Abstract
Silver nanoparticles were successfully incorporated into a melamine-based polymer, resulting in the synthesis of (Ag NPs@Bipy-PAN) through a reverse double solvent approach. The synthesised Ag NPs@Bipy-PAN polymer underwent extensive characterisation through Powder X-ray Diffraction (PXRD), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy and Energy Dispersive X-ray (EDX) and Thermal Gravimetric Analysis. PXRD analysis confirmed the successful encapsulation of Ag nanoparticles and provided insights into the amorphous nature of the polymer following encapsulation. SEM and EDX analyses further corroborated the presence and distribution of Ag nanoparticles on the polymer surface. The biological efficacy of the Ag NPs@Bipy-PAN polymer was evaluated through antibacterial, anti-breast cancer, and biocompatibility assays. The results demonstrated notable antibacterial and anticancer activities, with significant efficacy against bacterial strains and breast cancer cells. Biocompatibility assessments indicated acceptable compatibility, particularly at a concentration of 2.5 mg/mL, compared to untreated control cells. These findings suggest that Ag NPs@Bipy-PAN has considerable potential as a candidate for cancer-targeted and antimicrobial drug delivery systems. The incorporation of silver nanoparticles into the melamine-based polymer enhances the safety profile of these systems in in vivo conditions, making them a viable option for advanced therapeutic applications.
Similar content being viewed by others
Introduction
One of the most crucial modern day health concerns is the antibacterial resistance, as pathogens continually adapt to resist traditional antibiotic remedies, resulting in diminished treatment effectiveness. However, despite the imperative need to address this crisis, the discovery of new antimicrobial compounds has notably declined. Conversely, the prevalence of pathogen resistance to widely prescribed antibiotics has significantly increased. This alarming trend underscores the critical need for the exploration and development of novel therapeutic and preventive antimicrobial interventions1,2.
In response to this urgent challenge, the exploration of novel antimicrobial agents such as melamine-based polyaminal polymers (PANs) has gained considerable attention in recent years3. PANs possess distinctive properties, including a high nitrogen content and the presence of amino groups, which impart them with antimicrobial activity4. Melamine, a compound consisting of cyanamide and 1,3,5 triazine, is recognised for its nitrogen-rich structure, allowing it to impart excellent sorption capabilities and chemical stability when used in the creation of porous polymers4. These polymers are synthesised through a one-pot polycondensation reactions and microwave methods, resulting in various interconnected networks such as azolinked polymer5, polyamide5,6, polyimide5,6, and polyaminal networks7. PANs are particularly of a significant importance due to their nitrogen-rich composition from triazine rings and aminal linkages8, along with features like abundant micropores9, a large surface area9, and simplified synthesis via one-pot catalyst-free polycondensation of aldehyde monomers combined with varieties of amines10.
Empirical investigations have demonstrated the broad-spectrum inhibitory effects of PANs against diverse bacterial strains, encompassing both Gram-positive and Gram-negative species. The underlying mechanisms of PANs' antibacterial action primarily involve interactions with bacterial cell membranes and intracellular components11,12. Positively charged amino groups within PANs facilitate electrostatic interactions with negatively charged bacterial cell surfaces, leading to membrane disruption and subsequent cellular lysis13. Furthermore, the nitrogen-rich composition of PANs may disrupt bacterial metabolic processes, contributing to their antimicrobial efficacy14. These multifaceted mechanisms underscore the potential of PANs to serve as promising candidates for combating antibacterial resistance.
Several subclasses of polyaminal polymers (PANs) have emerged, and each with their own distinctive characteristics and potential applications or antibacterial applications due to their ability to facilitate interactions with bacterial membranes and intracellular components15. Polymers with intrinsic microporosity (PIMs) exhibit inherent microporosity owing to their rigid and contorted structures16, which may contribute to enhanced antibacterial activity through mechanisms such as membrane disruption and inhibition of bacterial metabolic processes17,18,19. Covalent organic frameworks (COFs) represent another subclass with potential antibacterial properties, characterized by crystalline porous materials formed through the self-assembly of organic building blocks, offering tuneable porosity and surface functionality advantageous in combating bacterial infections20,21. Covalent triazine-based frameworks (CTFs), featuring triazine units linked by covalent bonds, provide high surface areas and thermal stability22, which may contribute to their efficacy in antibacterial applications by facilitating interactions with bacterial surfaces and promoting antimicrobial activity. Hyper-cross-linked polymers (HCPs)23 and porous aromatic frameworks (PAFs) also hold promise for antibacterial applications, offering high porosity and surface areas conducive to interactions with bacterial membranes and intracellular components, thus potentially enhancing their antibacterial efficacy24.
On the other hand, silver nanoparticles (AgNPs) are well-known for their antimicrobial properties, capitalize on their elevated surface area to volume ratio to outstrip bulk silver in antimicrobial efficacy25. Silver nanoparticles exhibit antibacterial effects through multiple mechanisms. Firstly, they release silver ions continuously, which adhere to bacterial cell walls and cytoplasmic membranes, enhancing permeability and disrupting the bacterial envelope26. Within cells, silver ions deactivate respiratory enzymes, generate reactive oxygen species, and interfere with DNA replication and protein synthesis. Additionally, silver nanoparticles themselves can kill bacteria by accumulating in cell wall pits, causing membrane denaturation, and disrupting organelles, leading to cell lysis. They can also penetrate bacterial cell walls and alter membrane structure, affecting signal transduction and promoting cell apoptosis26,27. The suspension of silver nanoparticles in exposure media impacts their antibacterial efficacy, with smaller nanoparticles and certain shapes releasing silver ions more efficiently26,28. Gram-negative bacteria possess thinner cells walls and are thus highly susceptible to silver nanoparticles. However, biofilms can hinder the effectiveness of silver nanoparticles by impeding their transport and reducing their diffusion coefficients, thereby allowing bacteria within the biofilm to remain tolerant to silver nanoparticle exposure29.
Functioning through multiple mechanisms such as membrane disruption, interference with cellular processes, and generation of reactive oxygen species, AgNPs have been effectively utilized in textiles, medical devices, and coatings as antimicrobial agents30,31,32. The incorporation of PANs and AgNPs may synergistically improve their antimicrobial properties33. Specifically, the positively charged amino groups inherent in PANs can facilitate the binding and subsequent release of AgNPs, fostering enhanced interaction with microorganisms. In this study, we demonstrate the antibacterial potential of one-pot polycondensation bipyridine-based Polyaminal encapsulated with Ag nanoparticles (Ag NPs@Bipy-PAN).
Material and methods
Materials
The chemicals used in this study did not undergo any further purification. [2,2′-Bipyridine]-5,5′-dicarbaldehyde was purchased from Shanghai Sunchem Inc., Shanghai, China. Melamine (97.5%), silver acetate (CH3COOAg 98.5%), and dimethyl sulfoxide (DMSO 99%) were supplied by BDH Laboratory Reagents, England, UK. Tetrahydrofuran (THF ≥ 99.5%), acetone (99.5%), dichloromethane (DCM ≥ 99.9%), and Pd(NO3)2 (99%) were purchased from Fisher Scientific, Chicago, USA. NaOH (98%), NaBH4 (99%), and (CH3.COO)2 Cd.2H2O (99%) were supplied by BDH Chemicals, England, UK. NiSO4.6H2O (98%) and (CH3COO)2 Cu.H2O (≥ 99.8%) were obtained from BDH Laboratory Supplies, England, UK. Ba(NO3)2 (99%) was provided by Ward's Natural Science, Rochester, NY, USA, and finally, HCl (35%) was supplied by LOBA Chemie, Mumbai, India.
Synthesis of bipyridine-based polyaminal-linked porous organic polymer (Bipy-PAN)
Melamine-based porous polyaminal was prepared according to a previous study34. The synthesis involved utilizing a dry three-necked flask equipped with a magnetic stirrer, and the condenser was degassed through an evacuation-argon-backfill cycle. Initially, a vacuum was applied to evacuate the flask. Melamine (0.5 g, 3.96 mmol) and [2,2′-Bipyridine]-5,5′-dicarbaldehyde (0.7 g, 5.94 mmol) were dissolved in 30 mL of DMSO. The resulting mixture was then subjected to heating at 175 °C for 3d, with continuous stirring under an argon flow. The precipitate was obtained by filtration and subjected to washing with dimethylformamide (3 times), dichloromethane (3 times), and acetone (3 times). Subsequently, the white solid product was dried under vacuum at 70 °C for 2 h, resulting in a yield of 72%.
Synthesis of the Ag NPs@Bipy-PAN
To encapsulate Ag NPs by Bipy-PAN, 170 mg of Bipy-PAN powder was suspended in 40 mL of deionized water as a hydrophilic solvent, and the mixture was sonicated for 1 h until it became homogeneous. After stirring for 30 min, a solution of \({\text{CH}}_{3}\text{COOAg}\)(0.02 mmol) dissolved in \(\text{DCM}\) 0.04 mL) as the hydrophobic solvent was added dropwise for 10 min with constant vigorous stirring. The resulting solution was continuously stirred for 5 h. Then, the as-prepared mixture was reduced by adding a highly concentrated \({\text{NaBH}}_{4}\) aqueous solution (2.7 M, 1 mL). Finally, the mixture was filtered, washed with deionized water, and dried at 70 °C for 2 h to obtain the solid sample.
Instrumentation
Using a Maxima XRD-7000X Powder X-ray Diffraction (PXRD) and Dispersive X-ray Spectroscopy (EDX) equipments (Shimadzu, Kyoto, Japan), Ag@BiPy-PAN was investigated. Using nickel-filtered Cu-Kb reduction, X-rays were produced at 40 kV and 100 mA during the process. Ten degrees per minute was the scan speed, and the scan range (2θ) was 5 to 70 degrees. Ag@BiPy-PAN was investigated using Thermal Gravimetric analysis (TGA) on a TG-DTA6300 (Shimadzu, Kyoto, Japan) with a heating rate of 10 ̊C min-1 at intervals of 25–500 ̊ C in an N2 environment. The structure and particle distribution of Ag@BiPy-PAN in powder form were investigated using Scanning Electron Microscopy (SEM) (FEI Inspect F50, FEI, Tokyo, Japan). The dry substance was examined at a voltage of 30 kV. Additionally, the Ag@BiPy-PAN's size and morphology were examined using Transmission Electron Microscopy (TEM) (JEM-F200, Jeol, Tokyo, Japan). Following negative staining (phosphotungstic acid, 2%) with one drop of the substance applied to a copper grid at the proper dilution, TEM images were obtained35.
Antibacterial assay
Gram-negative Escherichia coli (E. coli, ATCC 25,922) and gram-positive Staphylococcus aureus (S. aureus, ATCC 25,923) were selected to examine the antibacterial effects of BiPy-PAN and Ag@BiPy-PAN using the agar well diffusion method. In this method, bacterial cells were sub-cultured in nutrient broth agar for 24 h before compound antibacterial testing to ensure bacterial cells were in the log phase. Mueller–Hinton agar (MH) media were prepared and inoculated by spreading bacterial inoculum over the whole agar surface. Then holes of 8 mm diameter were punched using a sterile Cork borer to add 100 µl of 0.25 mg, 0.5 mg, and 1 mg of the compound under investigation. Bacterial cells were incubated in a humified incubator under sterile conditions at 37 °C for 24 h in the presence, or absence, of the compounds. The inhibition zone around the hole was measured using a calibre36.
The cell viability assay
Breast cancer cells (MCF-7) and immobilized human embryonic kidney cells (HEK) were maintained, and cultures following the American Type Culture Collection (ATCC) guidelines and purchased from ATCC, Manassas, Virginia. The cultured cell lines were grown under 37 °C, 80–90% confluence, and 5% CO2. For the cell viability, the MTT assay kit was procured from Invitrogen, USA. Cells were initially left for incubation at 37 °C for 24 h using a 96-well plate37. In each well, 2 × 103 of each cell type was placed and kept inside the humified carbon dioxide incubator, maintained at 5% humidity. The treatment group of of BiPy-PAN and Ag@BiPy-PAN was added to the well at different concentrations and left for 48 h. After this, all the samples were centrifuged, and precisely 100 µl of obtained supernatant was replaced with that of DMSO and again left for incubation at 5% carbon dioxide and 37 °C for 4 h. The absorbance was recorded at 570 nm in a triplicate using a microplate reader35,36.
Results and discussion
Synthesis of the Ag NPs@Bipy-PAN
The Bipy-PAN polymer was utilized as an effective material for stabilizing metal ions by leveraging the binding sites provided by the nitrogen atoms in Bipyridine, triazine units, and aminal linkage. This indicates that the Bipy-PAN polymer has the potential to serve as an outstanding support for immobilizing metal nanoparticles. The reverse double solvent method (RDSM) was employed to investigate the immobilization of silver nanoparticles onto the polymer. The process involved dispersing the Bipy-PAN polymer in deionized water and adding a solution of CH3COOAg/CH2Cl2 (0.5 M). The mixture was then subjected to sonication, and after 5 h of stirring, NaBH4 was used as a reducing agent to convert the silver ions into atoms, resulting in the formation of a grey powder known as Ag NPs@Bipy-PAN (Scheme 1). Various characterization techniques, including PXRD, SEM, TEM, EDX, TGA, and N2 adsorption–desorption methods, were employed to confirm the successful formation of the Ag NPs@Bipy-PAN adsorption.
PXRD analysis
The powder X-ray diffraction (PXRD) was used to determine the crystal type of polymer and the concentration ratio of silver nanoparticles within the polymer matrix. From (Fig. 1), it was observed that the Ag NPs@BiPy-PAN exhibited broad diffraction peaks at 2θ of 21°, which are characteristic of the amorphous structure of the BiPy-PAN polymer, as well as sharp diffraction peaks at 2θ of 38.2°, which correspond to the (111) diffraction planes of the face-centred cubic structure of silver nanoparticles (JCPDS No. 04–0783). These results suggest that the silver nanoparticles were able to distribute and encapsulate within the BiPy-PAN polymer without significantly altering its original structure34.
Morphological analysis by SEM, TEM and EDX
The morphology, structure, and particle size distribution were thoroughly investigated using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) analysis. The SEM image of Ag NPs@BiPy-PAN (Fig. 2a–c) showed a compact, irregular shape. However, the morphology of Ag@BiPy-PAN remained unchanged during AgNPs loading. The TEM images of Ag@BiPy-PAN displayed a spherical morphology of BiPy-PAN, with black dots representing Ag particles uniformly distributed on BiPy-PAN surface (Fig. 2d–f). The average particle size of Ag nanoparticles was 9.2 nm, likely due to the coordination between AgNPs and nitrogen active sites in BiPy-PAN (Fig. 2h). Energy dispersive X-ray (EDX) analysis indicated the presence of dispersed C, N, and Ag on the surface of Ag@BiPy-PAN, as shown in (Fig. 2e–g). Overall, the results demonstrated the successful anchoring of Ag nanoparticles onto BiPy-PAN without significant changes in its morphology34.
TGA analysis
The thermogravimetric analysis (TGA) was used to assess the thermal stability of Ag NPs@BiPy-PAN. (Fig. 3) illustrates that the TGA curve of Ag NPs@BiPy-PAN showed an initial weight loss (about 10 wt%) in the temperature range of 35–80 °C, which can be attributed to the removal of absorbed water and solvent molecules. Furthermore, the material exhibits high thermal stability up to 360 °C, beyond which a significant weight loss (39.78 wt%) occurs in the temperature range of 360–460 °C, ascribed to the decomposition of the polymer network. The TGA analysis also indicates that Ag NPs@BiPy-PAN retains about 36.7% of its total weight at 500 °C. Overall, the results demonstrate that Ag NPs@BiPy-PAN exhibits outstanding thermal stability up to 360 °C34.
Antibacterial effects
The effects of Ag@BiPy-PAN were examined against gram-negative E. coli and gram-positive S. aureus bacteria, in which selected bacteria were incubated either in the presence or in the absence of 100 µl of 0.25, 0.5 and 1 mg of the compounds. Table 1 represents the anti-bacterial effects of Ag@BiPy-PAN on E. coli and S. aureus by observing a clear inhibition zone around the compounds. Results in both Table 1 and Fig. 4 demonstrate that the Ag@BiPy-PAN compound created clear inhibition zones impacting the growth of both bacteria in comparison to the BiPy-PAN, especially at 0.25 mg, that could add to the safety profile. Ag@BiPy-PAN inhibited the growth of E. coli by creating an inhibition zone of 9 mm when using 0.25 mg and 0.5 mg of the compound but the clear inhibition zone was not detected despite that the bacterial growth was reduced by 14 mm around the compound with the addition of 1 mg (light zone). Moreover, Ag@BiPy-PAN is effective on S. aureus creating a 10 mm clear inhibition zone around the compound regardless of increasing concentrations. Structural differences between both types of bacteria underlie the different effects Ag@BiPy-PAN exhibited against them.
Due to the electrostatic interaction between the positively charged resin and negatively charged bacteria cell membrane, the Ag@BiPy-PAN resin could interfere with the balance and integrity of the bacterial cell wall exhibiting anti-microbial activity towards microorganisms. In addition, the free radicals, derived from the resin surface, could disturb the membrane lipids and structure of microorganism cells, leading to a breakdown of membrane functions 36.
Anticancer and biocompatibility polymer effects
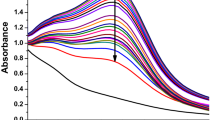
The cytotoxic effects of Ag@BiPy-PAN and BiPy-PAN are apparent in Fig. 5 against the breast cancer cells. The 50%-inhibitory concentrations (IC50) were 1.15 ± 0.11 mg/mL and 0.68 ± 0.21 mg/mL of Ag@BiPy-PAN and BiPy-PAN, respectively. As a result, the potency of the BiPy-PAN was higher than the Ag@BiPy-PAN. The BiPy-PAN in physiological conditions could be positively charged on the secondary amine functionality, which could enhance the BiPy-PAN cellular adherence to the bilayer phosphate group. Afterwards, the BiPy-PAN would imbalance the cellular membrane integrity causing cell death37,39. On the other hand, Ag@BiPy-PAN could follow the same mechanism, but the silver nanoparticles might interfere with the cellular adherence of the polymer, especially at concentrations lower than 2 mg/mL.
Regarding the polymers’ effects on the immobilized kidney cells, the cytotoxic effects of Ag@BiPy-PAN and BiPy-PAN are apparent in Fig. 6 against the normal kidney cancer cells. The 50%-inhibitory concentrations (IC50) were 1.45 ± 0.11 mg/mL and 0.91 ± 0.21 mg/mL of Ag@BiPy-PAN and BiPy-PAN, respectively. As a result, the potency of the BiPy-PAN was higher than the Ag@BiPy-PAN. The mechanism of actions and behavior could be related to the above explanation. The mechanism of action could be confirmed to be un-specific to the cancer cells37,39. As a result, a cancer-targeted drug delivery systems of Ag@BiPy-PAN and BiPy-PAN could enhance their safety profile when used against cancer38,39.
Conclusion
This study successfully synthesised and characterised silver nanoparticle stabilised by a bipyridine-based polymer (Ag NPs@Bipy-PAN). The Bipy-PAN polymer demonstrated significant potential as a support material for immobilising metal nanoparticles, utilising the binding sites provided by its nitrogen atoms. The reverse double solvent method (RDSM) facilitated the embedding of silver nanoparticles within the polymer matrix, as confirmed through various characterisation techniques including PXRD, SEM, TEM, EDX, TGA, and N2 adsorption–desorption methods.
PXRD analysis revealed that the silver nanoparticles were well-distributed within the amorphous structure of the Bipy-PAN polymer, maintaining its original integrity. SEM and TEM analyses showed consistent morphology and uniform distribution of Ag nanoparticles, with an average particle size of 9.2 nm. EDX confirmed the elemental composition, verifying the presence of Ag, C, and N on the polymer surface. TGA analysis demonstrated that Ag NPs@Bipy-PAN exhibited remarkable thermal stability up to 360 °C, indicating its suitability for high-temperature applications.
Functionally, Ag NPs@Bipy-PAN exhibited effective antibacterial activity against both gram-negative E. coli and gram-positive S. aureus, as evidenced by clear inhibition zones that increased with the compound concentration. This antibacterial effect is likely due to electrostatic interactions and free radicals derived from the resin surface, disrupting bacterial cell membranes. However, variations in nanoparticle size and polymer matrix can influence antibacterial efficacy.
The anticancer properties of Ag NPs@Bipy-PAN were assessed against breast cancer cells and normal kidney cells. The results indicated that while the BiPy-PAN polymer alone exhibited higher cytotoxicity, Ag NPs@Bipy-PAN also showed significant inhibitory effects. The data suggested a potential mechanism involving the interference of silver nanoparticles with cellular adherence, indicating a non-specific action on cancer cells.
Ag NPs@Bipy-PAN demonstrate considerable promise as a multifunctional material with applications in antibacterial treatments and cancer therapy. Future studies should focus on developing targeted drug delivery systems to enhance the specificity and safety profile of Ag NPs@Bipy-PAN and BiPy-PAN in clinical applications. The continued exploration of nanoparticle-polymer composites could lead to significant advancements in biomedical applications, reflecting a broader trend in the development of multifunctional nanomaterials for medical use.
Data availability
Data will be made available on request.
References
World Health Organisation (WHO), 2023, https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance#:~:text=As%20a%20result%20of%20drug,through%20genetic%20changes%20in%20pathogens
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance, a systematic analysis. The Lancet 399(10325), 629–655 (2019).
Huang, K. S. et al. Recent advances in antimicrobial polymers: a mini-review. Int J Mol Sci. 17(9), 1578 (2016).
Dorieh, A. et al. A review of recent progress in melamine-formaldehyde resin based nanocomposites as coating materials. Progr. Org. Coat. 165, 106768 (2022).
Shao, L., Liu, M., Sang, Y. & Huang, J. One-pot synthesis of melamine-based porous polyamides for CO2 capture. Microporous Mesoporous Mater. 285, 105–111 (2019).
Fawaz, J., & Mittal, V. Synthesis of polymer nanocomposites: review of various techniques. Synthesis techniques for polymer nanocomposites, 2014: 1–30.
Fajal, S., Dutta, S., & Ghosh, S. K. Porous organic polymers (POPs) for environmental remediation. Materials Horizons, 2023: 4083.
He, D. et al. Synthesis and study of low-cost nitrogen-rich porous organic polyaminals for efficient adsorption of iodine and organic dye. Chem. Eng. J. 446, 137119 (2022).
Wang, B. et al. Nitrogen-rich porous biochar for highly efficient adsorption of perchlorate: Influencing factors and mechanism. J. Environ. Chem. Eng. 11(3), 110293 (2023).
Yuan, K. et al. Facile synthesis and study of functional porous organic polyaminals with ultrahigh adsorption capacities and fast removal rate for rhodamine B dye. Microporous Mesoporous Mater. 344, 112234 (2022).
Dutta, D., Cole, N., Kumar, N. & Willcox, M. D. Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses. Invest. Ophthalmol. Visual Sci. 54(1), 175–182 (2013).
Carmona-Ribeiro, A. M. & Araújo, P. M. Antimicrobial polymer− based assemblies: a review. Int. J. Mol. Sci. 22(11), 5424 (2021).
Qiu, H. et al. The mechanisms and the applications of antibacterial polymers in surface modification on medical devices. Front. Bioeng. Biotechnol. 8, 910 (2020).
Sun, C., Wang, X., Dai, J. & Ju, Y. Metal and metal oxide nanomaterials for fighting planktonic bacteria and biofilms: a review emphasizing on mechanistic aspects. Int. J. Mol. Sci. 23(19), 11348 (2022).
Fan, D. et al. Functional insights to the development of bioactive material for combating bacterial infections. Front. Bioeng. Biotechnol. 11, 1186637 (2023).
McKeown, N. B. Polymers of intrinsic microporosity (PIMs). Polymer 202, 122736 (2020).
Soria, R. B., & Luis, P. Antifouling membranes for polluted solvents treatment. In Current Trends and Future Developments on (Bio-) Membranes 2023,: 295–334. Elsevier.
Yang, S. et al. Self-assembled short peptides: recent advances and strategies for potential pharmaceutical applications. Mater. Today Bio. 1(20), 100644 (2023).
Antunes, J. C. et al. Recent trends in protective textiles against biological threats: a focus on biological warfare agents. Polymers 14(8), 1599 (2022).
Younis, S. A., Lim, D. K., Kim, K. H. & Deep, A. Metalloporphyrinic metal-organic frameworks: controlled synthesis for catalytic applications in environmental and biological media. Adv. Colloid Interface Sci. 2020(277), 102108 (2020).
(a)-Song, Y., Phipps, J., Zhu, C., & Ma, S. Porous materials for water purification. Angewandte Chemie, 2023, 135(11). (b)- He, J., Feng, Y., Jiang, J. et al. Preparation and characterization of a sustained-release antibacterial melamine-impregnated paper based on Ag-BTC. J Mater Sci ,2023, 58, 6727–6742. https://doi.org/10.1007/s10853-023-08436-0
(a)- Liao L. , Li M. , Yin Y. , Chen J. , Zhong Q. , Du R. , Liu S. , He Y. , Fu W. , Zeng, F. Advances in Synthesis of Covalent Triazine Framework, ACS Omega, 2023 8, 5, 4527–4542 https://doi.org/10.1021/acsomega.2c06961 (b)- Liu, M., Guo, L., Jin, S., & Tan, B. (2019). Covalent triazine frameworks: synthesis and applications. Journal of materials chemistry A, 2019, 7(10): 5153–5172.
Shifrina, Z. B., Matveeva, V. G. & Bronstein, L. M. Role of polymer structures in catalysis by transition metal and metal oxide nanoparticle composites. Chem. Rev. 120(2), 1350–1396 (2019).
Yang, C. et al. Nanofibrous porous organic polymers and their derivatives: from synthesis to applications. Adv. Sci. 11(19), 2400626 (2024).
(a)- Abbott, S., & Holmes, N. Nanocoatings: Principles and Practice: From Research to Production. DEStech Publications, Inc., 2013.(b)- Goda, E.S Abu Elella M. H., Sohail M., Singu B. S., Pandit B., El Shafey A.M., Aboraia A. M. , Gamal H. , Hong S. E., Yoon K. R., N-methylene phosphonic acid chitosan/graphene sheets decorated with silver nanoparticles as green antimicrobial agents, Int.J. Bio. Macromolecules, 2021,182, 680–688
Yin, I. X. et al. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 15, 2555–2562 (2020).
Li, L. et al. Silver nanoparticles induce protective autophagy via Ca 2+ /CaMKKβ/AMPK/mTOR pathway in SH-SY5Y cells and rat brains. Nanotoxicology 13(3), 369–391 (2019).
Shanmuganathan, R. et al. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: green approach. Environ. Sci. Pollut. Res. Int. 25(11), 10362–10370 (2018).
Hosnedlova, B., Kabanov, D., Kepinska, M. & Narayanan, B. Effect of biosynthesized silver nanoparticles on bacterial biofilm changes in s aureus and E coli.. Nanomater. Basel 12(13), 2183 (2022).
Saallah, S. & Lenggoro, I. W. Nanoparticles carrying biological molecules: recent advances and applications. KONA Powder Part J. 35, 89–111 (2018).
Fernando, S., Gunasekara, T. & Holton, J. Antimicrobial nanoparticles: applications and mechanisms of action. Sri Lankan J. Infect. Dis. 8(1), 2–11 (2018).
(a)- Moritz M, Geszke-Moritz M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem Eng J. 2013, 228: 596–613 (b)- Goda E. S., Abu Elella, M.H., Hong, S.E. Pandit, B., Yoon K.R., Gamal H., Smart flame retardant coating containing carboxymethyl chitosan nanoparticles decorated graphene for obtaining multifunctional textiles. Cellulose 2021, 28, 5087–5105. https://doi.org/10.1007/s10570-021-03833-7 (c)- Abu Elella, M. H, Goda E. S, Yoon K.R., Hong, S. E. Morsy, M. S., Sadak R. A., Gamal H. , Novel vapor polymerization for integrating flame retardant textile with multifunctional properties, Composites Comm., 2021, 24, ,100614, https://doi.org/10.1016/j.coco.2020.100614.
Barroso-Solares, S., Cimavilla-Roman, P., Rodriguez-Perez, M. A. & Pinto, J. Non-invasive approaches for the evaluation of the functionalization of melamine foams with in-situ synthesized silver nanoparticles. Polymers (Basel) 12(5), 996 (2020).
Alkayal N.S.l, Alotaibi M.M., Tashkandi N.Y., Alrayyani M. A.,. Synthesis and characterization of bipyridine-based polyaminal network for CO2 capture. Polym. Basel 14(18), 3746 (2022).
Md, S., Abdullah, S., Awan, Z. A. & Alhakamy, N. A. Smart Oral pH-responsive dual layer nano-hydrogel for dissolution enhancement and targeted delivery of naringenin using protein-polysaccharides complexation against colorectal cancer. J. Pharm. Sci. 111(11), 3155–3164 (2022).
Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharm. Anal. 6(2), 71–79 (2016).
Yang, Z. et al. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab. Dispos. 40(10), 1883–1893 (2012).
Md, S. et al. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in a549 lung cancer cells. Pharmaceuticals 13(7), 152 (2020).
Bahrami, A. Effect of curcumin and its derivates on gastric cancer: molecular mechanisms. NuCancer 73(9), 1553–1569 (2021).
Martocq, L. & Douglas, T. E. L. Amine-rich coatings to potentially promote cell adhesion, proliferation and differentiation, and reduce microbial colonization: strategies for generation and characterization. Coatings 11(8), 983 (2021).
Abdullah, S., El Hadad, S. & Aldahlawi, A. The development of a novel oral 5-Fluorouracil in-situ gelling nanosuspension to potentiate the anticancer activity against colorectal cancer cells. Int. J. Pharm. 613, 121406 (2022).
Md, S. et al. Ambroxol hydrochloride loaded gastro-retentive nanosuspension gels potentiate anticancer activity in lung cancer (A549) cells. Gels 7(4), 243 (2021).
Funding
This research work was funded by Institutional Fund Projects under grant no. G:376–247-1443 Therefore, the author gratefully acknowledges the technical and financial support from the Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University (KAU), Jeddah, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, N.S.A.; Methodology, M.M. A. and B.A.; Validation, N.T.; Investigation, N.S.A., F.B. and S.A.; Resources, M.M. A.; Data curation, N.S.A. and F.B.; Writing—original draft, M.M.A. .F.B. and S.A.; Writing—review & editing, N.S.A., N.T. and M.M.A.; Supervision, N.S.A. ; Funding acquisition, M.M.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alotaibi, M.M., Almalki, B., Tashkandi, N. et al. Synthesis of silver nanoparticles embedded into melamine polyaminal networks as antibacterial and anticancer active agents. Sci Rep 14, 20008 (2024). https://doi.org/10.1038/s41598-024-70606-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70606-0