Abstract
To evaluate the safety and the potential antiviral treatment of inhaled enriched heparin in patients with COVID-19. The specific objectives were to investigate the anticoagulation profile, antiviral and anti-inflammatory effects, and respiratory evolution of inhaled enriched heparin. We conducted a randomized, triple-blind, placebo-controlled Phase I/II clinical trial in hospitalized adults with COVID-19 receiving inhalation of enriched heparin or saline (placebo) every 4 h for 7 days. Among the 27 patients who completed the study, no changes in blood coagulation parameters were observed, indicating the safety of inhaled enriched heparin. The group receiving enriched heparin showed a significant reduction in the need for supplemental oxygen and improvement in respiratory parameters, such as the PaO2/FiO2 ratio. Inhalation of enriched heparin is shown to be safe and has also demonstrated potential therapeutic benefits for patients with COVID-19. These promising results justify the continuation of the study to the next phase, Phase II/III, to further evaluate the therapeutic efficacy of inhaled enriched heparin in the treatment of COVID-19-associated viral pneumonia.
Trial registration: ClinicalTrials.gov. 08/02/2021. Identifier: NCT04743011.
Similar content being viewed by others
Introduction
Coronavirus 2019 disease (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), an enveloped ribonucleic acid (RNA) virus member of the Coronaviridae family1. SARS-CoV-2 is highly contagious and rapidly spread worldwide, leading the World Health Organization (WHO) to declare it a pandemic in March 2020. Since then, it has devastated and overwhelmed many healthcare systems worldwide. Although there are concentrated global efforts for mass vaccination, SARS-CoV-2, like other respiratory RNA viruses, adapts and can develop threatening mutations that increase its infectivity and spread2. For this reason, the development of specific or adjunctive drug therapies for COVID-19 is essential to prevent and treat severe complications of the disease. Despite COVID-19 primarily affecting the lungs and inhalation-based drug administration offering several advantages, to date, no inhaled medication has been approved for the treatment of COVID-193.
Heparin is a sulphated heterogeneous polysaccharide, a member of the glycosaminoglycans family. Due to its negative charge, heparin can bind to various proteins in the body, including antithrombin-III (AT), which is responsible for heparin's anticoagulant activity. Additionally, heparin has been widely studied for its potential antiviral action, demonstrating inhibition against various enveloped viruses, including coronaviruses4,5,6. Although the complete pharmacological mechanism of heparin's antiviral action remains unclear, previous studies have suggested that it may act by binding to the SARS-CoV-2 spike protein, thereby inhibiting the virus infection5,7. In another direction, one group posits that unfractionated heparin (UFH) could compete with SARS-CoV-2 for heparan sulphate (HS) binding, thus impeding virus attachment and entrance to the cells8,9. Additionally, another potential mechanism involves heparin inhibiting SARS-CoV-2 replication and transcription by targeting the activity of the virus's Mpro protein, a crucial enzyme for its replication and transcription9,10.
Comparative studies between unfractionated heparin (UFH) and low molecular weight heparin (LMWH) have shown that UFH exhibits significantly more effective antiviral activity against SARS-CoV-211. In this context, we previously conducted an in vitro study using enriched unfractionated heparin. This process involves the depletion of low-molecular-weight molecules, thereby concentrating the higher molecular-weight components of heparin. Our results revealed about 80% decrease in SARS-CoV-2 viral load in Vero cells with no cytotoxicity. This suggests that the heparin fraction with higher molecular weight molecules may hold greater therapeutic potential against SARS-CoV-2, with a possible dual function, offering both antiviral and antithrombotic effects3,5,12.Building upon our innovative heparin enrichment process and promising preliminary findings, we hypothesized that inhaled enriched heparin could be more effective against SARS-CoV-2 viral replication in infected patients. Due to the absence of similar studies, this research aimed to evaluate the safety of inhaled enriched heparin and its potential positive effects in patients with COVID-19.
Methods
Study design and participants
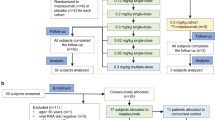
A phase I/II, single-center, randomized, triple-blind, placebo-controlled clinical study was carried out from May to August 2021 at the Clinical Hospital and the Clinical Research Unit (UPECLIN), both from the Botucatu Medical School in the São Paulo State University (UNESP). This study was carried out under applicable Brazilian laws, regulations, and international guidelines, following the ethical principles established at the 18th World Medical Assembly, Helsinki, 1964, and its subsequent amendments. The study was approved by the Research Ethics Committee (CAAE 39,872,920.0.0000.5411). The trial protocol for this study has been previously published13 and underwent some changes. All participants or their legal representatives were fully informed about the objectives and risks of the study and provided written informed consent as shown previously13. The protocol changes and a full list of inclusion, exclusion, and discontinuation criteria are found in the complementary study protocol (Supplementary Material 1). The protocol was registered at the National Institute of Health (NIH) United States National Library of Medicine ClinicalTrials.gov platform ID: NCT04743011.
Sample calculation
The prevalence of clinically significant bleeding resulting from heparin nebulization is estimated at 3%14. Therefore, the sample size calculation was 24 participants (12 for each group), considering two independent samples with the main objective of evaluating the treatment safety, with a confidence level of 95% and an error rate of 10%. Estimating a loss rate of more than 50%, the total sample initially proposed was 37 participants.
Randomization and blinding
The randomization was prepared by a technician not involved in the study execution or data processing, using the Stat Trek (Stat Trek, version 5.1, available at https://stattrek.com), a program to construct random number tables. Thirty-seven positions were drawn randomly and consecutively among placebo or heparin groups. Participants were randomly assigned in a 1:1 ratio. The envelope containing the randomization was held by an independent professional (pharmacist) without the knowledge of the study team members. The clinical trial was conducted following a triple-blind protocol. Participants, researchers, and data analysts had no access to the allocation distribution. The solutions were prepared by the Clinical Hospital of Botucatu Medical School laboratory team and labelled by an employee who did not participate in the study. The placebo and heparin vials were visually indistinguishable and had identical characteristics.
Interventions
Pharmaceutical unfractionated heparin formulations contain polysaccharide chains of molecular weight ranging from ∼5,000 to ∼30,000 Da15. The enriched unfractionated heparin was generated in sterile environment from a commercially available unfractionated heparin (Sodium heparin, injectable solution 5,000 UI/mL HEMAPAX®—Blau Farmacêutica™—São Paulo, Brazil) by filtration process using Amicon Ultra—10 kDa® centrifuge filter (Merck Millipore™ Merck, Burlington, Massachusetts, US), following the manufacturer's recommendations. Final enriched unfractionated heparin is a buffered solution depleted of low-molecular-weight, less sulfated heparin chains and low-molecular-weight heparin formulation components (Fig. S1). Enriched unfractionated heparin was the subject of two patents by Dr. Matheus Bertanha (BR 102,014,027,804–4 A2 and BR 102,020,011,964–8).
The placebo group received inhalation with 5 mL of 0.9% saline through a nebulization mask connected to oxygen, while the heparin group received inhalation of 12.5 mg of enriched heparin diluted in 5 ml of 0.9% saline through a nebulization mask connected to oxygen. The nebulization time was approximately 15 min. Both groups received the inhalation dose every 4 h, at 8 am, 12 pm, 4 pm, 8 pm, and midnight, except for the early morning dose. This regimen was followed over a 7-day period, totalling 5 doses per day and 35 doses throughout the study. All patients received standard supportive care administered by the attending team, who were not involved in the study design or randomization process. Patients were admitted to specialized COVID-19 wards equipped to provide non-invasive respiratory support.
Antibiotics were administered when bacterial co-infections were present or suspected, with this decision made by the attending medical team and not by the study team. Ten patients received antibiotic treatment alongside the study treatment (37% in total), evenly distributed between the groups (p = 0.9999), with only one patient from each group starting antibiotics after inclusion in the study. Therefore, the antibiotic regimen varied slightly from case to case but showed no statistical difference between the groups (Table 1). Supplemental oxygen therapy was present in all patients at the beginning of the study (inclusion criteria—moderate respiratory failure—Supplementary 1), using an oxygen mask with flow varying between 2 and 10 L per minute. In the most serious cases, patients were transferred to the ICU, where they received intensive treatment, including orotracheal intubation and mechanical ventilation, being excluded from the study, according to exclusion criteria (Supplementary 1). Due to the absence of suitable equipment and conditions for delivering inhaled medication to intubated patients, 3 cases in the control group and 5 cases in the treatment group experienced early worsening of the disease within 24 h of inclusion in the study, resulting in their exclusion (Fig. 2). Glucocorticoids (Dexamethasone 6 mg IV/day) were administered by the care team to the majority of patients: all in the control group and 11 patients in the treatment group (84.6%), with no statistical difference between the groups (p = 0.2222). Analgesics and antipyretics were provided for pain or fever relief (Dipyrone 500 mg IV every 6 h as needed). All patients received a prophylactic dose of 40 mg daily of enoxaparin subcutaneously to prevent thromboembolic (VTE) events. No other anticoagulants, anti-inflammatory medications, investigational drugs, or antiviral drugs were used concomitantly with the study.
Outcomes
The primary outcome was the safety of the use of inhaled enriched heparin in patients with SARS-CoV-2 by assessment of haemorrhagic events of any nature, alteration in the coagulation test, such as alteration in aPTT > 1.5, heparin-induced thrombocytopenia and death. In addition, the assessment of local and systemic adverse events, defined by Common Terminology Criteria for Adverse Events (CTCAE), was assessed16. The secondary outcome evaluated the patient's improvement through clinical, laboratory, and respiratory exams, e.g., assessment of the SARS-CoV-2 viral load, inflammatory and infectious profile, renal failure and pancreatitis development, deterioration of arterial blood gas PaO2/FiO2 ratio (< 200), and respiratory evolution through tomographic radiological imaging and by clinical parameters.
Clinical and laboratory monitoring
The schedule of visits and evaluations, epidemiological and personal antecedents form, clinical assessment and physical examination form, laboratory results and tomographic findings form, and adverse events form were previously available by Bertanha et al. (2021). Additional exam protocols are found in Supplementary Material 1.
Statistical analysis
To analyse the data from groups, descriptive and inferential statistical methods were used. Qualitative variables were presented using absolute and relative frequency distributions. Two-way ANOVA with Dunnett's multiple comparisons test was used to compare the in vitro viral inhibition and cytotoxicity. Fisher's Exact, Chi-squared or G-test of independence tests were used to compare the distribution of qualitative variables between the placebo and heparin groups. For paired data, the McNemar or G-test of independence tests was employed. Quantitative variables were described using central tendency and variation measures, and normality was assessed using the Shapiro–Wilk test. Results were expressed using the median and interquartile range (IQR) for non-parametric distributions, while the mean and standard deviation (SD) were used for parametric distributions. To compare quantitative variables between the placebo and heparin groups, unpaired t-tests or paired t-tests were applied for parametric distribution data; in the case of non-parametric or heteroscedastic distributions, the Mann–Whitney U test or Wilcoxon test was used. For the longitudinal analysis comparing moments D0, D2, D5, and D7 about quantitative variables, One-way ANOVA, followed by Tukey’s post-test was applied for parametric distributions, and the Kruskal–Wallis test followed by Dunn's post-test was applied for non-parametric distributions. The IQR method was used to remove outliers in the cytokine data, where there was a significant variation in the results. A significance level (alpha error) of 5% was previously established to reject the null hypothesis. Statistical data was processed using BioEstat version 5.3, SPSS version 27, and GraphPad Prism version 10.
Results
First, the impact of enriched heparin on SARS-CoV-2 infection was assessed. A cell viability assay was conducted using a range of concentrations from 15.625 to 250 μg/mL. No signs of cytotoxicity were observed up to 250 μg/mL. However, 125 μg/mL of the enriched heparin was found to inhibit virus replication by 82% (Fig. 1A). The antiviral effect of enriched heparin was observed during viral adsorption and replication (Fig. 1B, AD and FT bars), with no significant impact post-infection (PI bars). Interestingly, commercial unfractionated heparin did not show a similar antiviral activity in the experimental conditions. Overall, the findings suggest that enriched heparin acts against SARS-CoV-2 during viral adsorption in Vero cells.
Enriched heparin inhibits SARS-CoV-2 infection in Vero cells. (A) Percentage of virus load inhibition in the supernatant of cells infected with SARS-CoV-2 (102 TCID50/mL) cultured with increasing enriched heparin concentration (up to 250 ug/ml) for 72 h. (B) Three different times of heparin addition were evaluated, comprising the virus absorption (AD), post-infection (PI), and adsorption plus post-infection (FT). Data are representative of mean (SEM) of 4 replicates from 2 independent experiments. Data were analysed using Two-Way ANOVA with Dunnett’s multiple comparisons test. *p < 0.05; **p < 0.01.
In the screening phase, 70 patients were initially considered, but 33 were excluded for not meeting eligibility criteria. This left 37 patients in the study, with 18 in the placebo group and 19 in the heparin group. Two patients (one from each group) showed clinical improvement and were discharged on the third day. Unfortunately, eight patients (3 placebo, 5 heparin) experienced rapid deterioration, necessitating ICU transfer and intubation, leading to their study exclusion. Thus, 27 patients completed the seven-day follow-up, with 14 in the placebo group and 13 in the heparin group (Fig. 2). Epidemiological data showed group homogeneity (Table 1). Symptoms, predominantly dry cough (96%), asthenia, myalgia, dyspnoea (74%), fever (67%), and hypoxia (53%), were assessed (Table S1). Most symptoms had resolved before study inclusion, and the remaining symptoms improved by day 7. Both groups showed a significant reduction in dry cough and dyspnoea during treatment. Notably, no cases of bleeding were observed throughout the study period.
Primary outcomes
The primary outcome of this study was to evaluate the safety of administering inhaled enriched heparin to patients, focusing on potential changes in coagulation tests. Regarding safety parameters related to blood coagulation, the results are within the established reference ranges, and no significant alterations were observed in both the Activated Partial Thromboplastin Time (aPTT) and the International Normalized Ratio (INR) (Table 2).
Placebo n = 14; Heparin n = 13.
Results expressed as mean (standard deviation) on parametric analysis.
Results expressed as median (IQR) on nonparametric analysis.
* Unpaired t test.
** Mann–Whitney test.
# One-way ANOVA.
## Kruskal–Wallis test.
a Dunn's post-hoc test: D0xD5 (p< 0.0396); D0xD7 (p<0.0001); D2xD7 (p< 0.0086).
b Dunn's post-hoc test: D0xD7 (p< 0.0141).
c Dunn's post-hoc test: D0xD5 (p<0.0034); D0xD7 (p<0.0001); D2xD7 (p<0.0066).
d Dunn's post-hoc test: D0xD5 (p<0.0033); D0xD7 (p<0.0001). Statistical differences are highlighted (bold).
Secondary outcomes
The results of the serum laboratory tests collected on days D0, D2, D5, and D7 are presented in Table 2. Both the placebo and heparin patient groups showed a decrease in COVID-19 viral load over the course of the study; however, there was no difference between the groups by day. Significant differences were observed on days D0 and/or D2 for amylase, urea, neutrophils, and lymphocytes among the groups, but without clinical relevance, as the values remained within the normal reference range. C-reactive protein showed a significant decrease in both groups throughout the treatment. D-dimer levels remained elevated in all measurements for both groups, with no significant difference during the treatment. The inflammatory profile assessed by the analysis of serum cytokines demonstrated a high numerical variability. Despite some statistical differences, no result differed from the placebo profile (Fig. S2).
For the analysis of respiratory parameters, vital signs were obtained, including peripheral oxygen saturation, the need for supplemental oxygen therapy, and arterial blood gas analysis. The statistical disparity in pH reflects the exact values within the samples, leading to any variations in decimal points being deemed significant. There was no difference in the evolution of vital signs for both groups, which remained stable during the follow-up. However, patients who received enriched heparin inhalation showed a significant decrease in the need for supplemental oxygen therapy compared to the placebo group. While the placebo group showed a difference only between days D0 and D7, the heparin group showed a decrease in the need for oxygen earlier, demonstrating a difference between days D0 x D5, D0 x D7, and D2 x D7 (Table 3). Additionally, it is noteworthy that patients in the heparin group showed a significant increase in the PaO2/FiO2 ratio, reaching normal values earlier than patients in the placebo group.
Placebo n = 10; Heparin n = 9.
Results expressed as mean (standard deviation) on parametric analysis.
Results expressed as median (IQR) on nonparametric analysis.
* Unparied t test.
** Mann–Whitney test.
# One-way ANOVA.
## Kruskal–Wallis test.
a Dunn's post-hoc test: D0xD7 (p<0.0313)
b Dunn's post-hoc test: D0xD7 (p<0.0026)
c Dunn's post-hoc test: D0xD5 (p<0.0001); D0xD7 (p<0.0001); D2xD5 (p<0.0200); D2xD7 (p<0.0126). Statistical differences are highlighted (bold).
The results of the tomographic evaluations are presented in Table 4 with representative images in Fig. S3. It was observed that patients treated with enriched heparin inhalation showed a significant decrease in pulmonary congestion between days D0 and D7. Regarding the analysis of pulmonary involvement scores, there was a significant decrease between D0 and D7 in both the placebo and heparin groups, but there was no difference between the groups.
Placebo n = 14; Heparin n = 13.
Results expressed as n (%).
Score: 0 (0% or none), 1 (1–25%), 2 (26–50%), 3 (51–75%), 4 (76–100%).
* Fisher's exact test.
# McNemar test.
## G-test of Independence. Statistical differences are highlighted (bold).
Discussion
Unfractionated heparin (UFH) possesses relevant anticoagulant, anti-inflammatory, and possibly antiviral properties, which have recently been evaluated for SARS-CoV-2 infection17. In pre-pandemic clinical trials conducted in various clinical situations, including lung injury from smoke inhalation, inhaled heparin demonstrated a reduction in pulmonary coagulopathy, lowering the risk of microvascular thrombotic events. In addition, it was also associated with improved ventilatory support, reduced atelectasis, and optimized CO2 elimination18,19,20.
In this study, we employed an innovative approach using inhaled enriched unfractionated heparin. This unique enrichment method aimed to deplete low molecular weight components of UFH while concentrating high molecular weight heparin molecules. Our findings indicate that the enriched heparin did not induce any alterations in coagulation parameters compared to the placebo group. Additionally, no haemorrhagic adverse events were observed, suggesting its potential as a safe medication under the applied conditions. In support of our results, another randomized, placebo-controlled study involving 60 patients with severe Acute Respiratory Distress Syndrome (ARDS), where participants were assigned to inhaled UFH, streptokinase, or placebo, also reported no effects on systemic coagulation markers21. Furthermore, the statistical difference between the placebo and heparin groups in laboratory tests found in our study has no clinical relevance. This suggests that there was no significant systemic absorption of the medication, and no side effects were observed in other organ systems, supporting the safety of inhaled enriched heparin.
The most commonly reported symptoms in the literature regarding the clinical progression of COVID-19 patients include dry cough, dyspnoea, asthenia, and fever22, all of which were also observed in our study. Throughout the treatment course, there was a significant reduction in both dry cough and dyspnoea for both groups, signifying the natural progression of patients who were appropriately treated and did not experience a rapid unfavourable evolution due to risk factors or constitutional susceptibility. However, our sample indicated that, for the majority of clinical parameters, significant improvement occurred after the 7-day hospitalization period, aligning with literature findings where clinical support treatment, coupled with the use of glucocorticoids and supplemental oxygen therapy, alongside necessary antibiotic therapy, played a pivotal role in controlling the disease.
In the assessment of the inflammatory process induced by COVID-19, our study observed the values of C-reactive protein (CRP), a marker of acute-phase inflammation widely used in the context of COVID-19 infection due to its correlation with inflammation. In the literature, there is an established correlation between elevated CRP levels and a higher mortality rate23. Our study showed a significant decrease in CRP in both groups, suggesting a reduction in inflammation throughout the hospitalization period. This decline could be attributed to the supportive treatment involving glucocorticoids and/or the natural course of disease recovery.
Previous investigations have reported an increase in inflammatory cytokines in severe manifestations of coronavirus infections, including IL-2, IL-6, TNF-α, and MCP-117. This process, commonly referred to as a "cytokine storm", has been largely implicated in the development of respiratory complications in SARS caused by COVID-1924,25. Here, no significant differences were found between the placebo and heparin for all evaluated cytokines, which may be attributed to small sample size, molecular instability, and rapid degradation of samples. However, CXCL10 levels significantly decreased in both groups throughout the treatment (Fig. S2), which is generally associated with a favourable progression in patients. It is important to emphasize that the use of concomitant medications could influence the results, particularly the use of glucocorticoids. However, it has been showed that high doses of glucocorticoids do not provide additional clinical benefits over time26,27. In our study, concomitant medications were used similarly in both groups, which could account for the improvements observed in both groups. On the other hand, this similar distribution of medications allowed us to observe differences between the study groups and highlights the potential for adjuvant use of inhaled enriched heparin. No patients experienced thromboembolic events, and no anticoagulant medication was prescribed except for prophylactic enoxaparin. Although certain antivirals, such as Remdesivir, and biologic immunomodulators are known to play an important role in treating severe SARS-CoV-2 infections, these treatments were not available during the study period28,29.
According to our findings, there is an early and significantly noteworthy reduction in the need for supplemental oxygen therapy in the group treated with inhaled enriched heparin. Moreover, there was a particularly significant early increase in the PaO2/FiO2 ratio in the heparin group, suggesting a potential anti-inflammatory/antithrombotic effect on lung tissue and vascularization. In the CHARLI14 randomized clinical trial for treating non-COVID-19 acute respiratory distress syndrome, high doses of commercial nebulized heparin did not show significant improvements in patients' self-reported ability to perform daily physical activities. However, the treatment was well tolerated, with no adverse events reported. Exploratory results also suggested less progression and recurrence of lung injury, as well as earlier hospital discharge. Further research is needed to determine whether nebulized heparin can accelerate recovery in individuals with or at risk of acute respiratory distress syndrome. According to Ball et al. (2021)30, the CHARLI study underscores the need for further research on nebulized heparin in patients with COVID-19 pneumonia. In addition, DeNucci et al. (2023)31 investigated the safety and impact of nebulized unfractionated heparin (UFH) on mortality, length of hospitalization, and clinical progression in hospitalized COVID-19 patients. This randomized, open-label, parallel-group study compared standard-of-care therapy with standard care plus nebulized UFH. The study found that adding nebulized UFH to the standard treatment was well tolerated and demonstrated clinical benefits, particularly in patients who received at least six doses of heparin, without significant adverse events. Our study, however, employed a slightly different methodology, using much smaller doses of nebulized enriched heparin. It was limited to patients admitted to an infirmary due to characteristics specific to our health service. However, we were able to observe that pulmonary involvement scores via lung CT (computerized tomography) analysis indicated a substantial improvement in both placebo and heparin groups during hospitalization. Notably, patients receiving inhaled enriched heparin exhibited a significant reduction in pulmonary congestion compared to the placebo group. This discovery implies that enriched heparin may play an essential role in rearranging pulmonary architecture, particularly in mitigating the inflammatory process. This improvement might be attributed to the specific properties of heparins in inflammatory process, representing a highly significant finding in our study.
In a study by Camprubí-Rimblas et al. (2019)32, rats treated with nebulized anticoagulants exhibited reduced lung protein concentration. Additionally, the treatment decreased injury-induced coagulation factors (such as tissue factor, plasminogen activator inhibitor-1, plasminogen, and fibrinogen degradation products) and inflammatory markers (including tumour necrosis factor α and interleukin 1β) in the alveolar space. Importantly, systemic coagulation was not affected by this treatment. While our study did not directly address these specific outcomes, the results may align with our findings regarding safety and tomographic outcomes. It is important to note that our study aimed to assess the safety and explore the effectiveness of nebulized enriched heparin as an adjunct to standard clinical treatment for hospitalized patients with SARS-CoV-2 and moderate respiratory failure. Preliminary results suggest that this treatment may be a safe supplemental option, potentially reducing the pulmonary inflammatory process. However, further research is needed to confirm these findings and evaluate its efficacy in larger studies.
Limitations
This study has some limitations that should be considered: the severity of the disease led to the early exclusion of some participants, which may have affected the results of certain investigated parameters; two participants were excluded due to early discharge, a decision made by the assisting team that did not follow the study protocol, interfering with the study. It is important to note that the effective mass vaccination in the city of Botucatu, where the study was conducted, resulted very positively in the reduction of hospitalized patients, reducing the number of patients who could be included in the study after August 202133.
Conclusion
The administration of inhaled enriched heparin for treating SARS-CoV-2 has demonstrated its safety, as indicated by the absence of significant changes in coagulation parameters or any haemorrhagic adverse events. Noteworthy therapeutic effects were also observed, including a significant reduction in the need for supplemental oxygen therapy and an early, significant increase in the PaO2/FiO2 ratio.
Data availability
Data availability statement Data available: Yes. Data types: All deidentified participant data. Types of analyses: Study protocol, primary outcome and secondary outcome. When available: Immediately following publication. Who can access the data: Qualified researchers. Mechanisms of data availability: Data request with description of proposed research will be reviewed. If proposal is approved, data will be available after a signed data sharing agreement. How to access data: Send email to: matheusbertanha@gmail.com.
References
Dhont, S., Derom, E., Van Braeckel, E., Depuydt, P. & Lambrecht, B. N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 21, 198 (2020).
Cascella, M., Rajnik, M., Aleem, A., Dulebohn, S. C. & Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). StatPearls (2024).
Sahin, G. et al. Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment. J. Pharm. Sci. 111, 2652–2661 (2022).
Conzelmann, C. et al. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin. Med. (Northfield. Il). 20, e218–e221 (2020).
Mycroft-West, C. J. et al. Heparin inhibits cellular invasion by SARS-CoV-2: Structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb. Haemost. 120, 1700–1715 (2020).
Yu, X. Another new application of heparin in Covid-19: More than anticoagulation and antiviral. J. Investig. Med. 69, 1258–1258 (2021).
Paiardi, G. et al. The binding of heparin to spike glycoprotein inhibits SARS-CoV-2 infection by three mechanisms. J. Biol. Chem. 298, 101507 (2022).
Clausen, T. M. et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183, 1043-1057.e15 (2020).
Mangiafico, M., Caff, A. & Costanzo, L. The role of heparin in COVID-19: An update after two years of pandemics. J. Clin. Med. 11, 3099 (2022).
Li, J., Zhang, Y., Pang, H. & Li, S. J. Heparin interacts with the main protease of SARS-CoV-2 and inhibits its activity. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 267, 120595 (2022).
Tree, J. A. et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br. J. Pharmacol. 178, 626–635 (2021).
Kim, S. Y. et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 181, 104873 (2020).
Bertanha, M. et al. Nebulized enriched heparin to treat no critical patients with Sars-Cov-2. Medicine (Baltimore). 100, e28288 (2021).
Dixon, B. et al. Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 9, 360–372 (2021).
Mulloy, B., Hogwood, J., Gray, E., Lever, R. & Page, C. P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 68, 76–141 (2016).
Institute, N. C. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. (2017).
van Haren, F. M. P. et al. Can nebulised HepArin Reduce morTality and time to Extubation in patients with COVID-19 Requiring invasive ventilation Meta-Trial (CHARTER-MT): Protocol and statistical analysis plan for an investigator-initiated international meta-trial of prospective ra. Br. J. Clin. Pharmacol. 88, 3272–3287 (2022).
Camprubí-Rimblas, M. et al. Role of heparin in pulmonary cell populations in an in-vitro model of acute lung injury. Respir. Res. 18, 89 (2017).
Lan, X. et al. Nebulized heparin for inhalation injury in burn patients: a systematic review and meta-analysis. Burn. Trauma 8, (2020).
Phelps, M. K., Olson, L. M., Patel, M. A. V. B., Thompson, M. J. & Murphy, C. V. Nebulized heparin for adult patients with smoke inhalation injury: A review of the literature. J. Pharm. Technol. 36, 130–140 (2020).
Abdelaal Ahmed Mahmoud, A., Mahmoud, H. E., Mahran, M. A. & Khaled, M. Streptokinase Versus Unfractionated Heparin Nebulization in Patients With Severe Acute Respiratory Distress Syndrome (ARDS): A Randomized Controlled Trial With Observational Controls. J. Cardiothorac. Vasc. Anesth. 34, 436–443 (2020).
Stokes, E. K. et al. Coronavirus disease 2019 case surveillance—united states, January 22–May 30, 2020. MMWR. Morb. Mortal. Wkly. Rep. 69, 759–765 (2020).
Sadeghi-Haddad-Zavareh, M. et al. C-reactive protein as a prognostic indicator in COVID-19 patients. Interdiscip. Perspect. Infect. Dis. 2021, 1–5 (2021).
WONG, C. K. et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136, 95–103 (2004).
Mahallawi, W. H., Khabour, O. F., Zhang, Q., Makhdoum, H. M. & Suliman, B. A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104, 8–13 (2018).
Salton, F. et al. Prolonged higher dose methylprednisolone versus conventional dexamethasone in COVID-19 pneumonia: a randomised controlled trial (MEDEAS). Eur. Respir. J. 61, 2201514 (2023).
Salvarani, C. et al. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia: a double-blind, randomised, placebo-controlled trial. Eur. Respir. J. 60, 2200025 (2022).
Libra, A. et al. Use of remdesivir in patients hospitalized for COVID-19 pneumonia: Effect on the hypoxic and inflammatory state. Viruses 15, 2101 (2023).
van de Veerdonk, F. L. et al. A guide to immunotherapy for COVID-19. Nat. Med. 28, 39–50 (2022).
Ball, L., Schultz, M. J. & Pelosi, P. Nebulised heparin for patients on ventilation: implications for COVID-19 pneumonia. Lancet Respir. Med. 9, 321–322 (2021).
DeNucci, G. et al. Inhaled nebulised unfractionated heparin (UFH) for the treatment of hospitalised patients with COVID-19: A randomised controlled pilot study. Pulm. Pharmacol. Ther. 80, 102212 (2023).
Camprubí-Rimblas, M. et al. Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J. Thromb. Haemost. 18, 571–583 (2020).
Costa Clemens, S. A. et al. Effectiveness of the Fiocruz recombinant ChadOx1-nCoV19 against variants of SARS-CoV-2 in the Municipality of Botucatu-SP. Front. public Heal. 10, 1016402 (2022).
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP. Grant number 2020/12165-8. M.A.L M.A.L. also thanks The Royal Society (IEC\NSFC\201116) and the Academy of Medical Sciences/Wellcome Trust (Springboard grant, SBF007\100054) for project grant funding.
Author information
Authors and Affiliations
Contributions
1. Study design 2. Data collection 3. Data analysis 4. Writing 5. Obtained funding 6. Supervision 7. Administrative, technical, or material support 8. Critical reviewer Vinicius T. R. S. Grillo: 1, 2, 3, 4. Matheus Bertanha: 1, 2, 3, 4, 5, 8, 6. Lenize S. Rodrigues: 1, 2, 3, 4, 7. Marcelo A. de Lima: 1, 2, 3, 4, 8, 6. Pedro L. Mellucci Filho: 1, 2, 3. Rafael R. Guaragna Machado: 2, 3. Edson Luiz Durigon: 2, 3. Rita C. Alvarado: 2, 3, 7. Nathália D. Sertorio: 2, 3. Marjorie A. Golim: 2, 3, 4. Andrei Moroz: 2, 3. Aline Márcia M. Braz: 2, 3. Leonardo N. de Moraes: 2, 3, 7. Marco Antonio Leite: 2, 3. Helena B. Nader: 2, 3, 7. Gustavo C. de Campos: 2, 3. Cristiane R. Guzzo: 2, 3. Fábio F. Cardoso: 2, 3. Angelo José Magro: 2, 3. Helga C. Nunes: 2, 3. Rejane Maria T. Grotto: 2, 3, 5. Rita C. Alvarado: 2, 3. Maria Inês M. C. Pardini: 2, 3. Marcone L. Sobreira: 8. Erika Alessandra P. N. da Costa: 2, 3. Alexandre N. Barbosa: 8. Carlos Magno C. B. Fortaleza: 1, 8, 5, 6.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ramos da Silva Grillo, V.T., Bertanha, M., da Silva Rodrigues, L. et al. Nebulized enriched heparin improves respiratory parameters in patients with COVID-19: a phase I/II randomized and triple-blind clinical trial. Sci Rep 14, 19902 (2024). https://doi.org/10.1038/s41598-024-70064-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70064-8