Abstract
Biocide treatments are commonly employed to mitigate unwanted microbial activities in industrial water systems. This study illuminates the intriguing phenomenon wherein sub-minimum inhibitory concentration (sub-MIC) of tetrakis(hydroxymethyl)phosphonium sulfate (THPS), a frequently used biocide, stimulates the formation of biofilms by Pseudomonas aeruginosa, consequently intensifying the corrosion of carbon steel. Introducing 160 µg/ml THPS, constituting a sub-MIC level, into the culture medium resulted in a notable increase in biofilm thickness and corrosion rate, elevating them from 82 µm and 10 mpy to 97 µm and 18.7 mpy, respectively. Electrochemical impedance spectroscopy, Tafel polarization and linear polarization resistance measurements substantiated the extent of corrosion. Furthermore, the treated biofilm exhibited a heightened presence of extracellular polymeric substances, improved adherence to the metal surface, enhanced structural integrity, and an extended dispersal phase. Confocal laser scanning microscopy (CLSM) images revealed a greater abundance of viable sessile cells within the inner layers of the treated biofilm. These findings underscore the role of sub-MIC levels of biocides as a potential driving force for developing more corrosive biofilms on industrial materials, emphasizing the critical importance of precise biocide dosing.
Similar content being viewed by others
Introduction
In recent decades, an expanding body of scientific investigation has delved into the integral role of microorganisms in material corrosion1,2,3. This emerging field, known as microbiologically influenced corrosion, is characterized by its definition as "corrosion influenced by the presence or activity, or both, of microorganisms"4. Microbiologically influenced corrosion has garnered considerable attention within the sphere of industrial materials and facilities. Central to this phenomenon is the biofilm, a sophisticated microbial community enclosed within a polymeric matrix. This biofilm environment creates conditions conducive to accelerating corrosion2,3,5,6.
A holistic understanding of biofilm structure, its interactions with metal surfaces, and the factors influencing biofilm development is crucial for mitigating microbiologically influenced corrosion7.
Pseudomonas aeruginosa, a ubiquitous bacterium, has evolved intricate mechanisms to access iron for survival in diverse environments8. Numerous recent studies have demonstrated that the presence or activity of P. aeruginosa biofilm can trigger corrosion in a range of alloys and metals9,10,11,12,13,14,15,16,17,18,19,20. Additionally, P. aeruginosa is a promising model organism for biofilm research21.
To mitigate microbiologically influenced corrosion, biocides are commonly employed6,22,23,24. THPS, a non-oxidizing broad-spectrum biocide, has gained prominence due to its low environmental toxicity and effectiveness against a broad spectrum of bacteria, fungi, and algae. Consequently, it has found extensive use across industries, including the oil and gas sector, since the 1980s25,26. THPS's active functional group interacts with the disulfide bonds in microbial cell walls, disrupting their integrity and structure, ultimately leading to cell death27. Previous experiments have demonstrated THPS's potential as an efficient and rapid-acting biocide capable of penetrating biofilms26,28.
Minimum Inhibitory Concentration (MIC) indicates the minimal concentration of an antimicrobial substance required to inhibit the observable growth of the target microorganism following a designated incubation period29. A notable study by Hoffman et al. in 200530 revealed that tobramycin when used at concentrations below the minimum inhibitory concentrations (sub-MIC), could induce the formation of P. aeruginosa biofilms. Subsequently, numerous clinical studies have highlighted that various sub-MIC-level antibiotics can enhance biofilm formation in pathogenic bacteria31,32. The rate of biofilm formation induction and the inducer dose varied across different antibiotics and diverse bacterial species31. For instance, clarithromycin increased P. aeruginosa biofilm formation by 25, whereas ampicillin and vancomycin increased biofilm formation in Streptococcus intermedius and Staphylococcus epidermidis by 231.
However, there is a dearth of information regarding the impact of sub-MIC levels of commonly used non-oxidizing biocides in industrial settings. Microorganisms in industrial environments may be exposed to sub-MIC biocide concentrations due to inadequate biocide administration or uneven distribution, stemming from the intricacies of industrial systems and mixing processes. The research conducted in this study marks an early exploration of this subject matter, delving into the impacts of sub-MIC levels of THPS on the corrosion of carbon steel initiated by the presence of P. aeruginosa. Beyond employing microbiological methods, various analytical instruments and techniques were meticulously utilized to scrutinize and corroborate the observed outcomes. These methods encompassed scanning electron microscopy, confocal laser scanning microscopy, loss on ignition, weight loss analysis, linear polarization resistance, and electrochemical impedance spectroscopy.
Materials and methods
Bacterium, culture media, chemicals, and steel coupons
P. aeruginosa PAO1 (ATCC 15692) was employed in this study. To facilitate its growth and conduct the Minimum Inhibitory Concentration (MIC) and biofilm assessments, a modified Luria–Bertani (LB) broth supplemented with KNO3 was utilized. This specialized medium consisted of 5 g/L yeast extract, 5 g/L NaCl, 10 g/L tryptone, and 10 g/L KNO3, as indicated in previous studies10,33. When solid media were required, 1.5% agar (w/v) was introduced to the medium. For subculturing the strain, LB agar was employed. To maintain a pH level of 7.0, the medium was adjusted with 5N NaOH and subjected to sterilization via autoclaving at 121 °C for 15 min.
THPS (Sigma-Aldrich® Solutions-15175) was utilized as a biocide in the study. Different concentrations of THPS were introduced into screw-capped tubes measuring 75 × 17 mm, each containing 10 ml of LB broth. These concentrations covered a range of values: 10, 20, 40, 80, 160, 320, and 640 µg/mL.
For the corrosion experiments, disk-shaped C1018 (AISI 1018) carbon steel coupons with dimensions of 20 mm in diameter and 3 mm in height were employed. These coupons were procured from Mirab Sanat Inc. in Shiraz, Iran, and their chemical composition is detailed in Table 1. The coupon surfaces underwent polishing using 200, 400, and 600-grit emery papers. Subsequently, the coupons were weighed precisely (0.1 mg, Mettler-Toledo). The carbon steel coupons were securely mounted using epoxy resin to ensure that only the top surface was exposed to the culture medium during corrosion tests.
MIC determination of THPS
The frozen vial of strain PAO1, stored at − 80 °C, was reactivated through inoculation onto LB agar and subsequently incubated at 37 °C for 24 h. For inoculum preparation, a few revitalized colonies were inoculated into a 25 ml rubber stopper vial, filled with 20 ml of LB broth, and incubated at 37 °C until the culture achieved turbidity equivalent to 0.5 McFarland standard (~ 107 CFU/ml)29. Screw-capped tubes containing modified LB broth (prepared as described in Bacterium, culture media, chemicals, and steel coupons) were inoculated at a rate of 1% v/v and incubated at 37 °C for 16 h. The concentration of THPS at which visible growth inhibition of PA01 occurred was identified as the minimum inhibitory concentration (MIC)29.
Effect of sub-MIC concentration of THPS on biofilm formation
The formation of biofilms by P. aeruginosa was investigated following the protocol outlined in the work of Jia et al.10,33, with slight modifications. Initially, polished coupons were subjected to a degreasing process using acetone and rinsing with 100% ethanol. Subsequently, all surfaces of the coupons were thoroughly dried and sterilized by exposure to UV light for 20 min.
Each coupon was then positioned at the bottom of a 100 ml laboratory screw-capped bottle containing 70 ml of modified LB broth, which was supplemented with a sub-minimal inhibitory concentration (sub-MIC) of THPS. Inoculation was performed using an appropriate inoculum culture at a 1% volume/volume ratio. The bottles were meticulously sealed with parafilm and placed in an incubator at 37 °C. Notably, the control bottles employed in this study mirrored the test bottles in all aspects, except for the absence of THPS supplementation.
Following the designated incubation period, the coupons were carefully retracted from the bottles and transferred into sterile screw-capped tubes. The biofilms were gently washed with a sterile standard saline solution to eliminate planktonic or loosely adherent cells. The washing procedure was carried out thrice to ensure the elimination of any remaining planktonic cells.
Scanning electron microscopy
The biofilm specimens adhered to the coupons underwent a fixation process in a 2% v/v glutaraldehyde solution, lasting for 2 h. Subsequently, these coupons were immersed in a 25% ethanol solution for 1 h. This ethanol treatment was then iterated using 50%, 75%, 90%, and 100% ethanol concentrations.
After the ethanol treatments, the coupons were thoroughly desiccated within a fume hood until dry. Subsequently, the dried specimens were prepared for imaging by applying a gold coating. The imaging process utilized Tescan Mira3 field emission scanning electron microscopes (FESEM).
Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) images offer valuable insights into the thickness and spatial arrangement of live and dead sessile cells within biofilm structures. To visualize these biofilms, we utilized the FilmTracer™ LIVE/DEAD® Biofilm Viability Kit, incorporating SYTO 9 and propidium iodide (PI) as staining agents. The biofilm samples adhered to the test coupons were stained with the manufacturer's prescribed protocols. Subsequently, image acquisition was conducted using the Leica TCS SPE confocal laser scanning microscope and analyzed by Leica LAS-X software. COMSTAT 2 software was employed for quantification of biofilm parameters34,35.
Loss on ignition (LOI)
The coupons, which held the biofilm, were subjected to a temperature of 60 °C for two days. Following this period, once it was ascertained that the weight had remained constant, the organic component (comprising the biofilm) and the inorganic component (consisting of corrosion products) were removed from the coupons. These constituents were subsequently transferred into a platinum crucible and introduced into a furnace, where they were exposed to a temperature of 550 °C for one hour. Subsequently, the samples were placed in a desiccator and cooled before being weighed. The quantification of the biofilm and corrosion products' weight was achieved by taking into account the weight of the samples both before and after the combustion process. The results were then expressed in milligrams per square centimeter (mg/cm2).
Bacterial enumeration and total matrix polysaccharide assay
After washing the biofilm, the microbial mass was removed by adding and pipetting 1 ml of a 1.5 M NaCl sterile solution36. Subsequently, the cells were dislodged and resuspended through vortexing for 30 s. The separated cells were then thoroughly isolated from one another through sonication, employing a Branson ultrasonic bath for 10 min. The enumeration of total and viable sessile cells within the homogenized biofilm suspension was done using a Neubauer Chamber and a standard plate count method. Following this, the homogenized suspension was centrifugated at 5000 × g for 10 min at 25 °C, with the resulting supernatant being utilized to measure the total matrix polysaccharides content through the phenol–sulfuric acid method37.
Weight loss analysis
Once the biofilms were removed, the coupons were washed according to ASTM G1-03 to remove any corrosion products. Then, the coupons were weighed, and the weight loss results were reported in terms of mg.cm-2. The corrosion rate is calculated using the equation38:
where W is the mass loss (g), A is the exposed area (cm2), K is 3.45 × 106, T is the exposure time (hr), and D is the corrosion coupon material density (g.cm-3). The corrosion rate is expressed as mils per year (mpy).
Electrochemical measurements
Electrochemical measurements were conducted utilizing the ZAHNER-Elektrik potentiostat/galvanostat IM6-ex instrument. A graphite rod, an Ag/AgCl electrode, and carbon steel were employed as the counter, reference, and working electrodes. The working electrodes were affixed and subsequently meticulously polished using 200, 400, and 600-grit emery papers. After thoroughly degreasing with acetone, the working and counter electrodes were submerged in 100% ethanol, meticulously dried, and sterilized under UV light. The working and counter electrodes were then immersed in PA01 culture, as specified in the biofilm formation section and incubated within a 37 °C water bath. Electrochemical assessments were conducted at 24-h intervals over 3 days.
The electrochemical impedance spectrum of the system was systematically scanned within a frequency range spanning from 100 kHz to 0.01 Hz, all while maintaining an open circuit potential (OCP) and utilizing a sinusoidal excitation signal with an amplitude of 10 mV. Subsequently, the obtained EIS data were analyzed using the Zview2 software, wherein the impedance spectra for both the control and treated samples were subjected to fitting procedures. An equivalent electrical circuit was applied to facilitate the interpretation and calculation of electrochemical parameters.
Linear polarization resistance (LPR) was assessed, commencing from a cathodic potential of -15 mV vs. OCP and extending to an anodic potential of + 15 mV vs. OCP. The values of Rp (polarization resistance) were determined by extracting the slopes from the LPR plots39,40. All measurements were meticulously repeated three times to ensure accuracy and reliability.
Tafel polarizations were measured in the range of − 200 mV to + 200 mV vs. the OCP at a scan rate of 0.5 mV/s.
Data analysis
The evaluation of data normality was performed utilizing the Shapiro–Wilk test. Following this assessment, subsequent statistical analyses were conducted using Student's t-test, with the software GraphPad Prism version 9.3.1 employed for analysis. Significance was established at a threshold of P < 0.05. It is crucial to emphasize that, unless explicitly stated otherwise, all experiments were conducted in three sets of experimental triplicates to ensure the robustness and reliability of the results.
Results
Determination of minimum inhibitory concentration
The impact of different concentrations of THPS on the proliferation of P. aeruginosa PA01 is detailed in Table 2. It is noteworthy that THPS, when present at a concentration equal to or greater than 320 µg/ml, effectively suppressed the growth of P. aeruginosa PA01. Consequently, following the protocol outlined by Andrews29, we have designated the concentrations of 320 µg/ml and 160 µg/ml as the MIC and sub-MIC concentrations, respectively, for THPS.
Effect of THPS at Sub-MIC concentration on coupon weight loss
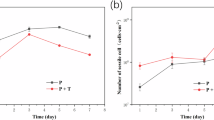
Figure 1a illustrates the influence of THPS (160 µg/ml) on the extent of weight loss in carbon steel coupons in the presence of PA01. It can be deduced from the acquired results that PA01 exhibited the capacity to generate biofilm and induce corrosion under experimental conditions. Interestingly, the sub-MIC THPS samples ("treated samples" hereafter) demonstrated more significant weight loss. The discrepancy in weight loss became more pronounced over time, with the weight loss in treated samples after 24, 48, and 72 h being 22%, 53%, and 85% higher, respectively than that observed in the control sample (comprising PA01, the coupon, and the medium). To illustrate, after 72 h, the average weight loss in control samples was approximately 1.6 mg/cm2, whereas it escalated to 3 mg/cm2 in samples containing 160 ppm THPS (P < 0.05).
The weight loss (a), the corrosion rate (b), the numbers of total (c) and live (d) sessile cells in biofilm of P. aeruginosa PAO1 in control ( ) and the samples containing sub-MIC of THPS (160 µg/ml) (
) and the samples containing sub-MIC of THPS (160 µg/ml) ( ) were shown. Also, significant (*) and non-significant (ns) data were shown. The control consisted of all components of test (including, bacterial medium, PA01, and carbon steel coupon,), without THPS addition.
) were shown. Also, significant (*) and non-significant (ns) data were shown. The control consisted of all components of test (including, bacterial medium, PA01, and carbon steel coupon,), without THPS addition.
Additionally, in the treated samples, corrosion rates exceeded those of the control samples at various time intervals Fig. 1b). In the control samples, corrosion rates after 24, 48, and 72 h were 18.2, 12.6, and 10 mpy, respectively. In contrast, the corrosion rate of the treated samples during the same timeframes increased to 22.1, 19.4, and 18.7 mpy, respectively (P < 0.05).
The effect of sub-MIC THPS on the corrosion of carbon steel in the medium without PAO1 (as positive control biocide) was also investigated. The results showed that the effect of 160 ppm THPS on corrosion rate was insignificant (P > 0.05) (data not shown).
The effect of THPS sub-MIC concentration on biofilm
Based on the weight loss results, it is possible to assert that sub-MIC concentrations of THPS can stimulate microbiologically influenced corrosion. Therefore, the impact of THPS on PAO1 biofilm formation and planktonic growth was meticulously investigated.
As illustrated in Fig. 1c, no statistically significant difference in the total number of sessile cells between the treated and control samples was evident after 24 h (P < 0.05). However, after 48 h of incubation, a substantial disparity became apparent (P < 0.05). Furthermore, after 72 h in the treated samples, the total number of sessile cells (4.2 × 108 CFU/cm2) exceeded that of the control (3.5 × 108 CFU/cm2) by 22%, signifying that sub-MIC concentrations of THPS stimulated PAO1 biofilm formation on carbon steel.
In the control samples, the total sessile cell count showed no significant difference between the 24 and 48-h samples (P < 0.05). Nevertheless, a slight decline of 10% (P < 0.05) and partial detachment of biofilm from the metal surface was observed at 72 h. Conversely, the biofilm of total sessile cells in the treated samples reached its peak population at 48 h and remained unchanged until 72 h (P < 0.05).
Figure 1d displays the results regarding the viability of the biofilm sessile cells. After 24 h, the highest number of live cells was present in both control and treated samples. No statistically meaningful difference was observed between the two study groups (P < 0.05).
Subsequently, after 48 h, the total number of viable cells amounted to 4.6 × 107 and 1.8 × 108 CFU/cm2 in control and treated samples, respectively (P < 0.05). Consequently, after 48 h, the viable sessile cell count in the treated samples exceeded that of the control by 3.9-fold. The most substantial discrepancy in viable sessile cells between treated and control samples became apparent after 72 h. Despite a marked decline in the number of living cells in both samples after 72 h, the treated samples exhibited a roughly five-fold higher count of viable sessile cells than the control samples.
Furthermore, the sub-MIC biocide also exerted an impact on PAO1 planktonic growth. For instance, in 48-h-old cultures, the number of planktonic cells in control and treated cultures was 1.5 × 109 and 1 × 109 cells/mL, respectively. In other words, the planktonic cell count in the control sample exceeded that of the treated sample by 50%.
Biofilm matrix assessments
The PA01 biofilm on carbon steel comprises two distinct segments. The outer gelly layer loosely adheres to the biofilm's main layer, which firmly attaches to the coupon surface. When exposed to sub-MIC concentrations of THPS, the biofilm outer layer becomes more gelly and exhibits a higher concentration of EPS (Fig. 2). As demonstrated in Fig. 2e, after 72 h, the treated biofilms showed a 55% increase in polysaccharide content compared to the control group (P < 0.05).
Biofilm integrity
The impact of THPS on biofilm integrity is depicted in Fig. 3. Following 30 s of vortexing, the biofilms subjected to THPS treatment exhibited fragmentation into substantial segments (Fig. 3a). In contrast, greater disintegration was observed in the control biofilm (Fig. 3b). Additionally, after 24 h of drying the biofilms on carbon steel coupons at laboratory temperature, the treated biofilm maintained its structural integrity (Fig. 3c), while inevitable cracks manifested in the control biofilm (Fig. 3d).
Dry weight of biofilm and corrosion products
As depicted in Fig. 4a, following a 72-h incubation period, the total dry weight of the biofilm and corrosion products in both control and treated samples amounted to 5.2 mg/cm2 and 8.7 mg/cm2, respectively. These findings indicate a significant increase, exceeding 67% (P < 0.05), in biofilm and the corresponding corrosion products in the presence of sub-MIC levels of THPS. The LOI results corroborate this trend, revealing elevated quantities of biofilm and corrosion products. Specifically, the corrosion products surged from 3.3 mg/cm2 in the control samples to 5.9 mg/cm2, marking a substantial 79% increase (P < 0.05) in the samples containing the biocide, thus indicating a heightened corrosion rate compared to the control samples (Fig. 4b).
Likewise, as illustrated in Fig. 4c, the dry weight of the biofilm increased by 47%, progressing from 1.9 mg/cm2 in the control group to 2.8 mg/cm2 in the treated biofilm (P < 0.05). These findings underscore the substantial impact of sub-MIC levels of THPS on biofilm formation and corrosion product accumulation.
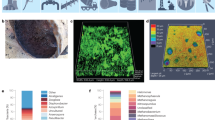
Confocal laser scanning microscopy (CLSM)
The results of the evaluation of biofilm thickness and the viability of sessile cells using confocal laser scanning microscopy were conducted on both 72-h treated and untreated biofilms, as depicted in Fig. 5. Using COMSTAT analysis, in the control sample, the biofilm average and maximum thicknesses were measured 78 and 80 µm, respectively. In contrast, the THPS-treated sample measured approximately 100 and 104 µm, respectively, confirming that sub-MIC levels of THPS led to a thicker biofilm. Moreover, live cells were more abundant within the biofilm formed in the presence of THPS. COMSTAT analysis revealed that live biomass increased from 15 μm3/μm2 in control sample to 20.9 μm3/μm2 in treated sample. In the same way, the live/dead ratio in the treated sample was higher than the control sample. The maximum live/dead ratio in the treated and control samples were 20.8% and 7.8%, respectively (Fig. 6). Remarkably, CLSM images revealed that in merged CLSM images (Syto 9 + PI), red dots were observed in the upper layers, while live cells were observed in the deeper layers of the biofilm (Fig. 5). The highest live/dead ratio in the control and treated samples were found at the depth of 30–50 and 70–100 µm, recpectively (Fig. 6).
Electrochemical studies
The effect of THPS sub-MIC on PA01 corrosion behavior of carbon steel in control and treated LB-NO3 media was studied by the EIS technique, and The Nyquist, Bode modulus, and Phase angle graphs of the working electrode are presented in Fig. 7. Semicircle diameters of the Nyquist plot indicate corrosion condition of carbon steel. Generally, the higher diameters of the Nyquist plot are accompanied by the higher electrical resistance at the metal/solution interface and the lower corrosion rate. As observed in Fig. 7A, all treated samples at various incubation times had much smaller semicircle diameters than control samples, indicating that treated samples had lower electrical resistance and higher corrosion rates, consistent with the weight loss results. Moreover, since the diameter of the Nyquist semicircles increased with time in both samples, it can be inferred that there is an inverse correlation between time and corrosion rate.
The equivalent electrical circuit obtained by interpretation of EIS analysis is shown in Fig. 8. The model consists of the solution resistance (Rs), biofilm or corrosion product resistance (Rb), constant phase element for biofilm or corrosion product (CPEb), charge-transfer resistance (Rct), and constant phase element of double-layer (CPEeff). The higher the Rct, the lower the corrosion rate would be.
The Constant phase element (ZCPE) can be defined as40:
In which j is an imaginary unit (j2 = -1), ω is the angular frequency, and Q is the capacitance of a capacitor.
The effective capacitance (Ceff) can be calculated by the equation41:
In which Re is Ohmic resistance, Rct is charge-transfer resistance, Q is the capacitance of a capacitor, and α (or n) is dispersion coefficient.
The electrochemical parameters obtained from the equivalent circuit are presented in Table 3. The charge-transfer resistance (Rct) is an important parameter inversely related to corrosion rate15,42,43. Rct values in the treated samples were lower than in the control ones. Rct values after 24, 48, and 72 h in treated samples were 934, 1238, and 5240 Ω.cm2, and in control samples were 1429, 3350, and 5627 Ω.cm2, respectively. The lower value of Rct indicates that THPS at sub-MIC can stimulate carbon steel corrosion by P. aeruginosa PAO1. Furthermore, Rct + Rb could reflect the corrosion rate, and the lower value indicates a higher corrosion rate15,43. The Rct + Rb values in treated samples were lower than those of controls in all incubation times, meaning higher corrosion rates in treated samples.
The polarization resistance (Rp) indicates the charge-transfer resistance between the corroding surface and the corrosive electrolyte. So, the greater the Rp values, the higher the corrosion resistance of the metal and the lower the corrosion rate44,45. The LPR data (Table 4 and Fig. 6c) showed that treated samples had a lower Rp value. For example, the Rp values of treated and control samples after 24 h were 935 and 1385 Ω.cm2, respectively. Therefore, the results showed that sub-MIC concentrations of THPS could decrease the Rp values, leading to higher corrosion rates.
Tafel polarization plots are depicted in Fig. 6d. Cathodic and anodic Tafel slope (βc and βa), corrosion current density (icorr), and the corrosion potential (Ecorr) were derived through extrapolation of both branches of the Tafel curves. The results were presented in Table 5.
Discussion
Comprehensive information concerning influential factors in biofilm formation and dispersion is essential for effective combat against microbiologically influenced corrosion. Pseudomonas aeruginosa, an opportunistic pathogen, has been extensively studied as a promising model organism for biofilm research21 and microbial corrosion experiments9,10,11,12,13,14,15,16,17,18,19,20. The mechanisms underlying microbial corrosion, particularly the role of extracellular electron transfer have been explored in P. aeruginosa46. However effects of biocides at sub-MIC on its corrosion rate have not been reported, yet. PAO1 is one of the most famous strains of this species which has the first completely sequenced P. aeruginosa genome and commonly used as a standard strain in microbiology research47. Consequently, P. aeruginosa PAO1 is a well documented bacterial strain for biofilm studies. Numerous studies have underscored the pivotal role of P. aeruginosa PAO1 biofilms in microbiologically influenced corrosion10,16,20,33,43,48,49,50,51,52.
Biocide treatment is one of the foremost methods for mitigating microbiologically influenced corrosion6,22,23,24. Given the intricate nature of industrial systems, achieving an even distribution of the appropriate biocide concentration may prove impractical, potentially leading to the exposure of microorganisms to sub-MIC of the biocide. The present study was meticulously crafted to elucidate the impact of sub-MIC concentrations of THPS biocide on microbiologically influenced corrosion.
This comprehensive investigation has unequivocally demonstrated that THPS, at a sub-MIC concentration of 160 ppm, significantly augments the corrosion of carbon steel induced by P. aeruginosa. Notably, the treated coupons exhibited an 85% increase in weight loss compared to the control samples. Moreover, the corrosion rates in the treated specimens exceeded those in the control specimens. Furthermore, while the corrosion rate in the control group decreased by 45% between the 24-h and 72-h time point, the treated samples only experienced a 16% reduction during the same period. This indicates that over time, the corrosion rate in the treated samples was significantly higher than that observed in the control samples. In summary, the results unequivocally demonstrate that sub-MIC treatments with THPS significantly enhance the microbiologically influenced corrosion of carbon steel by P. aeruginosa PAO1.
It is worth noting that electron transfer constitutes an indispensable aspect of metallic corrosion53. The heightened weight loss of carbon steel coupons and the reduction in the diameter of the Nyquist semicircle observed in the treated samples suggest that introducing sub-MIC concentrations of THPS increases the electron transfer rate, consequently enhancing the corrosion rate.
The second significant finding was that P. aeruginosa strain PA01 exhibited increased biofilm thickness when exposed to 160 ppm of THPS on carbon steel surfaces. As noted in prior investigations, the presence and activity of sessile cells play a pivotal role in microbiologically influenced corrosion5,6,7. Consequently, the augmentation in biofilm thickness can potentially enhance electron transfer and, subsequently, the corrosion rate. This notion is corroborated by the research of Gou et al. in 202218, which demonstrated that the extent of biofilm formation by P. aeruginosa can significantly impact microbiologically influenced corrosion. They elucidated that some aspects of low-alloy steel could enhance P. aeruginosa's chemotaxis toward metals, thereby increasing biofilm formation and the associated corrosion rate.
While the enhancement of P. aeruginosa biofilm formation in the presence of sub-MIC levels of antimicrobial agents has been documented in clinical studies, this phenomenon has rarely been addressed in industrial applications. For instance, Hoffman et al. reported that sub-MIC levels of tobramycin stimulated biofilm formation in P. aeruginosa PA0130. Additionally, it has been reported that sub-MIC levels of tetracycline, norfloxacin, and clarithromycin also stimulated biofilm formation in P. aeruginosa54,55. Due to the widespread use of antimicrobial agents in clinical and domestic settings, the impact of sub-MIC levels of certain biocides on P. aeruginosa biofilm formation has garnered attention. For example, Hemati et al. in 202056 demonstrated that sub-MIC levels of Savlon, chlorhexidine, and Deconex® could stimulate biofilm formation in clinical strains of P. aeruginosa. Furthermore, Yang and Alvarez in 201557 highlighted that exposure of P. aeruginosa PA01 to sublethal concentrations of silver nanoparticles resulted in increased biofilm formation, matrix polysaccharide content, and expression of antimicrobial resistance genes.
Biofilm formation represents a crucial antimicrobial defense mechanism, and research has indicated that biofilm sessile cells exhibit approximately 1000-fold greater tolerance to antimicrobial agents58. One of the pivotal mechanisms contributing to antimicrobial tolerance in biofilms involves restricting the penetration of these molecules across biofilm layers59. Consequently, it can be deduced that in thicker biofilms, a concentration gradient of THPS forms throughout the biofilm layers, with cells in the inner layers exposed to lower biocide concentrations. This, in turn, contributes to an increased survival rate among these cells.
Tracking changes in the population of planktonic and sessile cells during exposure to sub-MIC concentrations of THPS showed that, in contrast to the planktonic population, the sessile cells in the treated biofilm outnumbered those in the control sample. This suggests that the sub-MIC concentration of THPS, which was not lethal to planktonic cells, could be a driving force behind this transition to biofilm development. On the other hand, the higher number of planktonic cells in the control samples consumes more nutrient resources, leading to increased secretion of metabolic byproducts into the environment. In contrast, lower levels of wasted byproducts and increased nutrient availability in the treated samples result in enhanced biofilm formation.
The research has also demonstrated that the biofilms formed in the presence of sub-MIC levels of the biocide exhibit improved integrity and adhere better to the coupon surface. P. aeruginosa can uptake the necessary electrons for its metabolism from metals46. Therefore, it appears that tighter adherence to the coupon surface can enhance electron transfer and corrosion rates.
One of the more significant findings to emerge from this study is that the biofilm of PA01 developed in the presence of sub-MIC concentrations of THPS has a higher polysaccharide content and a more gelatinous consistency. Similarly, Bagge et al.60 demonstrated that P. aeruginosa PAO1 biofilm formed in sub-MIC concentrations of imipenem also had a higher polysaccharide content. The polysaccharide constituent of the biofilm matrix plays a crucial role in biofilm formation61,62. Additionally, numerous studies have emphasized the importance of polysaccharide structural scaffolds in P. aeruginosa for biofilm formation, integrity, surface adherence, intercellular interactions, and resistance to antimicrobial compounds63,64,65,66,67. It has also been reported that the polysaccharide content of the matrix can be one of the most critical factors contributing to biofilm antimicrobial tolerance by acting as a diffusion barrier68. According to the results of this study, it appears that PA01 secretes more extracellular polysaccharides to enhance biofilm adherence to the coupon surface, intercellular attachment and ultimately protect itself from THPS.
This study has shown that sub-MIC concentrations of THPS also affect the biofilm dispersion phase. Biofilm dispersion is the final phase in the biofilm developmental cycle69. Because planktonic cells are more susceptible to antimicrobial agents, inducing biofilm dispersion is a novel technique to mitigate biofilms, especially in clinical fields69. In the control samples, the total sessile cell number was not significantly different in 24- and 48-h-old samples (p > 0.05), but a 10% decrease was noticed at 72 h (p < 0.05), revealing that in control samples, the sessile population had reached its maximum level within 24 h and maintained its consistency up to 48 h. However, the 72-h-old biofilms of control samples entered the dispersal phase, and some bacterial cells evacuated the biofilm. On the contrary, the biofilm sessile population in samples containing THPS sub-MIC concentration reached its maximum at 48 h. Sessile cell count results showed no significant difference between 48- and 72-h-old biofilm (p > 0.05), and unlike control samples, no biofilm dispersal was seen. It is worth mentioning that treated biofilm did not enter the dispersal phase after 72 h. Sessile cells in treated biofilms seemed to have better integrity to delay the biofilm dispersal stage as much as possible to protect themselves from biocide.
The comparison of live and dead sessile cells showed that many died as biofilm aged. The difference between live and dead sessile cells was more notable in the control than in the treated sample. For instance, in the control samples, the total sessile cell density in 72-h-old biofilm was about 3.5 × 108 Cell/cm2, but only 1 × 106 CFU/cm2 was detected on the plate, highlighting the number of dead sessile cells. In a 72-h-old treated biofilm, live sessile cells were 5 times more than the control sample. Based on the CLSM data, the results indicate biofilms formed with a sub-MIC concentration of THPS might be better prepared to cope with unfavorable conditions. For instance, in the treated sample, live cells were present in the lower layers of the biofilm, which can increase their resistance to environmental antimicrobial compounds.
In biofilm structures, nutrient resources are predominantly consumed by sessile cells in the outer layers. This results in establishing a nutrient gradient across the various layers of the biofilm. This nutrient scarcity in the inner layers prompts a transition of inner cells into persister cells70. Persister cells are pivotal in developing antimicrobial tolerance58,70,71,72. These cells exhibit slow or negligible growth rates, displaying heightened antimicrobial tolerance58,70,71. Environmental stress at sublethal levels catalyzes the formation of persister cells72,73, exhibiting resistance to biocides68. This study showed that biofilms formed in the presence of sub-MIC levels of THPS exhibited improved structural integrity, increased polysaccharide content, and greater thickness. As a result, the availability of nutrients decreased even more significantly within the inner layers of the biofilm subjected to treatment, in contrast to the control biofilm. Consequently, there was a remarkable upsurge in the population of viable cells residing in the inner layer of the treated biofilm, a phenomenon supported by the imagery obtained through CLSM. Consequently, it is plausible to consider the emergence of persister cells of P. aeruginosa in sub-MIC levels of THPS. Such persister cells may contribute to an elevated resistance to antimicrobial agents.
Furthermore, as previously mentioned, live sessile cells were found to be more abundant in the inner layers, particularly near metal surfaces. P. aeruginosa can uptake the necessary electrons from metals46, and owing to the limited nutrient resources in the inner layers, these cells may obtain their required energy from the carbon steel substrate beneath them. Consequently, treating biofilm with THPS may lead to an increased rate of electron uptake by the cells in the inner layers, thereby resulting in elevated metal corrosion rates.
Recently, Xu et al.74 showed that inadequate dosing of THPS can induce Desulfovibrio hontreensis SY-21 biofilm formation and increase X70 steel corrosion rate in this anaerobic bacterium. The present study has confirmed the findings of Xu et al.74 but in another crucial corrosion-related microorganism, P. aeruginosa, a facultative aerobic bacterium. These findings demonstrate that sub-MIC biocides can frequently induce biofilm formation by various corrosion-associated organisms, including anaerobic and aerobic organisms. Considering this evidence, we can conclude that biofilm induction by sub-MIC concentrations of antimicrobial agents holds significant importance in industry as well as medicine. Additionally, the use of sub-MIC biocide concentrations in industrial facilities is not effective and increases costs associated with facilities maintenance.
Conclusion
This study is one of the first attempts to demonstrate that sub-MIC levels of THPS can induce biofilm formation by P. aeruginosa and thus significantly increase carbon steel corrosion. Moreover, it was observed that treated biofilm had higher thickness, more extracellular polymeric substances, better adherence to the metal surface, better integrity, and entered the dispersal phase for a more extended period. Furthermore, more live sessile cells were detected in the inner layers of the treated biofilm. All mentioned factors would increase electron transfer in treated samples, meaningfully intensifying carbon steel corrosion by P. aeruginosa. The current data highlight the importance of standard biocide evaluation in industrial settings. These results suggest that no biocidal treatment is better than incomplete treatment in mitigating microbiologically influenced corrosion.
The question raised by this study is: what are the effects of THPS and other biocides sub-MIC concentrations on other corrosion-related bacteria and corrosive consortia? Further studies could assess such effects on corrosion-related bacteria, including acid-producing, iron-reducing, and other critical bacterial groups and consortiums. Such experiments could shed more light on proper biocide application.
Data availability
All data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Little, B. et al. Microbially influenced corrosion—Any progress?. Corros. Sci. 170, 108641 (2020).
Knisz, J. et al. Microbiologically Influenced Corrosion-More than just Microorganisms. FEMS Microbiol. Rev. 47, fuad041 (2023).
Xu, D., Gu, T. & Lovley, D. R. Microbially mediated metal corrosion. Nat. Rev. Microbiol. https://doi.org/10.1038/s41579-023-00920-3 (2023).
ASTM-G193. Standard Terminology and Acronyms Relating to Corrosion, ASTM International (2022).
Javaherdashti, R. Microbiologically Influenced Corrosion (MIC) (Springer, 2017).
Jia, R., Unsal, T., Xu, D., Lekbach, Y. & Gu, T. Microbiologically influenced corrosion and current mitigation strategies: A state of the art review. Int. Biodeterior. Biodegrad. 137, 42–58 (2019).
Eckert, R. B. Emphasis on biofilms can improve mitigation of microbiologically influenced corrosion in oil and gas industry. Corros. Eng., Sci. Technol. 50, 163–168 (2015).
Schalk, I. J. & Perraud, Q. Pseudomonas aeruginosa and its multiple strategies to access iron. Environ. Microbiol. 25, 811–831 (2023).
Li, H. et al. Microbiologically influenced corrosion behavior of S32654 super austenitic stainless steel in the presence of marine Pseudomonas aeruginosa biofilm. J. Mater. Sci. Technol. 33, 1596–1603 (2017).
Jia, R., Yang, D., Xu, D. & Gu, T. Anaerobic corrosion of 304 stainless steel caused by the Pseudomonas aeruginosa biofilm. Front. Microbiol. 8, 2335 (2017).
Xu, D. et al. Enhanced resistance of 2205 Cu-bearing duplex stainless steel towards microbiologically influenced corrosion by marine aerobic Pseudomonas aeruginosa biofilms. J. Mater. Sci. Technol. 34, 1325–1336 (2018).
Zhao, Y. et al. Laboratory investigation of microbiologically influenced corrosion of 2205 duplex stainless steel by marine Pseudomonas aeruginosa biofilm using electrochemical noise. Corros. Sci. 143, 281–291 (2018).
Khan, M. S. et al. Microbiologically influenced corrosion of titanium caused by aerobic marine bacterium Pseudomonas aeruginosa. J. Mater. Sci. Technol. 35, 216–222 (2019).
Lou, Y. et al. Microbiologically influenced corrosion of FeCoCrNiMo0.1 high-entropy alloys by marine Pseudomonas aeruginosa. Corros. Sci. 165, 108390 (2020).
Huang, L. et al. Pyocyanin-modifying genes phzM and phzS regulated the extracellular electron transfer in microbiologically-influenced corrosion of X80 carbon steel by Pseudomonas aeruginosa. Corros. Sci. 164, 108355 (2020).
Pu, Y. et al. Microbiologically influenced corrosion of Cu by nitrate reducing marine bacterium Pseudomonas aeruginosa. J. Mater. Sci. Technol. 47, 10–19 (2020).
Liu, D. et al. Electron transfer mediator PCN secreted by aerobic marine Pseudomonas aeruginosa accelerates microbiologically influenced corrosion of TC4 titanium alloy. J. Mater. Sci. Technol. 79, 101–108 (2021).
Guo, Z. et al. Pseudomonas aeruginosa-accelerated corrosion of Mo-bearing low-alloy steel through molybdenum-mediating chemotaxis and motility. Bioelectrochemistry 144, 108047 (2022).
Li, C. et al. Effects of Pseudomonas aeruginosa on EH40 steel corrosion in the simulated tidal zone. Water Res. 232, 119708 (2023).
Jia, R., Yang, D., Xu, J., Xu, D. & Gu, T. Microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm under organic carbon starvation. Corros. Sci. 127, 1–9 (2017).
Ozer, E. et al. An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci. Adv. 7, eabg8581 (2021).
Skovhus, T. L., Eckert, R. B. & Rodrigues, E. Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry—Overview and a North Sea case study. J. Biotechnol. 256, 31–45 (2017).
Kannan, P., Su, S. S., Mannan, M. S., Castaneda, H. & Vaddiraju, S. A review of characterization and quantification tools for microbiologically influenced corrosion in the oil and gas industry: Current and future trends. Ind. Eng. Chem. Res. 57, 13895–13922 (2018).
Stillger, L., Viau, L., Kamm, L., Holtmann, D. & Müller, D. Optimization of antimicrobial peptides for the application against biocorrosive bacteria. Appl. Microbiol. Biotechnol. https://doi.org/10.1007/s00253-023-12562-9 (2023).
Videla, H. A. & Herrera, L. K. Microbiologically influenced corrosion: looking to the future. Int. Microbiol. 8, 169 (2005).
Moiseev, D. V. & James, B. R. Tetrakis (hydroxymethyl) phosphonium salts: Their properties, hazards and toxicities. Phosphorus Sulfur Silicon Relat. Elem. 195, 263–279 (2020).
McIlwaine, D. Oilfield application for biocides. In Directory of Microbicides for the Protection of Materials: A Handbook (ed. Paulus, W.) 157–175 (Springer Dordrecht, 2005).
Jones, C., Downward, B., Edmunds, S., Curtis, T. & Smith, F. THPS: a review of the first 25 years, lessons learned, value created and visions for the future. NACE CORROSION, NACE-2012–1505 (2012).
Andrews, J. M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48, 5–16 (2001).
Hoffman, L. R. et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436, 1171–1175 (2005).
Kaplan, J. B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 34, 737–751 (2011).
Ranieri, M. R., Whitchurch, C. B. & Burrows, L. L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 45, 164–169 (2018).
Jia, R., Yang, D., Xu, D. & Gu, T. Mitigation of a nitrate reducing Pseudomonas aeruginosa biofilm and anaerobic biocorrosion using ciprofloxacin enhanced by D-tyrosine. Sci. Rep. 7, 6946 (2017).
Heydorn, A. et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146, 2395–2407 (2000).
Vorregaard, M., Comstat2-a modern 3D image analysis environment for biofilms. Master's thesis, Technical University of Denmark, Kongens Lyngby, Denmark. (2008).
Chiba, A., Sugimoto, S., Sato, F., Hori, S. & Mizunoe, Y. A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol. 8, 392–403 (2015).
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
ASTM-G1–03. Standard Practice for Preparing, Cleaning and Evaluating Corrosion Test Specimens, ASTM International. (2011).
Odewunmi, N. A., Solomon, M. M., Umoren, S. A. & Ali, S. A. Comparative studies of the corrosion inhibition efficacy of a dicationic monomer and its polymer against API X60 steel corrosion in simulated acidizing fluid under static and hydrodynamic conditions. ACS Omega 5, 27057–27071 (2020).
Rahimi, A., Abdouss, M., Farhadian, A., Guo, L. & Neshati, J. Development of a novel thermally stable inhibitor based on furfuryl alcohol for mild steel corrosion in a 15% HCl medium for acidizing application. Ind. Eng. Chem. Res. 60, 11030–11044 (2021).
Hirschorn, B. et al. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 55, 6218–6227 (2010).
Li, H. et al. Microbiologically influenfced corrosion of 2707 hyper-duplex stainless steel by marine Pseudomonas aeruginosa biofilm. Sci. Rep. 6, 20190 (2016).
Jia, R., Yang, D., Xu, D. & Gu, T. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry 118, 38–46 (2017).
Shi, X., Yang, K., Yan, M., Yan, W. & Shan, Y. Study on microbiologically influenced corrosion resistance of stainless steels with weld seams. Front. Mater. 7, 83 (2020).
Rajala, P., Nuppunen-Puputti, M., Wheat, C. G. & Carpen, L. Fluctuation in deep groundwater chemistry and microbial community and their impact on corrosion of stainless-steels. Sci. Total Environ. 824, 153965 (2022).
Chugh, B. et al. Extracellular electron transfer by Pseudomonas aeruginosa in biocorrosion: A review. ACS Biomater. Sci. Eng. 8, 1049–1059 (2022).
Chandler, C. E. et al. Genomic and phenotypic diversity among ten laboratory isolates of Pseudomonas aeruginosa PAO1. J. Bacteriol. 201, 10–1128 (2019).
Batmanghelich, F., Li, L. & Seo, Y. Influence of multispecies biofilms of Pseudomonas aeruginosa and Desulfovibrio vulgaris on the corrosion of cast iron. Corros. Sci. 121, 94–104 (2017).
Zhao, X. et al. Influence of Pseudomonas aeruginosa and sulfate-reducing bacteria composite on the corrosion behavior of brass. Int. J. Electrochem. Sci. 14, 6468–6477 (2019).
Liu, H. & Cheng, Y. F. Corrosion of X52 pipeline steel in a simulated soil solution with coexistence of Desulfovibrio desulfuricans and Pseudomonas aeruginosa bacteria. Corros. Sci. 173, 108753 (2020).
Li, H., Sun, M., Du, M., Zheng, Z. & Ma, L. Mechanism underlying the acceleration of pitting corrosion of B30 copper–nickel alloy by Pseudomonas aeruginosa. Front. Microbiol. 14, 1149110 (2023).
Xu, L., Ivanova, S. A. & Gu, T. Mitigation of galvanized steel biocorrosion by Pseudomonas aeruginosa biofilm using a biocide enhanced by trehalase. Bioelectrochemistry 154, 108508 (2023).
Beech, I. B. & Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 15, 181–186 (2004).
Linares, J. F., Gustafsson, I., Baquero, F. & Martinez, J. Antibiotics as intermicrobial signaling agents instead of weapons. Proc. Natl. Acad. Sci. 103, 19484–19489 (2006).
Garey, K. W. et al. Increased bacterial adherence and biomass in Pseudomonas aeruginosa bacteria exposed to clarithromycin. Diagn. Microbiol. Infect. Dis. 63, 81–86 (2009).
Hemati, S. et al. Sub-minimum inhibitory concentrations of biocides induced biofilm formation in Pseudomonas aeruginosa. New Microbes New Infect. 38, 100794 (2020).
Yang, Y. & Alvarez, P. J. Sublethal concentrations of silver nanoparticles stimulate biofilm development. Environ. Sci. Technol. Lett. 2, 221–226 (2015).
Khan, F., Pham, D. T. N., Tabassum, N., Oloketuyi, S. F. & Kim, Y.-M. Treatment strategies targeting persister cell formation in bacterial pathogens. Crit. Rev. Microbiol. 46, 665–688 (2020).
Ciofu, O., Moser, C., Jensen, P. Ø. & Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 20, 621–635 (2022).
Bagge, N. et al. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob. Agents Chemother. 48, 1175–1187 (2004).
Flemming, H.-C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Flemming, H.-C. et al. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 21, 70–86 (2023).
Hentzer, M. et al. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183, 5395–5401 (2001).
Ryder, C., Byrd, M. & Wozniak, D. J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 10, 644–648 (2007).
Yang, L. et al. Polysaccharides serve as scaffold of biofilms formed by mucoid Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 65, 366–376 (2012).
Ma, L. Z., Wang, D., Liu, Y., Zhang, Z. & Wozniak, D. J. Regulation of biofilm exopolysaccharide biosynthesis and degradation in Pseudomonas aeruginosa. Annu. Rev. Microbiol. 76, 413–433 (2022).
Colvin, K. M. et al. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 14, 1913–1928 (2012).
Brown, D. C. & Turner, R. J. Biofilms and microbiologically influenced corrosion in the petroleum industry. In Introduction to Biofilm Engineering (eds Rathinam, N. K. & Sani, R. K.) 187–203 (ACS Publications, 2019).
Rumbaugh, K. P. & Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 18, 571–586 (2020).
Ciofu, O. & Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics. Front. Microbiol. 10, 913 (2019).
Pang, Z., Raudonis, R., Glick, B. R., Lin, T.-J. & Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37, 177–192 (2019).
Zhou, Y., Liao, H., Pei, L. & Pu, Y. Combatting persister cells: The daunting task in post-antibiotics era. Cell Insight 2, 100104 (2023).
Harms, A., Maisonneuve, E. & Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354, aaf4268 (2016).
Xu, L. et al. Inadequate dosing of THPS treatment increases microbially influenced corrosion of pipeline steel by inducing biofilm growth of Desulfovibrio hontreensis SY-21. Bioelectrochemistry 145, 108048 (2022).
Acknowledgements
The authors sincerely thank Alireza Rahimi from the Research Institute of Petroleum Industry (Tehran, Iran) for his support in electrochemical analyses.
Author information
Authors and Affiliations
Contributions
H.T: designed the experiments, carried out the experiments, data analysis, figures and tables preparations, writing the manuscript, resources and materials. J.H and S.M.M.D: designed the experiments, resources and materials, editing the manuscript, supervision. All authors participated in discussion, reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tirandaz, H., Dastgheib, S.M.M. & Hamedi, J. Sub-minimum inhibitory concentration of tetrakis(hydroxymethyl)phosphonium sulfate enhances biocorrosion of carbon steel by Pseudomonas aeruginosa. Sci Rep 14, 28918 (2024). https://doi.org/10.1038/s41598-024-70157-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70157-4
Keywords
This article is cited by
-
Sublethal THPS accelerates Pseudomonas-associated steel corrosion by stimulating biofilm development and microbial electron uptake
npj Materials Degradation (2025)