracal (Th12) and first lumbar (L1) vertebrae have excellent correlation with BMD as measured by DXA12,13. This enables identification of patients with or being at risk for osteoporosis. Especially in ICU patients, who frequently undergo CT scanning for other indications, this method may offer a valuable opportunistic screening tool.
The primary aim of this study was to investigate whether CT-based bone density analysis of the Th12 vertebral body can be used as an osteoporosis screening method in the ICU setting, evaluating its feasibility and reliability in terms of interobserver variability. Secondary aims were to determine the prevalence of CT-based low bone density upon ICU admission in the cohort of COVID-19 patients and to assess a potential decrease in bone density after ICU stay. For this exploratory study, a cohort of COVID-19 patients with extended ICU stay was instrumental, as in these patients CT scans obtained at different time points were readily available. Additionally, this specific population was chosen due to their hypothesized risk of decrease in bone density over time, owing to prolonged immobilization and administration of corticosteroid therapy.
Materials and methods
The study was exempted from requiring ethics approval by the medical ethics committee of the Leiden University Medical Center (Medisch-Ethische Toetsingscommissie Leiden-Den Haag-Delft) (waiver 2023–067), as it did not fall within the scope of the Dutch Medical Research Involving Human Subjects Act (WMO). All research was performed in accordance with relevant guidelines/regulations. Due to the retrospective nature of the study, Medisch-Ethische Toetsingscommissie Leiden-Den Haag-Delft waived the need of obtaining informed consent.
Study population
For this retrospective single-center cohort the study population consisted of patients aged 18 years or older admitted to the ICU of the Leiden University Medical Center (LUMC) between March 1st, 2020 and February 1st, 2022 with a diagnosis of COVID-19. Patients were included if their length of ICU stay was ≥ 21 days and if both a chest baseline CT scan and chest follow-up CT scan were available. A baseline CT scan was defined as being obtained ≤ 14 days before ICU admission and ≤ 3 days after ICU admission. A follow-up CT scan was defined as being obtained ≥ 21 days from the start of ICU stay and < 30 days after ICU discharge. For some patients, multiple CT scans were obtained throughout the admission. For the main analysis, the scans obtained closest to their date of admission and discharge, respectively, were used for the main analysis. All patients were scanned in supine position. Additional information regarding the obtained CT scans is described further. Patients who died during ICU admission or subsequent admission to the ward were excluded.
Clinical data collection
Clinical data was collected from electronic health records and included demographics, comorbidities, body mass index (BMI), length of ICU stay, days on mechanical ventilation, days on CVVH therapy, administration of corticosteroid therapy, and Acute Physiology and Chronic Health Evaluation (APACHE) IV score.
Radiologic assessment
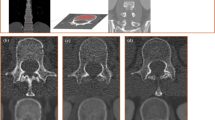
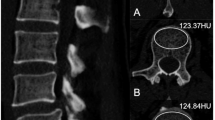
Analysis was performed on chest CT scans acquired on Acquillion One Genesis Edition (Canon Medical Systems, Otawara, Tochiqi, Japan). Contrast enhanced and non contrast enhanced CT images were acquired using a tube peak potential (KVp) of 100 and 120 KVp, respectively. The table feed was 32.5 mm per rotation (320 slice scanner with 80 × 0.5 mm collimation) with a spiral pitch factor of 0.813 and revolution time of 0.275 s using a 512 × 512 matrix. Reconstruction was performed using Adaptive Iterative Dose Reduction (AIDR3D) using an FC30 convolution kernel. CT scans were analyzed using the IDS7 viewer (version 25.3, Sectra, AB, Linköping, Sweden). Images were presented using a bone window (W:1800 L:400). Density of the vertebral body at the level of Th12 was assessed following the method as described by Pickhardt et al.12. In brief, a circular region of interest (ROI) was placed in the trabecular bone of the vertebral body stretching from the anterior part of the vertebral body (excluding cortical bone) and the basivertebral vein. One ROI for each scan was placed, in the axial image. Mean CT density (HU) was measured (Fig. 1). When placing the ROI, areas that would distort the CT density measurement (such as focal lesions, the internal posterior venous plexus or imaging-related artifacts) were avoided. Images with a slice thickness of 1.0 mm were used for analysis. Details on the assessment of bone density of Th12 is given in the Supplementary Materials. Density measurements were performed independently by three observers (EJ, DW, and NF; all intensive care physicians with varying levels of experience ranging from two to forty years) and mean values of the three observers were used for analysis. In some of the included patients, both contrast enhanced and non contrast enhanced CT images were obtained. If available, non contrast enhanced CT images were used for the main analysis. In additional analyses, we compared bone density between non contrast enhanced an contrast enhanced CT images, since venous enhancement could potentially influence measurement of CT density. For this comparison, the same ROIs were used (same size and location).
Measurement of CT-based bone density by manually placed ROI. CT-based bone density was measured by first locating the Th12 vertebral body in the sagittal plane (A). Subsequently, in the axial plane, a circular region of interest (ROI) was placed stretching from the anterior aspect of the vertebral body to the anterior side of the basivertebral vein (B). The mean CT-based bone density within the ROI was determined using the IDS7 software.
To distinguish osteoporosis from non-osteoporosis, a Th12 CT density cut-off value of 140 HU was used, based on the study of Pickhardt et al.12. Using this threshold, the authors found a balanced sensitivity and specificity of approximately 75% for each.
Statistical analysis
Statistical analyses were performed in SPSS (version 29, IBM, Armonk, New York, USA). For interobserver calculations, a total of 118 available CT scans in 38 patients were evaluated. Interobserver variability was assessed in different ways. First, for all sets of measurements (each set consisting of three HU values, one measured by each observer) the absolute difference between the individual measurements and the mean of three corresponding measurements were calculated. The overall mean absolute difference was calculated and presented as an absolute value and as a percentage of the overall mean bone density. The agreement between the three observers was also assessed graphically by a modified Bland–Altman plot as described by Jones et al.14. Secondly, interobserver variability was assessed by determining the intraclass correlation, using the single measures intraclass correlation coefficient (ICC) in a two-way mixed single measurement model with absolute agreement. For the third assessment, all HU measurements were classified as either osteoporotic or non-osteoporotic using the earlier mentioned threshold of 140 HU, and the level of full agreement between all observers was calculated (defined as the percentage of CT scans that were unanimously labeled as either osteoporotic or non-osteoporotic).
Population characteristics were described using mean and standard deviation (SD) for data following a normal distribution and median and interquartile range (IQR) for non-normally distributed data. Differences in HU between contrast enhanced and non contrast enhanced scans were compared using a paired samples t-test. Differences in bone density between baseline and follow-up CT scans were compared using a paired samples t-test. To assess correlations between bone density and patient characteristics, Pearson correlation coefficients (r) were calculated. P-values < 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 83 COVID-19 patients were admitted to the ICU of the LUMC for ≥ 21 days between January 1st, 2020 and February 1st, 2022. Baseline and follow-up CT scans were available in 38 patients, who were included in the study. Patient characteristics are shown in Table 1. The majority of patients were men (79%, n = 30/38). Mean age was 59 (SD = 12) and mean BMI was 29 (SD = 5.0). One-third of patients (32%, n = 12/38) had a pre-existent respiratory disease, mainly asthma, COPD, and/or obstructive sleep apnea syndrome (OSAS). The vast majority of patients (89%, n = 34/38) were treated with corticosteroid therapy during admission, only some patients admitted during the first phase of the COVID-19 pandemic were not, as during that time corticosteroid therapy was not yet part of standard care for COVID-19 patients.
Interobserver variability
Interobserver variability was first assessed by evaluation of the mean absolute difference between individual bone density measurements and the mean of the three measurements of the same CT-scan by the three observers. The overall mean absolute difference was 9.7 HU (SD = 8.0), which was 6.4% (SD = 6.1) of the overall mean bone density. The interobserver agreement of all bone density measurements is plotted in Fig. 2, showing the 95% CI limits of agreement with the mean, ranging from − 15 to 15 HU. The interobserver variability as expressed by ICC was 0.93 (95% CI 0.88–0.96, p < 0.01). When bone density measurements were treated as dichotomous variable (either osteoporosis or non-osteoporosis based on a threshold of 140 HU for bone density at Th12), the three had a diagnostic agreement of 88% (104/118 CT scans). Bland–Altman plots and correlation plots for pairwise comparisons of individual observers are shown in the Supplementary Material.
Interobserver agreement plot. Modified Bland–Altman plot showing the interobserver agreement between three observers for vertebral CT attenuation measurements. Differences between all measurements and the mean of the three observers (y-axis) are plotted against the mean HU values of the three observers for all CT scans (x-axis). The horizontal dashed lines represent the 95% CI limits of agreement with the mean of the three observers and range from -15 to 15 HU. HU Hounsfield Units
Effect of iodinated contrast administration on CT density analysis
For 42 CT scans both non contrast enhanced and contrast-enhanced portal venous phase images were available. On contrast-enhanced portal venous phase images mean CT density of trabecular bone of the vertebral body of Th12 was 7.5 HU (SD = 12.9) higher compared to non contrast enhanced images (p < 0.01). In the main analysis, 68% of used CT images were non contrast enhanced (in the other 32% only contrast-enhanced CT images were available). When bone density measurements were treated as dichotomous variable (osteoporosis or non-osteoporosis with 140 HU as cut-off value), 41/42 pairs (97%) of non contrast enhanced and contrast-enhanced CT images were classified in the same category.
Bone density at baseline
Distribution of bone density at baseline (upon ICU admission) is shown in Fig. 3. Mean bone density at baseline was 162 HU (± 56). More than one-third of patients (37%, n = 14/38) had a bone density of < 140 HU, being classified as osteoporosis.
Distribution of bone density upon ICU admission. CT-based bone density was measured at the vertebral body of Th12. Cut-off values were based on previous findings by Pickhardt et al. 12 for distinguishing osteoporosis from non-osteoporosis. For vertebral level Th12, the study of Pickhardt et al. showed that a CT-attenuation threshold of 115 HU or less was 90% specific and 51% sensitive for osteoporosis. A threshold of 165 or less was 89% sensitive and 49% specific for osteoporosis. A threshold of 140 or less showed a balanced sensitivity and specificity of 75 and 74%, respectively. ICU intensive care unit
Age was found to be negatively correlated with bone density (r = − 0.69, 95% CI 0.83–04.47). No significant correlations were found between bone density and either APACHE IV score or BMI (APACHE IV score: r = -0.20, 95% CI = − 0.49–0.14); BMI: r = 0.23, 95% CI − 0,10–0,51).
Change in bone density during ICU admission
Mean length of ICU stay was 49 days (SD = 17) and mean time between baseline and follow-up CT scan was 35 days (SD = 19). Overall, there was no significant difference between bone density at baseline (upon ICU admission) and bone density at follow-up (≥ 21 days after ICU admission). Also, in four subgroups (women, men, patients aged ≥ 50, and patients who received CVVH) no significant differences between bone density at baseline and bone density at follow-up were observed (Table 2). We conducted an additional check to ascertain the differences in HU between baseline and follow-up when considering measurements by single observers (3 × 38) rather than the averaged values. The observed differences were of comparable magnitude (very small, < 10HU). Difference between baseline mean bone density and follow-up mean bone density Observer 1: − 3.17 (95% CI − 11.9 − 5.58) Observer 2: − 5.44 (95% CI − 12.8–1.90) Observer 3: − 7.78 (95% CI − 14.95- -0.62).
Discussion
In this study, we evaluated CT-based bone density analysis at the Th12 vertebral level as a potential screening method for osteoporosis in ICU patients. Our results showed excellent interobserver variability, supporting the repeatability of manual CT measurements assessing bone density. These results align with previous studies on CT bone density analysis which reported similar estimates for interobserver variability15,16,17. We observed some effect of iodinated contrast administration on bone density measurements, however, the magnitude of this effect was minor (+ 7.5 HU for contrast-enhanced CT images, being smaller than the overall absolute mean difference between the three observers). This finding is consistent with prior research by Pickhardt et al., who also showed that the overall performance for predicting osteoporosis is similar between enhanced and non-enhanced scans, suggesting that both can be employed for opportunistic osteoporosis screening18.
Apart from the repeatability of CT attenuation analysis, our study revealed a significant proportion of 37% of patients in the cohort exhibiting low bone density (< 140 HU) upon ICU admission. This suggests a high prevalence of osteoporosis, which is remarkable considering the male predominance in our cohort. The available literature regarding bone density in ICU patients is notably limited. Three small studies conducted in (subpopulations of) ICU patients, documented rates of osteopenia or osteoporosis of 61% (osteopenia and osteoporosis), 45% (osteoporosis) and 37% (osteoporosis)8,19,20. Bone density was examined using DXA scan in two of these studies8,19 and using CT density analysis in one study. Our study focused on COVID-19 patients admitted to the ICU and the bone density measurements are therefore not generalizable to the broader ICU population. It should be noted that one third of the study cohort had a pre-existing respiratory condition such as asthma or COPD, which may imply regularly use of corticosteroids, potentially increasing their likelihood of low bone density. Yet, it seems highly plausible that the prevalence of low bone density within the general ICU population is substantial and merits further investigation.
Besides bone density at baseline, we also focused on potential changes in bone density between the time of ICU admission and the follow-up assessment, which was ≥ 21 days after admission. This study did not observe any difference in bone density between the baseline and follow-up time points. Although the amount of available literature is scarce, two previous studies reported on changes in bone density after a period of critical illness. Rawal et al. conducted a pilot study evaluating DXA-measured bone density changes in 46 ICU patients on day 1 and day 10 after admission, observing no change in bone density in the overall cohort19. Additionally, Hongo et al. investigated bone density alterations in 52 critically ill patients with sepsis using density analysis on abdominal CT scans, obtained upon ICU admission and during a follow-up moment that was not further specified20. They reported rather small yet significant decreases in CT attenuation values at various vertebral levels. Meanwhile, Orford et al.8 evaluated changes in DXA-measured bone density between ICU admission and 1-year post-ICU discharge, discovering a decrease in bone density among ICU patients, being higher than in a matched control population (1.5% decrease in ICU patients versus 0.11% increase in case–control subjects, P < 0.001). It is conceivable that the process of bone loss during critical illness might be a more prolonged one, which we therefore did not capture in our relatively short study period.
Some limitations of the study need to be addressed. First of all, we only focused on interobserver variability. We are not able to provide sensitivity and specificity data on low HU-values, as we didn’t perform a a gold standard bone density measurement (DEXA scan) for our patients. As mentioned above, a longer-term follow-up period, ideally extending to a year post-ICU discharge, would have provided a more comprehensive understanding of bone density changes. Unfortunately, an inadequate number of post-ICU discharge CT scans were available in this retrospective study. Secondly, CT-based bone density was measured solely at the Th12 vertebral level. This was a pragmatic choice as L1 visibility on chest CTs is not consistent, and Th12 is a reliable alternative as supported by the study of Pickhardt et al.12. Additionally, this exploratory study was constrained by a relatively small sample size. Within this population, there were no issues encountered in conducting the CT attenuation measurements. However, screening larger numbers of patients may occasionally pose challenges, such as focal lesions that could disturb measurements. In such cases, alternative vertebral sites (for instance L1 or Th11) could be chosen for measurements, possibly with corrective adjustments of the HU value for accurate comparison12. Furthermore, in this study we did not establish our own reference values for distinguishing osteoporosis as no DXA measurements were available. However, we have used the HU thresholds as determined by Pickhardt et al12. Finally, as this was a retrospective study using routine CT scans, a standardized CT phantom was not scanned with each subject to correct for HU values.
Conclusions
This study shows that low bone density measured using vertebral CT density analysis was highly prevalent in patients admitted to the ICU, even in male patients. Vertebral CT attenuation analysis could serve as a readily accessible screening tool that could seamlessly be incorporated in daily routine to identify those patients at risk for bone related pathology in the future after ICU recovery and enable enrollment in osteoporosis prevention programs.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the privacy sensitive data contained in the studied CT-scan images, but are available from the corresponding author on reasonable request.
References
Poole, K. E. & Compston, J. E. Osteoporosis and its management. BMJ 333(7581), 1251–1256 (2006).
Hollander, J. M. & Mechanick, J. I. Bisphosphonates and metabolic bone disease in the ICU. Curr. Opin. Clin. Nutr. Metab. Care 12(2), 190–195 (2009).
Lind, L. et al. Hypocalcemia and parathyroid hormone secretion in critically ill patients. Crit. Care Med. 28(1), 93–99 (2000).
Smith, L. M. et al. Increased bone resorption in the critically ill: Association with sepsis and increased nitric oxide production. Crit. Care Med. 30(4), 837–840 (2002).
Amrein, K. et al. Vitamin D and critical illness: What endocrinology can learn from intensive care and vice versa. Endocr. Connect. 7(12), R304-r315 (2018).
Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 61(1), 7–16 (2018).
de Jonge, E. et al. Negative calcium balance despite normal plasma ionized calcium concentrations during citrate anticoagulated continuous venovenous hemofiltration (CVVH) in ICU patients. J. Nephrol. 36(4), 1019–1026 (2023).
Orford, N. R. et al. Changes in bone mineral density in the year after critical illness. Am. J. Respir. Crit. Care Med. 193(7), 736–744 (2016).
Li, N. et al. The impact of fracture liaison services on subsequent fractures and mortality: A systematic literature review and meta-analysis. Osteoporos. Int. 32(8), 1517–1530 (2021).
Merlijn, T. et al. Fracture prevention by screening for high fracture risk: A systematic review and meta-analysis. Osteoporos. Int. 31(2), 251–257 (2020).
Kanis, J. A. et al. A reference standard for the description of osteoporosis. Bone 42(3), 467–475 (2008).
Pickhardt, P. J. et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 158(8), 588–595 (2013).
Deshpande, N. et al. Alternatives to DEXA for the assessment of bone density: A systematic review of the literature and future recommendations. J. Neurosurg. Spine 38(4), 436–445 (2023).
Jones, M., Dobson, A. & O’Brian, S. A graphical method for assessing agreement with the mean between multiple observers using continuous measures. Int. J. Epidemiol. 40(5), 1308–1313 (2011).
Pompe, E. et al. Inter-observer and inter-examination variability of manual vertebral bone attenuation measurements on computed tomography. Eur. Radiol. 26(9), 3046–3053 (2016).
Romme, E. A. et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J. Bone Miner. Res. 27(11), 2338–2343 (2012).
Pickhardt, P. J. et al. Simultaneous screening for osteoporosis at CT colonography: Bone mineral density assessment using MDCT attenuation techniques compared with the DXA reference standard. J. Bone Miner. Res. 26(9), 2194–2203 (2011).
Pickhardt, P. J. et al. Effect of IV contrast on lumbar trabecular attenuation at routine abdominal CT: Correlation with DXA and implications for opportunistic osteoporosis screening. Osteoporos. Int. 27(1), 147–152 (2016).
Rawal, J. et al. A pilot study of change in fracture risk in patients with acute respiratory distress syndrome. Crit. Care 19(1), 165 (2015).
Hongo, T. et al. Loss of bone mineral density following sepsis using Hounsfield units by computed tomography. Acute Med. Surg. 6(2), 173–179 (2019).
Author information
Authors and Affiliations
Contributions
NF, DW and EJ performed study conception and design; NF, WG, DW and EJ performed data analysis of the CT-scans, AN and NA contributed to data interpretation. All authors contributed to the manuscript; and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
du Fossé, N.A., Grootjans, W., Navas, A. et al. Exploring bone density analysis on routine CT scans as a tool for opportunistic osteoporosis screening. Sci Rep 14, 18359 (2024). https://doi.org/10.1038/s41598-024-69397-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-69397-1