Abstract
The resistance of foodborne pathogens to antimicrobial agents is a potential danger to human health. Hence, establishing the status of good agricultural practices (GAPs) and the antimicrobial susceptibility of major foodborne pathogens has a significant programmatic implication in planning interventions. The objective of this study was to assess the gap in attaining GAP and estimate the prevalence and antimicrobial susceptibility profile of Salmonella in vegetable farms fertilized with animal manure in Addis Ababa, Ethiopia. A total of 81 vegetable farms from four sub-cities in Addis Ababa were visited, and 1119 samples were collected: soil (n = 271), manure (n = 375), vegetables (n = 398), and dairy cattle feces (n = 75). Additional data were collected using a structured questionnaire. Isolation of Salmonella was done using standard microbiology techniques and antimicrobial susceptibility testing was conducted using disk diffusion assays. Carriage for antimicrobial resistance genes was tested using polymerase chain reaction (PCR). Among the 81 vegetable farms visited, 24.7% used animal manure without any treatment, 27.2% used properly stored animal manure and 80.2% were easily accessible to animals. The prevalence of Salmonella was 2.3% at the sample level, 17.3% at the vegetable farm level, and 2.5% in vegetables. The highest rate of resistance was recorded for streptomycin, 80.7% (21 of 26), followed by kanamycin, 65.4% (17 of 26), and gentamicin, 61.5% (16 of 26). Multidrug resistance was detected in 61.5% of the Salmonella isolates. Vegetable farms have a gap in attaining GAPs, which could contribute to increased contamination and the transfer of antimicrobial resistance to the vegetables. The application of GAPs, including proper preparation of compost and the appropriate use of antimicrobials in veterinary practices, are recommended to reduce the emergence and spread of antimicrobial resistance.
Similar content being viewed by others
Introduction
Foodborne infections are major public health problems worldwide1. For a healthy lifestyle, eating vegetables is encouraged to prevent different chronic diseases2. However, the number of foodborne outbreaks caused by contaminated vegetables is increasing from time to time3. Animal manure, which is widely used as fertilizer in different parts of the world to increase agricultural production4, can be contaminated with foodborne bacteria such as Salmonella, Escherichia coli, and Campylobacter, increasing the risk of infection by these pathogens5. Salmonella is the leading cause of produce-related outbreaks worldwide6.
The application of animal manure as fertilizer has been reported to contaminate soil with antimicrobial agents discharged from the feces or urine of animals7. In addition to the presence of foodborne pathogens in the food chain, antimicrobial-resistant bacteria carrying different genetic resistance markers enter the human food chain from food animals subjected to regular antibiotic treatments8. This is a major health concern because many of the antimicrobial agents used in animal production and human therapeutics are analogues9. These analogue antibiotics may select for resistant phenotypes of the pathogen that could cause more antibiotic-resistant bacterial illnesses in humans10.
The application of animal manure to agricultural soil during the production of food crops to improve soil fertility is a routine practice in many countries around the world, including Ethiopia11,12. In developing countries, animal manure is a major nutrient source for smallholder farmers who cannot afford mineral fertilizers4. In urban and peri-urban Addis Ababa, there are small-scale farms in close proximity to human residential areas, and in some cases, animal manure is used as fertilizer for the growth of different vegetables13.
Multiple studies have shown the occurrence of Salmonella and the increase in antimicrobial resistance among Salmonella isolates in Ethiopia. For instance, the prevalence of Salmonella in poultry farms was 14.6%14, and that in dairy farms was 8.3%15. The prevalence of Salmonella in diarrheic patients ranges from 1.1 to 6.2%16,17. In addition, several studies in Ethiopia have reported a high rate of antimicrobial resistance in Salmonella isolates from different sources18,19. However, there is a lack of data showing the impact of using animal manure as fertilizer on the dissimilation of Salmonella.
Here, we investigated the prevalence and antimicrobial resistance profile of Salmonella from animal manure amended vegetable farms in highly populated urban and peri-urban Addis Ababa, where vegetable farms are in close proximity to animals and humans. The good agricultural practices (GAPs) of these vegetable farms were also assessed.
Materials and methods
Study area and study design
This study was conducted in Addis Ababa, Ethiopia. Addis Ababa is the capital city of Ethiopia, located at 9° 1′ 48″ N, 38° 44′ 24″ E. The average altitude of Addis Ababa is 2400 m above sea level, with the highest elevation at Entoto Hill to the north reaching 3200 m. It has a subtropical highland climate, with average annual temperature of 16.3 °C and 1089 mm annual rainfall20. A cross-sectional study was conducted from February 2022 to March 2023.
Study farms
Four of the 11 sub-cities in Addis Ababa: Akaki-Kality, Nifas-Silk Lafto, Arada, and Gulelle sub-cities were randomly selected of which vegetable farms available in these sub-cities that reported the use of animal manure as fertilizer were recruited. Based on the availability of farms in each sub-city, a total of 81 vegetable farms were visited: 41 from the Akaki Kality sub-city, 23 from Nefas-Silk Lafto, 14 from the Gulele sub-city, and 3 from the Arada sub-city as shown in our previous study21.
Data and sample collection
A farm review consisting of onsite observation and face-to-face interviews with vegetable farmers was conducted in the visited 81 vegetable farms. The farm review was based on the USA Department of Agriculture (USDA) checklist for Good Agricultural Practices (GAPs)22. The focus was given to the source of water, animal activity, and handling of compost manure as part of the WHO requirement to grow safer vegetables1.
Manure, soil, and vegetable sample collection was performed as previously described23,24. Briefly, from each vegetable farm, 15 surface subsamples (0–20 cm) (3 plots of 1 m2 each, per farm, 6 m apart from each other, and 5 subsamples from each plot, including the four corners and the middle of the plot) were collected and made into a single composite soil. Six manure samples were collected from each farm and pooled to obtain one composite manure sample. The same sampling technique was followed, except that only six subsamples (for each vegetable type) were used in the case of vegetables. Moreover, fresh fecal droppings from dairy cattle living around vegetable farms were also collected.
A total of 1119 samples were collected: 521 samples from Akaki Kality, 268 from Nefas Selk Lafto, 258 from Gulele, and 72 from Arada. Based on sample type, 398 were vegetables, 375 were manure, 271 were soil, and 75 were dairy cattle feces. The number and type of samples collected in each sub-city are shown in Table 1.
Then samples were transported to the Microbiology Laboratory of the Aklilu Lemma Institute of Pathobiology, Addis Ababa University, in an icebox containing an ice pack and processed within 4–6 h of collection.
Isolation and identification of Salmonella
Salmonella was detected using the guidelines of the International Organization for Standardization (ISO) 657925. Ten grams of each sample was suspended in 90 mL of Buffered Peptone Water (BPW) and mixed by shaking to form a slurry. The slurry was incubated at 37 °C for 24 h, and 1 mL of the slurry was enriched in 10 mL tetrathionate broth (Oxoid Ltd., Cambridge, UK) and incubated at 37 °C for 24 h. One hundred µL of the grown culture was then transferred to 9.9 mL of Rappaport–Vassiliadis broth (RV; Oxoid Ltd., Cambridge, UK) and incubated at 42 °C for 24 h. A loopful of bacteria grown in RV were plated on XLT-4 plates and incubated at 37 °C for 24 h26. Identification of presumptive Salmonella colonies and biochemical tests were performed as described previously27.
Isolates showing specific biochemical characteristics of Salmonella were further confirmed using Salmonella genus-specific PCR as previously described28. PCR was based on the amplification of a 496-base pair (bp) segment of histidine transport operon gene, which is highly conserved among species of Salmonella. The forward and reverse primer sequences, from 5′ to 3′ were ACTGGCGTTATCCCTTTCTCTGGTG; and ATGTTGTCCTGCCCCTGGTAAGAGA. The reference strain of Salmonella enterica serovar Typhimurium ATCC 13311 was used as a positive control. PCR confirmed Salmonella isolates were preserved at − 80 °C in 20% glycerol until further analysis.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of the Salmonella isolates was performed according to the Clinical Laboratory Standards Institute guideline29 using the Kirby-Bauer disk diffusion method on Muller-Hinton agar plates (Oxoid, CM0337 Basingstoke). The antimicrobial agents used in the present study were ampicillin (10 μg), nalidixic acid (30 µg), sulfamethoxazole + trimethoprim (1.25/23.75 μg), sulfisoxazole(1000 μg), chloramphenicol (30 μg), ceftriaxone (30 μg), amoxicillin + clavulanic acid (20/10 µg), streptomycin (10 μg), kanamycin (30 μg), ciprofloxacin (5 μg), tetracycline (30 μg), gentamicin (10 μg), amikacin (30 μg) and azithromycin (15 μg). The antimicrobial disks used in this study were all from Sensi-Discs (Becton, Dickinson, Company, Loveton, USA).
Salmonella isolates were considered multidrug-resistant when they were resistant to three or more antimicrobial agents belonging to different classes30. Escherichia coli ATCC 25922 was used as a quality control strain when conducting antimicrobial susceptibility tests.
Salmonella isolates that were phenotypically resistant to tetracycline, ampicillin, gentamycin, streptomycin and sulfonamide were screened for: tetracycline resistant genes (tet(A), tet(B), and tet(C)), aminoglycoside resistance genes (aminoglycoside acetyltransferase genes (aac (3)-IV), and adenyl transferase gene (aadA), sulfonamide resistant genes (sulI and sulII), and beta-lactamase gene (bla TEM), using conventional PCR. The PCR conditions and primer sequences are described previously21. The PCR product was observed using agarose gel electrophoresis.
Statistical analysis
Descriptive statistical methods were used to summarize the characteristics of the farms. The chi-square test was used to assess the associations of different factors with Salmonella positivity. A p-value less than 0.05 was considered to indicate a statistically significant association. SPSS version 26 was used to perform the descriptive analysis and the association between the antimicrobial resistance pattern, their genetic determinants, and the source of the isolates was assessed as described previously21.
Ethical consideration
The Institutional Review Board of Aklilu Lemma Institute of Pathobiology reviewed the protocol for its ethical and methodological standards and approved the conduct of the study (Ref. No. ALIPB IRB/75/2014/22 on April 30, 2022). The plant collection and use was in accordance with all the relevant guidelines.
Results
Vegetable farm description and farm practices
Most of the vegetable farms, 95% (77 of 81) used dairy manure, while only 5% (4 of 81) used poultry litter to fertilize their farms. The majority (88.8%; 71/88) applied both organic and inorganic fertilizers, and the remaining 11.1% (9 of 81) used only organic fertilizer. Of the 81 vegetable farms visited, 75.3% (61 of 81) used compost, and the remaining 24.7% (20 of 81) of the farmers applied animal manure without any treatment. Only 27.2% (22 of 81) of the vegetable farms stored manure properly to prevent contamination.
Most of the vegetable farms (80.2%; 65/81) were not fenced and were easily accessible to domestic and wild animals. A total of 92.6% (75 of 81) of the vegetable farms reported that they use river water and 7.4% (6 of 81) of the farms use both rain water and tap water to irrigate their farms.
There are no steps taken to protect irrigation water from contamination or to restrict livestock access to the source or delivery of irrigation water. There was no monitoring system on the farms for the presence of animals entering the vegetable farms. Most of the vegetable farms use composted manure to fertilize their farms 75.3% (61 of 81), whereas 24.7% (20 of 81) farms use fresh, untreated manure. Only 27.2% (22 of 81) of the vegetable farms stored manure properly to prevent contamination (Table 2).
Prevalence and distribution of Salmonella
The farm level prevalence of Salmonella in vegetable farms was 17.3% (14 of 81). In terms of sub-city stratification, 17.0% (7 of 41) of the vegetable farms in Akaki Kality, 21.7% (5 of 23) of the vegetable farms in Nefas Selk Lafto, and 14.3% (2 of 14) of the vegetable farms in the Gulele sub-city were positive for Salmonella. The presence of Salmonella in vegetable farms was not significantly associated with any of the studied factors or sub-city classifications (p > 0.05).
The sample-level prevalence of Salmonella was 2.3% (26/1119). The 26 Salmonella isolates were obtained from samples collected in the Gulele sub-city (4/258; 1.6%), Akaki Kality sub-city (13/521; 2.5%), and Nefas Selk Lafto sub-city (9/268; 3.3%), and no Salmonella was isolated from the Arada sub-city. The prevalence of Salmonella on vegetable farms is summarized in Table 3.
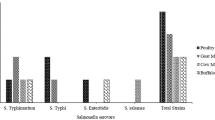
The highest prevalence (5.3%; 4/75) was detected in dairy cattle feces, followed by vegetables (2.5%; 10/398). A similar prevalence (1.8%) was detected in manure (7/375) and soil (5/271) samples. From the total of 398 vegetable samples, the highest prevalence was recorded in lettuce at 3.2% (5 of 153). However, the prevalence of Salmonella in kale was 2.5% (3 of 120) and, that in Swiss chard was 2.7% (2 of 72). Salmonella was not isolated from cabbage. The prevalence of Salmonella in different sample types and vegetables is shown in Fig. 1.
Antimicrobial susceptibility profile of Salmonella
Regardless of the source, all the Salmonella isolates were susceptible to chloramphenicol, ceftriaxone and meropenem. The highest resistance rate was recorded for streptomycin (80.7%, 21 of 26), followed by kanamycin (65.4%, 17 of 26), and gentamicin (61.5%, 16 of 26). The resistance to amoxicillin + clavulanic, amikacin, and ciprofloxacin was 30.7% (8 of 26), 15.3% (4 of 26), and 11.5% (3 of 26), respectively. Resistance to sulfamethoxazole + trimethoprim was 53.8% (14 of 26), and that of sulfisoxazole was 46.1% (12 of 26) (Fig. 2). A similar resistance rate of 26.9% (7 of 26) was recorded for tetracycline and nalidixic acid and 34.6% (9 of 26) for ampicillin and azithromycin. Multidrug resistance was observed in 61.5% (16 of 26) of the Salmonella isolates.
Antimicrobial resistance profile of Salmonella from vegetable farms in Addis Ababa, Ethiopia. Te tetracycline, C chloramphenicol, Amp ampicillin, Gm gentamicin, S streptomycin, K kanamycin, An amikacin, Na nalidixic acid, Cip ciprofloxacin, Cro ceftriaxone, Amc amoxicillin + clavulanic-acid, Stx sulfamethoxazole + trimethoprim, Su sulfisoxazole, Azm azithromycin, Mem meropenum.
Antimicrobial resistance genotypic profile of the Salmonella isolates
Resistance genes encoding for aminoglycoside resistance aadA were detected in 76.2% (16 of 21) of tested isolates, followed by blaTEM detected in 66.6% (6 of 9), and aac(3)-IV was detected in 62.5% (10 of 16) of isolates. On the other hand, tet (A) and tet (B) were detected in 28.6% (2 of 7), and 57.1% (4 of 7), respectively, and tet (C) gene was detected in none of the isolates. Moreover, sulI and, sulII were detected in 28.6% (4 of 14) and 50% (7 of 14), of the strains, respectively. The detailed distribution of AMR genes among Salmonella isolates from different sample sources is shown in Table 4.
Correlation between phenotypic and genotypic resistance profiles of Salmonella isolates
Hierarchal clustering showed that eleven Salmonella isolates that were susceptible to tetracycline were clustered together (Cluster A). Moreover, six isolates in this cluster were streptomycin resistant and contained the aadA gene. Another nine Salmonella isolates susceptible to nalidixic acid and ciprofloxacin and one tetracycline resistant isolate were clustered together (Cluster B). In Cluster B, there were six Salmonella isolates that were resistant to gentamycin and contained aac(3)-IV. Six isolates in Cluster B were from vegetable samples, one from a fecal sample and two from a manure sample. Similarly, six Salmonella isolates were clustered together, all of which were resistant to streptomycin and kanamycin (Cluster C).
Hierarchal clustering revealed that all the Salmonella isolates from the manure and feces samples were resistant to streptomycin. Similarly, all the Salmonella isolates from the soil samples were resistant to sulfamethoxazole + trimethoprim. Salmonella isolates from fecal samples showed the highest rate of resistance to ampicillin and amoxicillin + clavulanic acid. Among Salmonella isolates from manure samples, the highest rate of resistance was recorded for gentamicin and kanamycin. However, based on antimicrobial resistance patterns, isolates with different resistance profiles were shown to be distributed across farms, sub-cities, and specimen types. The antimicrobial susceptibility profiles of Salmonella isolates from different sub-cities, and sample types are summarized in Fig. 3.
Hierarchal clustering of Salmonella. Samples were clustered according to farm, sub-city, sample type, and phenotypic antimicrobial resistance profile. Amp ampicillin, Na nalidixic acid, Sxt sulfamethoxazole + trimethoprim, Su sulfisoxazole, Amc amoxicillin + clavulanic acid, S streptomycin, K kanamycin, Cip ciprofloxacin, Te tetracycline, Gm gentamycin, An amikacin, C chloramphenicol, Mem meropenum, Cro ceftriaxone, Azm azithromycin.
According to the correlation analysis, antimicrobial agents of the same antimicrobial class were strongly and positively correlated. Additionally, phenotypic resistance is positively and strongly correlated with genotypic resistance. For instance, in the aminoglycoside class of antimicrobials, streptomycin and kanamycin (r = 0.98) were strongly and positively correlated with corresponding resistance genes. Sulfamethoxazole + trimethoprim and sulfisoxazole were strongly and positively correlated (r = 0.9). Moreover, antimicrobials from different classes were correlated with each other. For instance, tetracycline and ampicillin were strongly and positively correlated (r = 0.9). Genotypic resistance to tetracycline was and strongly correlated with tet (A) and tet (B).
Discussion
Food safety has come a major public health concern driven by widespread outbreaks of Salmonella, E. coli O157, and Campylobacter31. Fresh vegetables can be contaminated at various stages, from farm to fork32. Leafy vegetables are associated with foodborne disease outbreaks, mainly due to Salmonella and E. coli infections33. These outbreaks could be attributed to environmental reservoirs of pathogens and possibly contaminated soil34.
Of the vegetable farms included in the current study, 24.6% used animal manure directly without any treatment, which is lower than that reported previously35. This result is greater than a report from Minnesota, where only 2% of the farmers applied raw manure36. The application of raw manure as fertilizer to vegetables could contribute to vegetable contamination because it is a common source of E. coli, Salmonella, Campylobacter, and Listeria spp.37.
Only 19.8% of the farms were fenced, and the majority were easily accessible by domestic and wild animals in the current study, which is lower than the 60% in Nigeria38 and 23% in the US36. Fencing vegetable farms is an important agricultural practice because both wild and domestic animals are sources of contamination with foodborne pathogens39.
Untreated wastewater is widely used in urban and peri-urban areas of developing countries40. In Ethiopia, a large volume of wastewater is released into water bodies that farmers use for irrigation41. In this study, rivers were the main source of water for 92.5% of the farms, which is higher than that reported in another study35. Moreover, none of the farms protect against the contamination from the water used for irrigation. In previous studies in Nigeria and South Africa, contamination level of water used for irrigation was higher than the WHO standard42,43. In addition, studies in Ethiopia showed that dirty water used for irrigation is the main source of contamination23,44.
The prevalence of Salmonella in this study (2.3%) is lower than that in reports from different countries35,45,46 and higher than that in other studies47,48. The variability of the prevalence of foodborne pathogens across different geographical areas could be due to seasonal variability and the level of GAP implementation on vegetable farms35. In the present study, the prevalence of Salmonella varied across the four sub-cities; some of these variabilities could be attributed to their geographic location, the concentration of farms in each location, differences in the number of farms represented, and their agricultural systems49,50.
The prevalence of Salmonella in the feces of dairy cattle in the current study (5.3%) was relatively higher than reports from previous studies in Ethiopia which ranged from 1.6 to 4.7%14,51,52,53 and lower than the 10.7% prevalence in another study in Ethiopia54. These differences might be due to variations in living and housing conditions and the type of feed provided to cattle55. The difference in the way feces were collected could also be the reason for the variation. In the current study, fresh feces were collected from the ground, while in some of the previous studies; feces were collected directly from the rectum of the animals.
The prevalence of Salmonella in vegetables (2.5%) in the current study was lower than previous report from China (3.4%)56. The prevalence of Salmonella in lettuce in the current study is higher than the report from a study in Rwanda (1%)35. Lettuce is the most consumed leafy vegetable, and Salmonella is one of the common pathogens identified in lettuce57.
The prevalence of Salmonella in soil and manure in the current study was lower than in the US24,58. Agricultural animal feeding regimens can affect manure composition and the survival of pathogens in manure59. Contaminated manure plays an important role in contaminating agricultural soil, irrigated water, and vegetables, and it enables the microorganisms to survive in soil for several months37.
The highest resistance rates were recorded for streptomycin, kanamycin, and gentamicin. Development of resistance to these antimicrobials poses significant public health consequences. High rate of resistance was recorded to ciprofloxacin in the current study. According to WHO classification, fluoroquinolones including ciprofloxacin is recommended to be used only in humans due to its high risk of antimicrobial resistance60. Detection of such high level of resistance to this antimicrobial might be due to contamination of the environment by microorganisms from public health facilities. Multidrug-resistant Salmonella is one of the leading veterinary and public health problems worldwide61. In this study, 61.5% of the Salmonella isolates were multidrug-resistant. This finding was lower than that reported in China (92.9%)47, and was higher than that reported in Ethiopia and elsewhere (17.9%–58.7%)24,51,52. The difference in the use of veterinary antimicrobials may be the reason behind the observed differences.
Sulfonamides, tetracyclines, and fluoroquinolones persist longer in soil and are detected in farm environments62. In addition to creating selection pressure on microorganisms in the soil and produce, veterinary antimicrobials could be transferred to the produce, contributing to antimicrobial resistance and possible toxicity in humans consuming these produces63. The application of animal manure on vegetable farms is recognized as an important route of transmission for antimicrobial resistance to foodborne pathogens64. Contamination of farmlands with antibiotics used for veterinary purposes is a global concern65.
The occurrence of a high rate of resistance to gentamicin, kanamycin, and streptomycin in this study was strongly and positively correlated, and the occurrence of sulfamethoxazole + trimethoprim and sulfisoxazole was strongly and positively correlated. This correlation could be due to the co-selection of resistance to these antimicrobials in Salmonella isolates and some of the resistance genetic markers may also be carried on same mobile genetic elements66. Studies have shown that there is clonal relatedness among multidrug-resistant Salmonella isolates in manure and soil, which highlights the potential role of manure application in the dissemination and persistence of multidrug-resistant Salmonella24.
Conclusion
Animal manure is a reservoir for different foodborne pathogens. Our results showed that vegetable farms that applied animal manure as fertilizer were contaminated with Salmonella. Moreover, most of these Salmonella isolates were multidrug-resistant. Good Agricultural Practice (GAP) was designed to address the source of vegetable contamination, but limited GAP efforts are being made in Addis Ababa to reduce food safety risks. Therefore, there is a need to treat and implement appropriate measures, including composting, before applying animal manure to agricultural farms. In addition, protecting farms from contamination by foodborne pathogens is recommended.
Data availability
The authors confirm that all the data are included in the manuscript.
References
WHO. World Health Organization of the United Nations. Five keys to growing safer fruits and vegetables. Promoting health by decreasing microbial contamination. Available at: http://apps.who.int//iris/bitstream/10665/75196/1/9789241504003_eng.pdf?ua=1 (2012).
Callejón, R. M. et al. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 12(1), 32–38 (2015).
Olaimat, A. N. & Holley, R. A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 32(1), 1–19 (2012).
Ndambi, O. A. et al. Manure management practices and policies in sub-Saharan Africa: Implications on manure quality as a fertilizer. Front. Sustain. Food Syst. 3, 29 (2019).
Heredia, N. & García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 4(3), 250–255 (2018).
Dyda, A. et al. Changing epidemiology of Salmonella outbreaks associated with cucumbers and other fruits and vegetables. Global Biosecurity 2(1), 1 (2020).
Iwu, C. D., Korsten, L. & Okoh, A. I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 9(9), e1035 (2020).
Lekshmi, M. et al. The food production environment and the development of antimicrobial resistance in human pathogens of animal origin. Microorganisms 5, 1. https://doi.org/10.3390/microorganisms5010011 (2017).
WHO, Critically important antimicrobials for human medicine. Available at https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf?ua=1 (2019).
O’Bryan, C. A., Crandall, P. G. & Ricke, S. C. Antimicrobial resistance in foodborne pathogens. In Food and Feed Safety Systems and Analysis 99–115 (Elsevier, 2018).
Jung, Y., Jang, H. & Matthews, K. R. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb. Biotechnol. 7(6), 517–527 (2014).
Tadesse, S. T. et al. Manure recycling from urban livestock farms for closing the urban–rural nutrient loops. Nutr. Cycling Agroecosyst. 119(1), 51–67 (2021).
Assefa Tuji, A. Constraints and opportunities in peri-urban and urban agriculture system in Addis Ababa, Ethiopia. Afr. J. Rural Dev. (AFJRD). 1(1978–2017–2069), 107–114 (2016).
Eguale, T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia: Prevalence and antimicrobial resistance. BMC Vet. Res. 14, 1–8 (2018).
Gebeyehu, A., Taye, M. & Abebe, R. Isolation, molecular detection and antimicrobial susceptibility profile of Salmonella from raw cow milk collected from dairy farms and households in southern Ethiopia. BMC Microbiol. 22(1), 84 (2022).
Andualem, T. et al. Prevalence and Antimicrobial Susceptibility Patterns of Shigella and Salmonella Species among Patients with Diarrhea Attending Gondar Town Health Institutions, Northwest Ethiopia. Sci. J. Public Health 2, 469–475 (2014).
Eguale, T. et al. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect. Dis. 15(1), 497 (2015).
Abebe, E., Gugsa, E. & Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 1, 1 (2020).
Asante, J., Noreddin, A. & El Zowalaty, M. E. Systematic review of important bacterial zoonoses in Africa in the last decade in light of the ‘One Health’concept. Pathogens 8(2), 50 (2019).
CDO, Addis Abeba climate (Ethiopia) (2021)
Hailu, W., et al. Escherichia coli isolates from vegetable farms in Addis Ababa, Ethiopia: Antimicrobial susceptibility profile and associated resistance genetic markers. Food Sci. Nutr. (2024).
USDA, United States department of agriculture. Good Agricultural Practices, Good Handling Practice audit verification checklist. Available at: https://www.ams.usda.gov/sites/default/files/media/GAPGHP_Checklist_no_spell_Checklist_Enabled%5B1%5D.pdf (2014).
Woldetsadik, D. et al. Farmers’ perceptions on irrigation water contamination, health risks and risk management measures in prominent wastewater-irrigated vegetable farming sites of Addis Ababa Ethiopia. Environ. Syst. Decis. 38(1), 52–64 (2018).
Pornsukarom, S. & Thakur, S. Assessing the impact of manure application in commercial swine farms on the transmission of antimicrobial resistant Salmonella in the environment. Plos One 11(10), e0164621 (2016).
Mooijman, K. A. The new ISO 6579–1: A real horizontal standard for detection of Salmonella, at last!. Food Microbiol. 71, 2–7 (2018).
Rybolt, M. L., Wills, R. W. & Bailey, R. H. Use of secondary enrichment for isolation of Salmonella from naturally contaminated environmental samples. Poultry Sci. 84(7), 992–997 (2005).
Eguale, T. et al. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC Microbiol. 16(1), 1–11 (2016).
Cohen, N. D. et al. Genus-specific detection of salmonellae using the polymerase chain reaction (PCR). J. Vet. Diagn. Invest. 5(3), 368–371 (1993).
CLSI. Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing: twenty-sixth informational supplement M100-S28 (2018).
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18(3), 268–281 (2012).
Bolton, D. J., O’Neill, C. J. & Fanning, S. A preliminary study of Salmonella, verocytotoxigenic Escherichia coli/Escherichia coli O157 and Campylobacter on four mixed farms. Zoonoses Public Health 59(3), 217–228 (2012).
Ssemanda, J. N. et al. Indicator microorganisms in fresh vegetables from “farm to fork” in Rwanda. Food Control 75, 126–133 (2017).
Machado-Moreira, B. et al. Microbial contamination of fresh produce: What, where, and how?. Comprehen. Rev. Food Sci. Food Saf. 18(6), 1727–1750 (2019).
Angelo, K. M. et al. Outbreak of Salmonella Newport infections linked to cucumbers—United States, 2014. Morb. Mort. Weekly Rep. 64(6), 144 (2015).
Ssemanda, J. N. et al. Foodborne pathogens and their risk exposure factors associated with farm vegetables in Rwanda. Food Control 89, 86–96 (2018).
Hultberg, A., Schermann, M. & Tong, C. Results from a mail survey to assess Minnesota vegetable growers’ adherence to good agricultural practices. HortTechnology Hortte 22(1), 83–88 (2012).
Ferguson, M. et al. A longitudinal study to examine the influence of farming practices and environmental factors on pathogen prevalence using structural equation modeling. Front. Microbiol. 14, 1141043 (2023).
Oyinlola, L. A. et al. Prevention of microbial hazard on fresh-cut lettuce through adoption of food safety and hygienic practices by lettuce farmers. Food Sci. Nutr. 5(1), 67–75 (2017).
Chen, H. et al. Produce growers’ on-farm food safety education: A review. J. Food Protect. 84(4), 704–716 (2021).
Qadir, M. et al. The challenges of wastewater irrigation in developing countries. Agric. Water Manag. 97(4), 561–568 (2010).
Weldesilassie, A. B., Amerasinghe, P. & Danso, G. Assessing the empirical challenges of evaluating the benefits and risks of irrigating with wastewater. Water Int. 36(4), 441–454 (2011).
Akinyemi, K. O. et al. Prevalence of multiple drug resistance and screening of enterotoxin (stn) gene in Salmonella enterica serovars from water sources in Lagos Nigeria. Public Health 125(2), 65–71 (2011).
Ijabadeniyi, O. A. et al. Irrigation water as a potential preharvest source of bacterial contamination of vegetables. J. Food Saf. 31(4), 452–461 (2011).
Gashaye, D. Wastewater-irrigated urban vegetable farming in Ethiopia: A review on their potential contamination and health effects. Cogent Food Agric. 6(1), 1772629 (2020).
Gu, G. et al. Diversity and dynamics of Salmonella enterica in water sources, poultry litters, and field soils amended with poultry litter in a major agricultural area of Virginia. Front. Microbiol. 10, 1 (2019).
Ni, P. E. et al. Prevalence and characterization of Salmonella serovars isolated from farm products in Shanghai. Food Control 85, 269–275 (2018).
Cao, C. et al. Microbiological analysis and characterization of Salmonella and ciprofloxacin-resistant Escherichia coli isolates recovered from retail fresh vegetables in Shaanxi Province China. Int. J. Food Microbiol. 387, 110053 (2023).
Pires, A. F. A. et al. Prevalence and risk factors associated with Campylobacter spp. and Salmonella enterica in livestock raised on diversified small-scale farms in California. Epidemiol. Infect. 147, e321 (2019).
Pagadala, S. et al. Assessment of region, farming system, irrigation source and sampling time as food safety risk factors for tomatoes. Int. J. Food Microbiol. 196, 98–108 (2015).
Sahin, O. et al. Campylobacter in poultry: Ecology and potential interventions. Avian Dis. 59(2), 185–200 (2015).
Ketema, L. et al. Prevalence and antimicrobial susceptibility profile of Salmonella serovars isolated from slaughtered cattle in Addis Ababa Ethiopia. BioMed Res. Int. 1, 1 (2018).
Tesfaw, L. et al. Prevalence and antimicrobial resistance profile of Salmonella isolates from dairy products in Addis Ababa Ethiopia. Afr. J. Microbiol. Res. 7(43), 5046–5050 (2013).
Gutema, F. D. et al. Prevalence, antimicrobial resistance, and molecular characterization of salmonella in cattle, beef, and diarrheic patients in Bishoftu Ethiopia. Foodborne Pathog. Dis. 18(4), 283–289 (2021).
Addis, Z. et al. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: A cross sectional study. BMC Infect. Dis. 11(1), 1–7 (2011).
Baer, A. A., Miller, M. J. & Dilger, A. C. Pathogens of interest to the pork industry: A review of research on interventions to assure food safety. Comprehen. Rev. Food Sci. Food Saf. 12(2), 183–217 (2013).
Yang, X. et al. Prevalence and characterization of Salmonella isolated from raw vegetables in China. Food Control 109, 106915 (2020).
Wadamori, Y., Gooneratne, R. & Hussain, M. A. Outbreaks and factors influencing microbiological contamination of fresh produce. J. Sci. Food Agric. 97(5), 1396–1403 (2017).
Alali, W. Q. et al. Prevalence and distribution of Salmonella in organic and conventional broiler poultry farms. Foodborne Pathog. Dis. 7(11), 1363–1371 (2010).
Sharma, M. et al. Survival of Escherichia coli in manure-amended soils is affected by spatiotemporal, agricultural, and weather factors in the Mid-Atlantic United States. Appl. Environ. Microbiol. 85(5), e02392-e2418 (2019).
WHO. WHO's List of Medically Important Antimicrobials: a risk management tool for mitigating antimicrobial resistance due to non-human use (World Health Organization, Geneva, 2024).
Gebreyes Wondwossen, A. et al. Molecular Epidemiology of Infectious Zoonotic and Livestock Diseases. Microbiol. Spect. 8(2), 822 (2020).
Peng, S. et al. Long-term application of fresh and composted manure increase tetracycline resistance in the arable soil of eastern China. Sci. Total Environ. 506–507, 279–286 (2015).
Wu, J. et al. Antibiotics and antibiotic resistance genes in agricultural soils: A systematic analysis. Crit. Rev. Environ. Sci. Technol. 53(7), 847–864 (2023).
Ongeng, D. et al. Fate of Escherichia coli O157:H7 and Salmonella enterica in the manure-amended soil-plant ecosystem of fresh vegetable crops: A review. Crit. Rev. Microbiol. 41(3), 273–294 (2015).
Wei, R. et al. Occurrence of 13 veterinary drugs in animal manure-amended soils in Eastern China. Chemosphere 144, 2377–2383 (2016).
Krüger, G. I. et al. Mobile genetic elements drive the multidrug resistance and spread of Salmonella serotypes along a poultry meat production line. Front. Microbiol. 14, 1072793 (2023).
Acknowledgements
The authors are thankful to all vegetable farmers in the study districts for allowing them to visit and collect samples from their farms. We are also grateful to the horticulturalists in the Addis Ababa city administration Urban Agriculture Development Commission and the sub-cities for providing information on vegetable farms and their support during sample collection.
Funding
This study was supported by Addis Ababa University thematic research project and Sustainable One Health Research Training Capacity (OHEART): Molecular epidemiology of zoonotic foodborne and waterborne pathogens in Eastern Africa, funded by the NIH Fogarty International Center (D43TW008650), through the Global One Health initiative (GOHi).
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript. Conceptualization, WH, and TE; methodology, WH, HA, GM, GR, LH, DW, WG, and TE; software, WH, and GM; validation, GM and TE; formal analysis, WH, and GM; investigation, WH, HA, GM, LH, DW, GR, WG, TE; data curation, WH, HA, GM, GR, WG, and TE; writing—original draft preparation, WH, HA, GM, TE; writing—review and editing, WH, HA, GM, LH, DW, GR, WG, and TE; supervision, GM, GR, WG, TE; funding acquisition, TE.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hailu, W., Alemayehu, H., Wolde, D. et al. Prevalence and antimicrobial susceptibility profile of Salmonella isolated from vegetable farms fertilized with animal manure in Addis Ababa Ethiopia. Sci Rep 14, 19169 (2024). https://doi.org/10.1038/s41598-024-70173-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70173-4