Abstract
The present study investigated the associations of serum gamma-glutamyl transferase (GGT), a marker of fatty liver and oxidative stress, and ALT/AST, a marker of fatty liver, with percentage trunk fat and postload glucose, insulin resistance, and β-cell function in middle-aged Japanese individuals, whose BMI averaged < 23.0 kg/m2. Pancreatic β-cell function was assessed using the disposition index calculated by a product of the insulinogenic index (IGI) and Matsuda insulin sensitivity index, a biomarker of early-phase glucose-stimulated insulin secretion and whole-body insulin sensitivity, respectively. Multivariate linear regression analyses revealed that the disposition index was associated inversely with GGT independently of percentage trunk fat, homeostasis model assessment insulin resistance (HOMA-IR), a marker of insulin resistance, and Matsuda index. When IGI was included instead of the disposition index, IGI (inversely) and HOMA-IR were associated with GGT independently of percentage trunk fat and Matsuda index. When the area under the glucose concentration curve (AUCg) during an oral glucose tolerance test was included instead of the disposition index, AUCg and HOMA-IR emerged as independent determinants of GGT. ALT/AST was associated with HOMA-IR alone. Results suggest a different pathophysiologic basis between GGT and ALT/AST in predicting diabetic risk in non-obese Japanese.
Similar content being viewed by others
Introduction
Type 2 diabetes is associated with obesity in Europe and North America and is characterized primarily by obesity-driven insulin resistance. However, lean type 2 diabetes is highly prevalent in Japan where more than half of the patients with this condition are considered normal weight (BMI <25 kg/m2)1,2. It is widely recognized that poor insulin secretion may be at play in the early natural history of type 2 diabetes in East Asians and insulin resistance may not be sufficient to explain the high prevalence of the disease3. For example, we demonstrated that middle-aged Japanese people with prediabetes4 and those with slower glucose disposal during an oral glucose tolerance test (OGTT)5 had reduced insulin secretion in the absence of insulin resistance.
Subcutaneous fat, mainly located in gluteofemoral regions, can expand to store excess fatty acids in response to chronic positive energy balance. Asian populations may have a reduced ability to expand gluteofemoral fat mass6. It is proposed that increased free fatty acid (FFA) overflow from dysfunctional adipose tissue associated with impaired expansion of subcutaneous adipose tissue in non-obese people may lead to ectopic lipid accumulation in the liver and consequently to nonalcoholic fatty liver disease (NAFLD)7. Azuma et al.8 reported that hepatic fat content is higher among Japanese than non-Hispanic whites, despite a lower mean BMI in Japanese men, suggesting that fatty liver is a sensitive marker for the failure of the adipose tissue to expand to accommodate an increased energy influx.
NAFLD is commonly revealed by elevations in serum alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) in large epidemiological studies. Many studies have shown associations of liver enzymes with type 2 diabetes9,10,11,12,13,14,15,16,17,18. Although ALT may be a biomarker of fatty liver, GGT has been investigated as a surrogate biomarker of oxidative stress as well19. Some studies suggested that GGT may be a better diabetes predictor than ALT9,10,12 Since chronic high plasma FFA levels are known to impair glucose-stimulated insulin secretion and ß-cell function by a process that likely involves the generation of oxidative stress20,21, we deduced that chronic FFA overflow from dysfunctional adipose tissue may impair glucose-stimulated insulin secretion in middle-aged Japanese people. We, therefore, hypothesized that GGT may be associated with impaired glucose-stimulated insulin secretion, β-cell function (insulin resistance-adjusted insulin secretion), and postglucose glycemia in addition to insulin resistance in the present study in non-obese young and middle-aged Japanese adults. We have recently found that ALT/ aspartate aminotransferase (ALT/AST), a marker of fatty liver, was associated with insulin resistance and β-cell function in non-obese middle-aged Japanese women whereas in young Japanese women, ALT/AST was associated with insulin resistance but not with β-cell function22 The present study investigated the associations of GGT and ALT/AST with postload glucose, insulin resistance and β-cell function in nonobese Japanese people.
Methods
After briefing sessions on the study for students of the Department of Food Sciences and Nutrition and Department of Health and Sports Sciences of Mukogawa Women's University, 485 Japanese female students and 207 of their biological parents (148 mothers and 59 fathers) participated in the present study as volunteers as previously reported in detail23,24,25. Subjects who reported having clinically diagnosed acute or chronic inflammatory diseases, endocrine, cardiovascular, hepatic, and renal diseases, and those on a diet to lose weight and hormonal contraception were excluded from the study. This research followed the tenets of the Declaration of Helsinki. All participants gave written informed consent after the experimental procedure had been explained.
Blood samples were obtained in the morning after 12-h overnight fasting. Serum liver enzymes were measured using an autoanalyzer (AU5232, Olympus, Tokyo, Japan). HbA1c values were determined by high-performance liquid chromatography (HLC723-G7, Tosoh, Tokyo, Japan). A 75-g OGTT was performed on 168 female students and 124 parents (65 mothers and 59 fathers). Blood samples were taken at min 0 (fasting), 30, 60, and 120 for glucose and insulin analysis. Homeostasis model assessment insulin resistance (HOMA-IR) was determined using fasting plasma glucose and insulin levels26 and insulin sensitivity by Matsuda index using glucose and insulin levels during OGTT27. Early-phase glucose-stimulated insulin secretion was estimated using the insulinogenic index (IGI), which was calculated as incremental insulin concentrations (μU/mL) divided by incremental glucose concentrations (mg/dL) during the first 30 min of OGTT28. The oral disposition index (ODI), reflecting β-cell function adjusted for insulin sensitivity, was calculated as the product of the IGI and Matsuda index29. The area under the glucose and insulin concentration curve during OGTT (AUCg and AUCi, respectively) was calculated using the trapezoidal method. Prediabetes and diabetes were diagnosed based on glycemia criteria (fasting and 2-h glucose concentrations) of the American Diabetes Association30. As previously reported, 7 daughters31 and 37 parents had prediabetes and 4 parents had newly diagnosed diabetes4.
Body weight, height, and waist circumference were measured after an overnight fast with a light cloth and shoes off and BMI was calculated. BMI ≥ 25 kg/m2 was present in 21 daughters (4.3%) and 40 parents (19.3%). Whole-body dual-energy X-ray absorptiometry (DXA) (Hologic QDR-2000, software version 7.20D, Waltham, MA) was used to measure lean tissue mass, fat mass, and bone mineral mass for arms, lower body, trunk, and the total body24.
Statistical analysis
Data were presented as mean ± SD unless otherwise stated. Due to deviation from normal distribution, fasting insulin, HOMA-IR, GGT, IGI and ODI were logarithmically transformed for analyses. Pearson correlation analyses were conducted to evaluate the relationship between GGT and ALT/AST and β-cell function, insulin resistance, insulin secretion and other metabolic parameters. Stepwise multivariate linear regression analyses were performed to further identify the most significant variables contributing to the variation of GGT and ALT/AST. Variables that showed significant associations with GGT were included as independent variables. Since GGT was lower in mothers than in fathers (median: 16 and 32 U/L, respectively, p < 0.001) they were divided into three groups according to respective GGT tertile and then data were combined for analyses. Although we reported associations of ALT/AST in daughters and mothers22, the present study reported associations in daughters and parents because associations between log GGT and log IGI did not reach statistical significance when mothers and fathers were analyzed separately probably due to small sample size in each group (data not shown). Comparisons between the two groups were made with a two-sample t-test. Differences among the three groups were analyzed using analysis of variance and then Bonferroni's multiple comparison procedure. A two-tailed p < 0.05 was considered statistically significant. All calculations were performed with SPSS system 23 (SPSS Inc, Chicago, IL).
Ethics approval and consent to participate
The study was approved by the Ethics Committees of the Mukogawa Women’s University (No. 07-28 on 19/02/2008).
Results
Female students and their parents were on average nonobese and the prevalence of BMI<25 kg/m2 was 95.7 % in daughters and 80.7 % in parents although anthropometric variables were higher in parents (Table 1). Mean serum liver enzyme concentrations were within normal reference ranges and median GGT was 13 U/L in daughters and 19 U/L in parents.
Glycemia at 4-time points, AUCg, and HbA1c were higher in parents (Table 1). However, fasting, 1-h and 2-h insulin, AUCi, HOMA-IR, and Matsuda index did not differ despite higher, albeit modest, BMI, % body fat, and %trunk fat in parents. In contrast, 30-min insulin, IGI, and hence ODI were lower in parents.
Because 7 daughters31 and 37 parents had prediabetes and 4 parents had newly diagnosed diabetes4, we compared 161 daughters and 83 parents with normal glucose tolerance (NGT) (Table 2). Insulin concentrations at 30 min and incremental insulin concentrations during the first 30 min of OGTT, a direct measure of early-phase glucose-stimulated insulin secretion, were lower and HbA1c, AUCg, and liver enzymes were higher in parents with NGT. As the Matsuda index was higher in parents, ODI did not differ between daughters and parents with NGT.
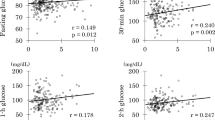
In young women, log GGT showed positive associations with fasting and 2-h insulin, HOMA-IR, and AUCi (Table 3). However, there were no associations with other variables studied. Multivariate analysis revealed that log GGT was associated with 2-h insulin independently of HOMA-IR and AUCi (standardized β: 0.229, p<0.001, R2: 0.049).
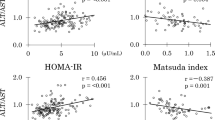
In middle-aged parents (Table 3), log GGT did not show an association with percentage body fat and trunk fat whereas ALT/AST showed positive associations although both enzymes were associated positively with BMI. In contrast, log GGT was inversely associated with log IGI whereas ALT/AST did not. As both enzymes were inversely associated with the Matsuda index, they were inversely associated with log ODI. However, associations with log ODI, post-glucose glycemia, and AUCg were stronger for log GGT. In contrast, associations with fasting insulin, HOMA-IR, Matsuda index, and AUCi were stronger for ALT/AST.
Multivariate analyses for log GGT as a dependent variable were done in parents (Table 4). All models included percentage trunk fat, log HOMA-IR, and Matsuda index as independent variables. When log ODI was added as an independent variable (model A), log ODI emerged as an independent determinant of log GGT. When log IGI was added (model B), log IGI and log HOMA-IR were associated with log GGT. When AUCg was added (model C), AUCg and log HOMA-IR were associated with log GGT. In multivariate analyses for ALT/AST as a dependent variable, which included the same independent variables as for log GGT, log HOMA-IR (standardized β: 0.521, p<0.001) emerged as a single determinant of ALT/AST in all three models (R2: 0.265).
Middle-aged mothers and fathers were grouped according to their respective tertile of log GGT and then combined together. Although fasting glucose and log IGI did not differ, parents with the highest compared to the lowest tertile had higher post-glucose glycemia and AUCg and lower log ODI (Fig. 1).
Left panel: Area under the glucose concentration curves (AUCg) and log oral disposition index (ODI) in 65 middle-aged Japanese mothers and 59 fathers grouped according to their respective tertile of serum gamma-glutamyl transferase concentrations. Mean ± SD. Means not sharing common letter are significantly different with each other at p < 0.05 or less by Bonferroni’s multiple comparison procedure. Right panel: glycemic responses to a 75 g oral glucose loading. Mean ± SE. #: high versus low tertile at p < 0.05 or less. Blue, yellow and red symbols represent the low, median and high tertile, respectively.
The same analyses described above were done in those subjects with BMI <25 kg/m2 and had normal glucose tolerance (Supplementary Table 1). Lower 30-minute insulin and incremental insulin concentrations in parents during the first 30 minutes of OGTT were confirmed. In addition, ODI was lower in parents as well. However, neither log GGT nor ALT/AST showed an association with ODI (Supplementary Table 2).
Discussion
The present study confirmed an independent association of ALT/AST (a marker of fatty liver) with HOMA-IR (a marker of insulin resistance) in non-obese Japanese women22. GGT (a marker of fatty liver and oxidative stress) was independently associated with 2-h insulin concentrations, a marker of insulin resistance in normal-weight Hispanic women32, in young Japanese women. It was associated with HOMA-IR in middle-aged Japanese people in the present study. In addition, GGT was associated inversely with β-cell function (ODI) in non-obese middle-aged Japanese. Further, GGT was associated directly and inversely with early-phase glucose-stimulated insulin secretion (IGI) and hence positively with glucose excursion during OGTT (AUCg).
An association of GGT with a marker of insulin resistance was evident even in young Japanese women, in whom BMI averaged 21 kg/m2 and median GGT was 13 U/L. In addition, GGT was associated with HOMA-IR in middle-aged nonobese Japanese. Further, we have reported an association between ALT/AST and HOMA-IR in young lean Japanese women and middle-aged nonobese Japanese22. These associations between markers of fatty liver and insulin resistance may be related to and may support the hypothesis that fatty liver is a sensitive marker for impaired adipose tissue expandability in Japanese8. We suggested that lower birthweight33 and a positive family history of type 2 diabetes (FHD)34 may be associated with reduced subcutaneous fat mass, a proxy of impaired adipose tissue expandability, in young Japanese women.
β-cell function is calculated as a product of insulin sensitivity and insulin secretion. As lower insulin sensitivity may initially be compensated by increased insulin secretion in obese people, impaired β-cell function may be associated with increased insulin secretion and much lower insulin sensitivity in some obese people. For example, Wang et al.35 reported that increased GGT and ALT were associated with increased risk of β-cell dysfunction in young obese patients. However, lower β-cell function was associated with higher insulin secretion and much lower insulin sensitivity in young nondiabetic obese patients. In contrast, increased GGT was associated with decreased insulin secretion (decreased IGI) in middle-aged Japanese despite lower insulin sensitivity (higher HOMA-IR) in the present study. Middle-aged Japanese parents with NGT had higher serum GGT concentrations, a marker of fatty liver, higher percentage trunk fat, a marker of ectopic fat accumulation36, and lower insulin secretion in response to oral glucose compared with their daughters with NGT. Although we did not measure liver and visceral fat, these findings suggest that ectopic fat accumulation and its related abnormalities associated with impaired adipose tissue expandability for decades may be related to reduced glucose-induced insulin secretion in middle-aged Japanese people.
The strength of this study includes homogeneous study population with scarce confounding factors23,24,25. Several limitations of this study include the cross-sectional design, relatively small sample size, and a single measurement of biochemical variables. We used many surrogates in the present study, which may be less accurate. ODI (β-cell function) was calculated from IGI and Matsuda index, both of which used 30-min insulin and 30-min glucose derived from OGTT for their calculation. Therefore, the insulin secretion derived from ODI is not totally independent of insulin sensitivity derived from Matsuda index. Finally, as we studied Japanese only, results may not be generalized to other races or ethnicities.
In conclusion, the different associations of serum GGT and ALT/AST with insulin secretion, β-cell function, and insulin resistance were found even in non-obese Japanese, suggesting a different pathophysiologic basis between GGT and ALT/AST in its prediction of diabetic risk. Further studies in other populations and other genres will be needed.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Del Prato, S. et al. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int. J. Clin. Pract. 64, 295–304 (2010).
Tanaka, S., Honda, M., Wu, B. & Kazumi, T. Clinical features of normal weight Japanese patients with type 2 diabetes who had formerly been obese. J. Atheroscler. Thromb. 18, 115–121 (2011).
Yabe, D., Seino, Y., Fukushima, M. & Seino, S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr. Diab. Rep. 15, 602. https://doi.org/10.1007/s11892-015-0602-9 (2015).
Tsuboi, A. et al. Higher circulating orosomucoid, an acute-phase protein, and reduced glucose-induced insulin secretion in middle-aged Japanese people with prediabetes. BMJ Open Diabetes Res. Care 8, e001392. https://doi.org/10.1136/bmjdrc-2020-001392 (2020).
Tsuboi, A. et al. Higher circulating orosomucoid and lower early-phase insulin secretion in midlife Japanese with slower glucose disposal during oral glucose tolerance tests. Diabetol. Int. 11, 27–32 (2019).
Smith, U. & Kahn, B. B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 280, 465–475 (2016).
Azzu, V. et al. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology. 158, 1899–1912 (2020).
Azuma, K. et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism 58, 1200–1207 (2009).
Nannipieri, M. et al. Liver enzymes, the metabolic syndrome, and incident diabetes: The Mexico City diabetes study. Diabetes Care 28, 1757–1762 (2005).
Fraser, A. et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: The British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 32, 741–750 (2009).
Nguyen, Q. M. et al. Elevated liver function enzymes are related to the development of prediabetes and type 2 diabetes in younger adults: The Bogalusa Heart Study. Diabetes Care 34, 2603–2607 (2011).
Schneider, A. L. et al. Liver enzymes, race, gender and diabetes risk: The Atherosclerosis Risk in Communities (ARIC) Study. Diabet Med. 30, 926–933 (2013).
Ko, S. H. et al. Increased liver markers are associated with higher risk of type 2 diabetes. World J Gastroenterol. 21, 7478–7487 (2015).
Doi, Y. et al. Liver enzymes as a predictor for incident diabetes in a Japanese population: The Hisayama study. Obesity (Silver Spring) 15, 1841–1850 (2007).
Sato, K. K. et al. Liver enzymes compared with alcohol consumption in predicting the risk of type 2 diabetes: The Kansai Healthcare Study. Diabetes Care 31, 1230–1236 (2008).
Kaneko, K. et al. Association of gamma-glutamyl transferase and alanine aminotransferase with type 2 diabetes mellitus incidence in middle-aged Japanese men: 12-year follow up. J. Diabetes Investig. 10, 837–845 (2019).
Choi, S. H., Kim, B. T., Shin, J. & Kim, K. N. Combined effect of serum alanine aminotransferase and gamma-glutamyltransferase on incidence of diabetes mellitus: A longitudinal study. Medicine (Baltimore) 99, e18963. https://doi.org/10.1097/MD.0000000000018963 (2020).
Hua, S. et al. Association of liver enzymes with incident diabetes in US Hispanic/Latino adults. Diabet Med. 38, e14522. https://doi.org/10.1111/dme.14522 (2021).
Brennan, P. N., Dillon, J. F. & Tapper, E. B. Gamma-Glutamyl Transferase (γ-GT)—An old dog with new tricks?. Liver Int. 42, 9–15 (2022).
Oprescu, A. I. et al. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: Evidence for a role of oxidative stress in vitro and in vivo. Diabetes 56, 2927–2937 (2007).
Lytrivi, M., Castell, A. L., Poitout, V. & Cnop, M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 432, 1514–1534 (2020).
Minato-Inokawa, S. et al. Associations of alanine aminotransferase/aspartate aminotransferase with insulin resistance and β-cell function in women. Sci. Rep. 13, 7853. https://doi.org/10.1038/s41598-023-35001-1 (2023).
Wu, B. et al. Different associations of trunk and lower-body fat mass distribution with cardiometabolic risk factors between healthy middle-aged men and women. Int. J. Endocrinol. 2018, 1289485. https://doi.org/10.1155/2018/1289485 (2018).
Tanaka, S. et al. Associations of lower-body fat mass with favorable profile of lipoproteins and adipokines in healthy, slim women in early adulthood. J. Atheroscler. Thromb. 18, 365–372 (2011).
Kitaoka, K. et al. Increased adipose and muscle insulin sensitivity without changes in serum adiponectin in young female collegiate athletes. Metab. Syndr. Relat. Disord. 15, 246–251 (2017).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Tura, A., Kautzky-Willer, A. & Pacini, G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res. Clin. Pract. 72, 298–301 (2006).
Ohn, J. H. et al. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: A community-based prospective cohort study. Lancet Diabetes Endocrinol. 4, 27–34 (2016).
American Diabetes Association 2. Diabetes Care. 41, S13-S27 (2018).
Tsuboi, A. et al. Association of serum orosomucoid with 30-min plasma glucose and glucose excursion during oral glucose tolerance tests in non-obese young Japanese women. BMJ Open Diabetes Res. Care 6, e000508. https://doi.org/10.1136/bmjdrc-2018-000508 (2018).
Vella, C. A. et al. Associations of insulin resistance with cardiovascular risk factors and inflammatory cytokines in normal-weight Hispanic women. Diabetes Care 36, 1377–1383 (2013).
Honda, M. et al. Birth weight was associated positively with gluteofemoral fat mass and inversely with 2-h postglucose insulin concentrations, a marker of insulin resistance, in young normal-weight Japanese women. Diabetol Int. 13, 375–380 (2021).
Honda, M. et al. Reduced gluteofemoral (subcutaneous) fat mass in young Japanese women with family history of type 2 diabetes: An exploratory analysis. Sci. Rep. 12, 12579. https://doi.org/10.1038/s41598-022-16890-0 (2022).
Wang, L. et al. New evidence for an association between liver enzymes and pancreatic islet β-cell dysfunction in young obese patients. Endocrine 44, 688–695 (2013).
Smith, U. Abdominal obesity: a marker of ectopic fat accumulation. J. Clin. Invest. 125, 1790–1792 (2015).
Acknowledgements
We are indebted to all the participants for their dedicated and conscientious collaboration.
Author information
Authors and Affiliations
Contributions
SMI, ATK, MH and MT collected data and prepared figures. KK, MY, MK and BW analyzed data and prepared tables. TK wrote the manuscript, and KF reviewed and edited it. All authors approved the final version of the manuscript to be published. TK supervised the study, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Minato-Inokawa, S., Tsuboi-Kaji, A., Honda, M. et al. The different associations of serum gamma-glutamyl transferase and alanine aminotransferase with insulin secretion, β-cell function, and insulin resistance in non-obese Japanese. Sci Rep 14, 19234 (2024). https://doi.org/10.1038/s41598-024-70396-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70396-5
Keywords
This article is cited by
-
Low leg fat mass is associated with low insulin sensitivity, inflammatory markers, and β-cell dysfunction in non-obese Japanese people
Scientific Reports (2025)
-
Elevated 1 h post-load plasma glucose associates with decreased serum lipoprotein lipase in youth and decreased leg fat mass in midlife
Scientific Reports (2025)