Abstract
Osteoblasts and osteoclasts play an important role in maintaining the structural integrity of bone tissue, in which osteoclasts degrade bone structure and osteoblasts restore bone tissue. The imbalance of osteoblast and osteoclast function can lead to many bone-related diseases, such as osteoporosis and inflammatory osteolysis. The drug that can both promote bone formation and inhibit bone loss will be able to treat those diseases. In this study, it was found that LMK-235, an selective HDAC4/5 inhibitor, inhibited the differentiation and maturation of osteoclasts by regulating NF-κB and p-Smad2/3 signaling pathways via inhibition of HDAC4. At the same time, we found that LMK-235 promoted osteoblast mineralization by upregulating Runx2 expression via inhibition of HDAC4. In vivo, LMK-235 was able to alleviate lipopolysaccharide (LPS)-induced calvarial osteolysis and promote the repair of bone defects. Taken together, LMK-235 suppresses osteoclast differentiation and promotes osteoblast formation by inhibiting HDAC4. This may provide a valuable treatment for bone diseases caused by abnormal osteoclast bone resorption and osteoblast bone regeneration.
Similar content being viewed by others
Introduction
Bone-related diseases, including fractures, bone defects, osteoporosis, osteolysis, and metabolic bone diseases, involve dynamic interactions between osteoclasts (OCs), which are responsible for bone degradation, and osteoblasts (OBs), which contribute to bone formation1,2. Current therapeutic approaches for these conditions fall into two categories: those that promote bone formation and those that inhibit bone resorption. The former includes calcium, vitamin D and parathyroid hormone and is usually used in combination. While small doses of parathyroid hormone can intermittently stimulate bone formation, large doses can have the opposite effect. The latter group includes bisphosphonates, calcitonin, and estrogen. Bisphosphonates inhibit bone resorption and enhance bone strength and bone cell longevity. However, long-term use may lead to an abnormal increase in bone density. Calcitonin mainly inhibits the activity and quantity of osteoclasts, but it has a potential carcinogenic effect3,4. Therefore, there is an urgent need to develop safer alternative drugs that can simultaneously promote bone formation and inhibit bone loss to treat such diseases.
Histone deacetylase (HDAC) functions by deacetylating histones, condensing chromatin, and inhibiting transcription. In mammalian cells, there are five types of HDACs (I, IIa, IIb, III, and IV). HDAC4, a member of the HDAC IIa family (HDAC4, 5, 7, and 9), plays a crucial role in chromosome structural modification and gene expression regulation by deacetylating lysine5. HDAC5 has been implicated in nerve injury and regeneration, osteogenesis, glucose metabolism, and is associated with the development of myocardial hypertrophy and various malignant tumors. HDAC4 decreased the acetylation level of histone H3 at the miR-200a promoter6. Knockdown of HDAC5 increased the acetylation level of Lys9 site of histone H3 (H3K9) of TAp63 promoter, indicating that HDAC5 inhibited the transcription of TAp63. HDAC5 knock-down will increase the H3K9ac modification on HIPK2 promoter, which will lead to its combination with HIF1α promoter and further inhibit the expression level of HIF1α7. HDAC4 is closely associated with cartilage formation, osteoblast differentiation, and chondrocyte hypertrophy. Importantly osteoblast-specific HDAC4 knockout mice exhibit significantly smaller sizes than their wild-type counterparts8,9. HDAC4 modifies Osterix and regulates the differentiation process of osteoblasts10. MiR-26a-5p derived from urine stem cells can induce osteoblast differentiation through the HDAC4/HIF-1α/VEGFA pathway and play a therapeutic role in diabetic osteoporosis11. Various other microRNAs are also involved in the development of osteoporosis by targeting HDAC412,13. A novel miRNA (miR-2861) inhibits HDAC5 expression and plays a role in osteoblast differentiation and osteoporosis14. HDAC4 or 5 is involved in TGF-β/ SMAD3-mediated inhibition of Runx2 function, thereby affecting OBs differentiation15. These collective studies highlight the critical importance of HDAC4/5 in bone-related diseases.
Previous studies have shown that histone deacetylase inhibitors (HDACi) have an inhibitory effect on osteoclast (OC) formation, suggesting that it has therapeutic potential to mitigate osteolytic bone destruction associated with bone-related diseases16. HDACi can also promote the expression of related genes during the differentiation of osteoblast precursor cells into mature OBs17. The important role of HDACi in regulating the differentiation and maturation of OBs and OCs has prompted consideration to investigate its effects on these cell types, as well as its effects on various bone diseases, providing potential alternatives for clinical treatment. Although the use of HDAC inhibitors (HDACi) in malignancies and other diseases has been widely explored, they also bring a variety of side effects. The most common side effects are fatigue, gastrointestinal disorder and reversible bone marrow suppression, most of which are mild to moderate, and do not increase cardiac toxicity. The reported adverse reactions of nonspecific HDACi in phase I and phase II clinical trials of tumors include nausea, vomiting, abnormal blood system and prolonged QT interval. Nonspecific HDACi such as SAHA, LBH589 and ITF-2357 may cause transient thrombocytopenia or bone marrow suppression18,19. The FDA approved vorinotha (SAHA) for the treatment of cutaneous T-cell lymphoma in 2006. Subsequently, Belinostat, Chidamide, and roidepsin were approved for the treatment of peripheral T-cell lymphoma, and Panobinostat was approved for the treatment of multiple myeloma. Although these pan-HDACI drugs have achieved initial clinical results, they also have various side effects. In contrast, specific HDAC inhibitors are well tolerated and are a promising direction for future research20,21,22.
LMK-235, an effective and selective HDAC4/5 inhibitor23, has demonstrated anti-tumor activity in multiple myeloma cells by down-regulating HDAC4-associated HO-1 and activating the JNK/AP-1 pathway12. LMK-235 also exhibits a synergistic effect with bortezomib in breast cancer cell lines24. LMK-235 reduced the accumulation of HDAC4 in the neuronal precursor cells of CDKL5-knocked out mice, completely restoring the reduced number of neurons25. In addition, LMK-235 can promote the induction of dentin cells and the regeneration of dental tissue26. However, there remains limited research on the potential therapeutic role of LMK-235 in bone-related diseases.
Given the systemic side effects of the drug, our study focused on the local therapeutic effect of the HDAC inhibitor LMK-235 in two mouse models, cranial osteolysis and tibial bone defect. Our study found that LMK-235 inhibits HDAC4 and subsequently suppresses the NF-κB and p-smad2/3 signaling pathways, thereby restraining osteoclast formation. Moreover, LMK-235 promotes osteoblast formation by inhibiting HDAC4 and enhancing the expression of the Runx2 transcription factor. Furthermore, animal experiments confirmed the therapeutic efficacy of LMK-235 in resolving bone loss and promoting bone defect repair in vivo. Therefore, LMK-235 may serve as a potential therapeutic agent for osteoclast-induced bone resorption abnormalities and osteoblast-promoted bone regeneration. This study will provide valuable insights for future clinical applications.
Materials and methods
Ethics statement
The study protocol were carried out in accordance with the guidelines of the Institutional Animal Care Committee and reported in compliance with the ARRIVE guidelines.
The animals were provided by the Experimental Animal Center of Shanxi Medical University. Animal research was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University, Taiyuan, China (No. DW2022055). All animals were housed in a room in which there was an adjusted climate (temperature, 22–24 °C ± 2 °C; humidity, 30–60% ± 5%) and special sun-substitution ultraviolet light (12 h photoperiod, 6–18 h) , and the noise was avoided.
Samples of human femoral head collected during hip replacement surgeries were approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (2022YXNo.136). Informed consent was obtained from all subjects and/or their legal guardian(s). Related experiments were conducted according to the guidelines approved by the Second Hospital of Shanxi Medical University. All methods were performed in accordance with the relevant guidelines and regulations.
Anesthesia and euthanasia
Animal surgery were performed under anesthesia and aseptic conditions. Anesthesia was induced by intraperitoneal injection of 0.8% sodium pentobarbital at a dose of 70 mg/kg.
After the animal experiments, the animals were euthanized by an overdose of gaseous carbon dioxide in an empty Plexiglas box and observed for at least 10 min after cardiac arrest and breathing cessation.
Primary cell culture
Primary bone marrow-derived mononuclear macrophages (BMMs) were isolated from the femur and tibia of C57BL/6 male mice aged 4–6 weeks. The culture medium was refreshed every two days, comprising α-minimal Eagle’s medium (α-MEM, HyClone), 1% penicillin–streptomycin (P/S, 100 U/ml penicillin, 100 μg/ml streptomycin, Thermo Fisher Scientific, USA), 10% fetal bovine serum (FBS, VWR) and 25 ng/mL mouse macrophage colony-stimulating factor protein (M-CSF, LifeTein, LLC., Beijing, China). The cells were incubated at 37 °C with 5% CO2.
Bone marrow-derived mesenchymal stem cells (hBMSCs) were obtained from human femoral head samples taken during hip replacement surgeries. The culture medium was changed every three days and consists of α-MEM, 1% penicillin–streptomycin and 10% fetal bovine serum. The cells were incubated at 37 °C with 5% CO2.
Toxicity evaluation of LMK-235
BMMs were seeded in 96-well plates at a density of 3 × 103 cells/well and cultured in α-MEM containing 25 ng /ml M-CSF for 24 h. Following this, the previous medium was refreshed but with varying concentrations of LMK-235 (0, 1.953125, 3.90625, 7.8125, 15.625, 31.25, 62.5, 125, and 250 nM). Cytotoxicity was assessed at 48-, 72-, and 96-h using Cell Counting Kit-8 (CCK-8, MCE, USA), and the absorbance at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, USA).
HBMSCs were plated onto 96-well plates at a density of 3 × 103 cells/well and cultured in complete medium (containing α-MEM, FBS and P/S) for 24 h to facilitate adhesion. Subsequently, the medium was replaced with different concentrations of LMK-235 (0, 0.9765625, 1.953125, 3.90625, 7.8125, 15.625, 31.25, 62.5, 125, and 250 nM), and the subsequent procedures were identical to those applied to BMMs.
Assessment of LMK235 efficacy in vitro
BMMs were seeded onto 96-well plates at a density of 8 × 103 cells/well and cultured in α-MEM complete culture solution (as described above) for 24 h to facilitate adhesion. Subsequently, the medium was replaced with complete culture medium containing 50 ng/ml receptor activator of nuclear factor-κB ligand (RANKL, LifeTein, LLC., Beijing, China) to induce BMMs differentiation into osteoclasts. Simultaneously, LMK-235 in different concentrations (0–62.5 nM) were added. The medium containing RANKL and LMK-235 was refreshed every 48 h until osteoclasts (OCs) were formed on the 6th day. Cell morphology was observed under a microscope, and TRAP staining was conducted using a TRAP staining kit (Sigma, USA). Cells with three or more nuclei were classified as OCs. The extent of OC differentiation and the therapeutic impact of LMK-235 were assessed by quantifying the number of TRAP-positive multinucleated cells.
HBMSCs were plated into a 24-well plate at a density of 4 × 104 cells/well. After 24 h of culture in complete medium to allow adherence, the cells were exposed to an osteoblast-inducing differentiation medium (HyCyteTM Starfish Biological Company, China) to stimulate their differentiation into osteoblasts. This induction medium contains MC3T3-E1 osteogenic differentiation basal medium, MC3T3-E1 osteogenic differentiation FBS, P/S solution, glutamine, β-glycerophosphate, ascorbate acid and dexamethasone. Various concentrations of LMK-235 (0–62.5 nM) were added during induction. Subsequent to this, the induction medium and LMK-235 were replaced every 72 h. After 7 and 14 days of induced differentiation, ALP (BCIP/NBT alkaline phosphatase chromogenic kit, Biyuntian Company, China) and AR (Alizarin red staining solution, HyCyteTM Starfish Biological Company, China) staining were performed. Cells were fixed with 4% paraformaldehyde for 30 min before staining according to the provided instructions. The extent of osteoblasts (OBs) differentiation and the therapeutic effects of LMK-235 were assessed based on the depth of coloring.
RNA extraction and Real-time quantitative polymerase chain reaction (RT-qPCR)
BMMs were seeded in 6-well plates at a density of 1.5 × 105 cells/well and cultured in α-MEM culture solution containing 25 ng/ml M-CSF and 50 ng/ml RANKL. Additionally LMK-235 (0 nM, 31.25 nM, 62.5 nM) was added. And the medium containing RANKL and LMK-235 was refreshed every 48 h. After 5 days of culture, cellular total RNA was extracted using TRIzol reagent. Reverse transcription with Primescript RT Master Mix (Takara, Beijing, China) was performed to generate single-stranded cDNA. Real-time quantitative polymerase chain reaction was performed on the Applied Biosystems QuantStudio 6 Flex Real-Time PCR system using the TB Green PreMix Ex Taq (Takara, Beijing, China) kit to assess the expression of the target gene. Employing the 2−ΔΔCT method, mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a reference gene for normalization, and the relative expression of the target gene was analyzed. Primer sequences are provided in Table 1.
HBMSCs were inoculated into a 6-well plate at a density of 1.5 × 105 cells/well, and cultured in osteoblast-induced differentiation medium. Simultaneously, LMK-235 (0 nM, 31.25 nM, 62.5 nM) was added, and the induction medium and LMK-235 were refreshed every 72 h. After 14 days of culture, cellular total RNA was extracted using TRIzol reagent, and subsequent experiments were conducted following the aforementioned methods.
Western blot
BMMs were seeded into a six-well plate at a density of 1.5 × 105 cells/well. The cells were treated with a medium containing 25 ng/mL M-CSF and 50 ng/mL RANKL, with simultaneous addition of LMK-235 (0 nM, 31.25 nM, 62.5 nM). The RANKL and drugs were refreshed every 48 h. Following 5 days of culture, the protein was extracted by using a whole protein extraction kit (KGI, China).
BMMs was seeded into a six-well plate at a density of 5 × 105 cells/well, and cultured in α-MEM containing 25 ng/ml M-CSF for 24 h. Then cells were starved and pretreated with LMK-235 (concentration of 62.5 nM) for 2 h, and then 50 ng/mL RANKL was introduced to stimulate cells for 30 min, after which the total protein was collected.
HBMSCs were seeded into a six-well plate at the density of 1.5 × 105 cells/well, and cultured in osteoblast-induced differentiation medium, concurrently LMK-235 (0 nM, 31.25 nM, 62.5 nM) was added, and the induction medium and LMK-235 were refreshed every 72 h. After 14 days of culture, the whole protein extraction kit was used to extract the protein.
Protein samples were separated through 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were subsequently transferred to a polyvinylidene fluoride (PVDF) membrane, which was then blocked with 5% skim milk for 1–2 h. Following this, the membrane was incubated with primary antibodies at 4 °C for 12–16 h. To remove the primary antibody, membrane was washed through TBS+Tween-20 (TSBT) for three times for 10 min each. The membrane was then incubated with anti-rabbit (1:5000, RS0001, immunology) or anti-mouse (1:5000, RS0002, immunology) secondary antibody at room temperature for 1 h. After another wash with TBST three times, each for 10 min, the membrane was subjected to the ultra-sensitive ECL chemiluminescence kit (Biyuntian, P0018AS) for visualization of immune bands. The primary antibodies used are as follows: Integrinβ3 primary antibody (Santa Cruz, sc-365679, 1: 1000), CTSK primary antibody (Santa Cruz, sc-48353, 1: 1000), β-actin primary antibody (Abclonal, AC026, 1: 1000), Osterix primary antibody (Bioss, bs-1110R, 1: 1000), HDAC4 primary antibody (Abclonal, A0179, 1: 1000), P-Smad2/3 primary antibody (Abclonal, AP0548, 1:1000), NF-κB p65 primary antibody (Affinity, BF0382, 1:1000), p-NF-κB p65 (Ser536) (Affinity, AF2006, 1:1000) and Runx2 primary antibody (Abways, CY5395, 1:1000). The immunoreactive bands were visualized by the Touch Imaging System of Bio–Rad (ChemiDoc™, Bio–Rad, CA, USA). Image J 1.46r software was used to analyze the gray value of stripes.
A mouse skull model with inflammatory osteolysis induced by LPS
Twenty-four male C57BL/6 mice aged 8–10 weeks were randomly divided into four groups: PBS control group, LPS group, LMK-235 low concentration (88.3 ug/kg) + LPS group, and LMK-235 high concentration (176.7 ug/kg) + LPS group. Mice were provided by the Experimental Animal Center of Shanxi Medical University. Animal research was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (No. DW2022055). Related experiments were conducted according to the guidelines approved by the Second Hospital of Shanxi Medical University. Anesthesia was induced by intraperitoneal injection of 0.8% sodium pentobarbital at a dose of 70 mg/kg. After anesthesia, the mice underwent skin disinfection with 75% alcohol, followed by a 0.5 cm incision in the middle of the skull. A gelatin sponge about 1 cm square was placed, the wound was sutured. Drug administration commenced after a 3-day interval. The LPS group received injections of LPS (2.5 mg/ml, 100 µl each time) three times a week, while the control group received PBS injections, all of which directly administered into the implanted gelatin sponge via local injection. The drug was delivered via intraperitoneal injection twice a week.
Six mice in each group were euthanized after 14 days of drug treatment. Animals were euthanized by excessive gaseous carbon dioxide in an empty plexiglass box, and observed for at least 10 min after cardiac arrest and respiratory arrest. The skulls of mice were collected, then fixed and stored in 4% paraformaldehyde for one day, and then stored in 75% alcohol. Microscopic CT and morphological experiments were used to analyze the osteolysis of skull.
Tibial bone defect model in mice
Eighteen male C57BL/6 mice aged 12 weeks were randomly divided into three groups: control group, LMK-235 (88.3ug/kg) group and LMK-235 (176.7ug/kg) group. Mice were provided by the Experimental Animal Center of Shanxi Medical University. Animal research was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (No. DW2022055). Related experiments were conducted according to the guidelines approved by the Second Hospital of Shanxi Medical University. Anesthesia was induced by intraperitoneal injection of 0.8% sodium pentobarbital at a dose of 70 mg/kg. The skin was sterilized with 75% alcohol. The incision were made in middle of the medial tibia of the right leg, and a 0.8 mm hole was drilled in the tibia of mice to establish a bone defect model. From the second day after surgery, normal saline or drugs were injected locally every other day for one week.
Six mice in each group were euthanized after 7 days of drug treatment. Animals were euthanized in an empty plexiglass box by excessive gaseous carbon dioxide, and observed for at least 10 min after cardiac arrest and respiratory arrest. The tibia of mice was collected, then fixed and stored in 4% paraformaldehyde for 24 h. Micro-CT and morphological experiments were used to analyze the bone defect.
Micro CT analysis
Skull samples were scanned using Skyscan 1275 Micro CT scanner (Bruker Micro CT, Kontich, Belgium). The scanning software was X-ray Microtomograph 1275 1.6.3.0. The parameters were set as source current: 46 μA, source voltage: 75 kV, rotation step size: 0.2, pixel size: 12 μm, and no filter. Whereupon, the bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) of skull suture were analyzed using CT Analyser1.17.7.2 software.
The tibia samples were scanned with VivaCT80 Micro CT scanner (SCANCO Medical AG, Switzerland). The scanning software was μCT Tomography V6.3-4. The bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) of tibial bone defect were analyzed with μCT Evaluation Program V6.5-3 software.
Histological analysis
The skull and tibia of mice were fixed and preserved in 4% paraformaldehyde for 24 h, and followed by a 2-week decalcification through 10% EDTA. Paraffin-embedded Sects. (4 μm thick) were stained with hematoxylin–eosin (H&E), saffron O- fast green and TRAP (Methyl green counterstain). Images were collected by Magscanner (KF-PRO-120), a scanning equipment.
Statistical analyses
All experiments were conducted three or more times, and data were expressed as mean ± standard deviation. GraphPad Prism 8 software was used for statistical analysis. One-way analysis of variance (ANOVA) was employed for statistical comparisons. Statistical significance was indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control group.
Data availability statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Results
Inhibition of RANKL induced osteoclastogenesis by LMK-235
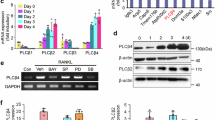
LMK-235 is a potent and selective inhibitor of HDAC4/5. BMMs were induced with RANKL, and subsequent gene-level changes were assessed. Five days after RANKL stimulation, our results revealed an upregulation of osteoclast marker genes (ACP-5, DC-STAMP, TNFRSF11a), consistent with our general understanding of osteoclasts. Subsequently, we conducted PCR analysis on the same set of samples for HDAC4/5. The analysis demonstrated the expression of both HDAC4 and HDAC5 in mature osteoclasts, with HDAC4 being more abundant than HDAC5 (Fig. 1A). These findings affirm the presence of HDAC4 and HDAC5 in osteoclasts, highlighting the predominance of HDAC4. This suggests that HDAC4 is of primary importance in the subsequent drug experiments.
LMK-235 inhibits RANKL-induced osteoclast generation. (A) Five days after RANKL stimulation, the expression of HDAC4, HDAC5 and osteoclast-specific genes ACP-5, DC-STAMP and TNFRSF11a were assessed by qRT-PCR, and GAPDH served as the reference gene (n = 3). (B) CCK-8 assay was employed to evaluate the cytotoxicity of LMK-235 on BMMs (n = 3). (C) After six days of RANKL stimulation, TRAP staining results of OCs. (D) Quantitative analysis of TRAP-positive OCs with three or more nuclei (n = 3). (E) The molecular structural formula of LMK-235. *P < 0.05, **P < 0.01, ***P < 0.001 represents comparison with the control group.
Figure 1E illustrates the chemical structure of LMK-235. In order to ascertain that the inhibitory effect of LMK-235 is not attributable to its cytotoxicity on BMMs, we initially conducted a CCK-8 assay to assess the impact of LMK-235 on BMM viability. BMMs were exposed to varying concentrations of LMK-235 for 48, 72, and 96 h. The results indicated that the survival of BMMs remained unaffected at concentrations below 250 nM (Fig. 1B). Consequently, LMK-235 concentrations below 250 nM were employed in subsequent experiments to evaluate the drug's influence on osteoclasts in vitro.
To assess the impact of LMK-235 on the differentiation and maturation of osteoclasts induced by RANKL, we conducted a TRAP staining experiment. As illustrated in Fig. 1C and D, after 6 days of RANKL stimulation, the number of TRAP-positive cells in LMK-235 treated wells (31.25 nM, 62.5 nM) was lower than that in the positive control group. These results unequivocally demonstrate that LMK-235 has the capacity to inhibit the in vitro formation of osteoclasts.
LMK-235 leads to downregulation of osteoclast-related genes and proteins via NF-κB and p-smad2/3 signaling pathways
We evaluated the expression levels of several genes closely associated with the formation and maturation of osteoclasts, including ACP-5 (TRAP), DC-STAMP, C-fos, TNFRSF11a, NFATC1, CTSK, and V-ATPase-d2. As illustrated in Fig. 2A, our experimental results show that after 5 days of RANKL stimulation, compared to the negative control group, the gene expression levels of these relevant genes increased in the RANKL treatment group. However, LMK-235 effectively inhibited the expression of these genes. Notably, HDAC4 and HDAC5 were also downregulated. Combined with the previous Fig. 1A, HDAC4 is more abundant in mature osteoclasts than HDAC5, so this study mainly considers the influence of drugs on HDAC4. The results indicating that LMK-235 genetically interfered with RANKL-induced osteoclast differentiation and function by downregulating HDAC4.
LMK-235 suppresses HDAC4, HDAC5 and related genes and proteins in osteoclasts, inhibiting the p-NF-κB and p-Smad2/3 signaling pathways. (A) Gene Expression Analysis: The expression of relevant genes, and after 5 days of RANKL stimulation, including HDAC4, HDAC5, ACP5, DC-STAMP, C-fos, TNFRSF11a, NFATC1, CTSK, and V-ATPase-d2, was down-regulated, with GAPDH serving as the reference gene (n = 3). (B) Western blots analysis of the HDAC4, Integrin β3, CTSK and p-smad2/3 expression induced by treatment with RANKL (50 ng/mL) for 5 days in the presence or absence of LMK-235 (31.25 nM and 62.5 nM), with β-actin used as the internal reference protein. In addition, the cells were starved and pretreated with LMK-235 (concentration 62.5 nM) for 2 h, then the cells were stimulated by 50 ng/mL RANKL for 30 min, and the expression of p-NF-κB (Ser536) was analyzed by protein blot. Quantitative analysis of protein expression levels demonstrated significant differences compared to the control group (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
Furthermore, we examined the expression levels of several associated proteins through Western Blot analysis. As shown in Fig. 2B, the expression of HDAC4 protein decreased after drug treatment, and the expression of other osteoclast-related proteins, including CTSK and Integrinβ3, was also suppressed. This suggests that LMK-235 exerts a certain inhibitory effect on the biosynthesis of relevant proteins. Both Western Blot and qPCR results corroborated that LMK-235 effectively curtailed the expression of key genes and proteins involved in the formation and differentiation of osteoclasts.
BMMs was starved and pretreated with LMK-235 (concentration 62.5 nM) for 2 h, then 50 ng/mL RANKL was introduced to stimulate cells for 30 min, and then the total protein was collected. We detected the expression level of related proteins by protein blot analysis, and observed that LMK-235 reduced the phosphorylation level of NF-κB (Ser536) under RANKL stimulation (Fig. 2B). In the same figure, LMK-235 also reduced the level of p-smad2/3 in cells after 5 days of RANKL stimulation. These collective results demonstrate that LMK-235 inhibits the NF-κB pathway and p-smad2/3 pathway, consequently impeding the differentiation and maturation of osteoclasts.
LMK-235 Attenuates LPS-Induced Cranial Osteolysis
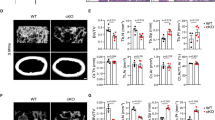
To assess the influence of LMK-235 on osteoclasts in vivo, we established a model of cranial osteolysis induced by LPS. Micro-CT analysis, as illustrated in Fig. 3A, highlights significant osteolysis in the cranial specimens of the LPS group. However, treatment with LMK-235 noticeably reduced the extent of osteolysis. The analysis results were further quantified, revealing changes in several important bone parameters (BV/TV, Tb.Th, Tb.N, Tb.Sp) following drug treatment, notably an increase in BV/TV, while other indicators showed no statistically significant differences (Fig. 3D). These results suggest that LMK-235 can mitigate LPS-induced skull osteolysis in vivo.
LMK-235 mitigates LPS-induced osteolysis. (A) Micro CT image of skull. (B) H&E staining. (C) TRAP staining. (D) Micro-CT quantitatively analyzed bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp). The experimental groups were PBS control group (n = 6), LPS group (n = 6), LMK-235 low concentration (88.3ug/kg) + LPS group (n = 6) and LMK-235 high concentration (176.7ug/kg) + LPS group (n = 6). Statistical significance indicated as: *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group.
Histological results provide additional support for this perspective. H&E staining results (Fig. 3B) indicate a decrease in bone mass in the LPS group, which an improvement can be observed with the intervention of LMK-235. As depicted in Fig. 3C, the LPS group exhibited numerous osteoclasts, whereas the addition of LMK-235 led to a reduction in osteoclasts. Collectively, these findings indicate that LMK-235 inhibits osteoclasts to some extent, contributing to its therapeutic effect on in vivo osteolysis.
LMK-235 enhances osteoblast production and the expression of related genes and proteins
The cytotoxicity of LMK-235 on human bone marrow-derived mesenchymal stem cells (hBMSCs) was assessed through a CCK-8 experiment to rule out any adverse effects on cell viability. HBMSCs were exposed to varying concentrations of LMK-235 for 48 h, 72 h, and 96 h. The viability of hBMSCs remained unaffected by LMK-235 within the concentration range below 125 nM, as depicted in Fig. 4A. Consequently, LMK-235 concentrations below 125 nM were utilized in subsequent experiments to evaluate the drug’s impact on osteoblasts in vitro.
LMK-235 can promote the induction of osteoblasts by hBMSCs. (A) CCK-8 assay was used to evaluate the cytotoxicity of LMK-235 to hBMSCs (n = 3). (B) ALP staining and Alizarin red staining results of OBs. (C) Western blots analysis of the HDAC4, Runx2 and osterix expression induced by treatment with osteoblast-induced differentiation medium for 14 days in the presence or absence of LMK-235 (31.25 nM and 62.5 nM), with β-actin used as the internal reference protein. Quantitative analysis of protein expression levels demonstrated significant differences compared to the control group (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001). (D)QRT-PCR was used to analyze the gene expression, including ALP, Runx2 and col1a1, with GAPDH as the reference gene (n = 3).
To assess the influence of LMK-235 on the differentiation and maturation of osteoblasts induced by an osteogenic induction medium, we conducted ALP and Alizarin red staining. As shown in Fig. 4B, ALP staining in LMK-235-treated wells exhibited increased color intensity, and more mineralized “nodules” were observed. The results indicated that LMK-235 can enhance the formation of osteoblasts in vitro.
We evaluated the expression levels of key genes associated with osteoblast formation, including ALP, Runx2, and col1a1. As illustrated in Fig. 4D, the experimental results indicate that the LMK-235 treatment group exhibited higher expression levels of ALP, Runx2, and col1a1 genes compared to the positive control group. This suggests that LMK-235 enhances the differentiation and functionality of osteoblasts induced by the osteogenic induction medium at the gene level.
Additionally, we examined the expression levels of several relevant proteins through Western-blot analysis. In Fig. 4C, the protein expression of HDAC4 decreased, while the expression of other osteogenesis-related proteins, Runx2, and osterix, increased. This suggests that LMK-235 plays a role in promoting protein levels. The results from Western-blot and RT-PCR collectively demonstrate that LMK-235 effectively enhances the expression of key genes and proteins related to osteoblast formation.
LMK-235 enhances the bone defect repair
To investigate the impact of LMK-235 on osteoblasts in vivo, we established a bone defect model. Micro-CT analysis, as illustrated in Fig. 5A, revealed that LMK-235 treatment significantly promoted bone defect repair. Quantitative analysis of the results (Fig. 5E) indicated changes in several indexes (BV/TV, Tb.Th, Tb.N, Tb.Sp) after treatment, with increases in BV/TV and Tb.N, while other indicators showed no statistically significant differences. These findings suggest that LMK-235 enhances bone defect repair in vivo.
LMK-235 promotes bone defect repair. (A) Micro CT image of tibia. (B) H&E staining. (C) Saffron O-fast green staining. (D) TRAP staining. (E) Micro CT quantitative analysis, including bone volume/tissue volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp). The experimental groups were control group (n = 6), LMK-235 (88.3ug/kg) group (n = 6) and LMK-235 (176.7ug/kg) group (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 represents comparison with the control group.
Furthermore, histological results substantiate the aforementioned observations. As portrayed in Fig. 5B, C, H & E staining and Saffron O-Fast Green staining demonstrated accelerated bone defect repair and increased bone mass following LMK-235 treatment. Osteoclasts were also assessed using TRAP staining. In Fig. 5D, the addition of LMK-235 accelerated bone remodeling and faster bone formation. Collectively, these findings demonstrate that LMK-235 promotes osteoblast activity, thereby contributing to therapeutic effects on bone defects in vivo.
Discussion
The formation and maintenance of bone is a highly intricate process involving the interaction of multiple cell types and molecular mechanisms. Then imbalances between bone formation and absorption contribute to the occurrence of certain bone diseases, resulting in bone loss or deterioration1,2. HDAC4 is indispensable in the process of bone development, which can affect the proliferation and differentiation of chondrocytes and play an important role in the differentiation of OBs and related diseases such as osteoporosis8,12,13,27. It shows the importance of exploring the role of HDAC4 in LMK-235 inhibiting osteoclast generation and promoting osteoblast generation. The molecular mechanism of regulating HDAC4 on OCs has not been reported, and some progress has been made in OBs28. This triggered our study.
In our study, we present comprehensive and logically analyzed conclusions elucidating the mitigating effects of LMK-235 on bone loss. CCK-8 results affirm the bio-safety of LMK-235, underlining our experimental strategies design. Our results from TRAP staining demonstrate a reduction in the number of multinucleated giant cells stained in red in cell cultures treated with LMK-235. This, coupled with the earlier CCK-8 assay results, visually illustrates that LMK-235, without compromising cell viability, decreased osteoclast differentiation. PCR and Western blot analyses provided compelling evidence that LMK-235 significantly reduced the expression of HDAC4 in osteoclastogenesis compared to the positive control group. Simultaneously, genes closely associated with osteoclastogenesis are suppressed. These include characteristic genes such as ACP5, encoding the TRAP gene, and TNFRSF11a, encoding the receptor activator of NF-κB (RANK). Additionally, the gene expression of key molecules in osteoclast differentiation, namely NFATc1 and cFos, experiences a marked reduction under the influence of the drug. The above results indicate that LMK-235 exerts inhibitory effects on osteoclastogenesis at the molecular level by suppressing HDAC4 expression.
Activation of NF-κB p65 is known to be a crucial early event in RANKL-induced osteoclast differentiation, essential for the activation of NFATC129. Previous reports have indicated that LMK-235 mediates apoptosis in diffuse large B-cell lymphoma cell lines OCI-LY10 and OCI-LY3 by inhibiting NF-κB signaling via upregulation of BCLAF1 expression20. Additionally, HDAC4 deficiency enhances the sirt1/NF-κB signaling pathway by increasing protein levels of sirt1 induced by IL-13 in human nasal epithelial cells and decreasing levels of p-NF-κB, thereby alleviating IL-13-induced inflammatory responses and mucus production associated with allergic rhinitis (AR)30. These studies underscore the close relationship between HDAC4 and the NF-κB signaling pathway. Our results further demonstrate that LMK-235 can reduce p-NF-κB levels in osteoclasts by inhibiting HDAC4, thereby regulating osteoclast differentiation and maturation.
Moreover, extensive research has demonstrated that the Smad signaling pathway plays a critical regulatory role in osteoblast and osteoclast differentiation during bone development, bone formation, and bone homeostasis, indicating its close association with bone remodeling31. TGF-β not only recruits HDAC4 to interact with the Runx2 molecule, affecting acetylation of osteocalcin and signaling pathways associated with osteoblasts, but also collaborates with Smad2 to regulate HDAC415. Pharmacological and genetic HDAC4 inhibition reduces renal tubule epithelial cell arrest, TGF-β1 expression, smad3 phosphorylation, and ERK1/2 activation32. UUO (Unilateral Ureteral Obstruction) injury induces elevated expression of HDAC4, fibrin fibronectin, and α-smooth muscle actin, mitigated by tasquinimod or HDAC4 knockout32. LCZ (sacubitril/valsartan) safeguards kidneys from oxidative stress (OS), inflammation, and fibrosis, improving renal function and rebalancing TAC/ROS33. LMK235 enhances BMP-SMAD-dependent transcription in SH-SY5Y cells, promoting neurite growth via BMP-SMAD pathway activation. MC1568, a pan-IIa class HDAC inhibitor, activates p-smad1/5/8 and upregulates SMAD1 and BMP2 transcription in SH-SY5Y cells34. HDAC4 interacts with p-smad3 and is implicated in chondrocyte hypertrophy regulation35,36,37. LMK-235 treatment decreases p-smad2/3 levels in osteoclasts, suggesting an interaction with HDAC4. In our experiments, LMK-235 inhibited the expression of p-Smad2/3, suggesting that the drug influences osteoclastogenesis through this pathway. Therefore, we propose that LMK-235 exerts dual regulation on osteoclast differentiation via the NF-κB and p-Smad2/3 signaling pathways.
To further validate the results of our in vitro experiments, we conducted in vivo experiments using an LPS-induced bone resorption model. We found that compared to the untreated group, the LMK-235 intervention group exhibited reduced levels of bone resorption and a decrease in osteoclast numbers, showing a concentration-dependent effect. These observations confirm our in vitro findings. LMK-235 inhibits HDAC4 to suppress inflammatory pathways, thereby reducing inflammation-induced osteoclast differentiation and alleviating bone resorption. Interestingly, micro-CT analysis revealed a significant increase in bone volume/total volume (BV/TV) in the LMK-235 intervention group compared to the untreated group. This increase in bone mass post-treatment has sparked our interest.
Next, we conducted in vitro experiments to investigate whether LMK-235 has a role in promoting osteoblast differentiation. Previous studies have shown that HDAC4 and Runx2 play an important role in chondrocyte hypertrophy and osteoblast differentiation, and HDAC4 can be used as an upstream regulator of Runx2. In the absence of TMCO1, the decrease of phosphorylation of HDAC4 leads to the nuclear enrichment of HADC4, which leads to the deacetylation and degradation of Runx2, the main regulator of osteogenesis38. Other studies showed that PTHrP inhibited the signal pathways of Runx2 and MEF2C after HDAC4 was nucleated, which resulted in chondrocyte hypertrophy and bone formation inhibition5,39,40. HDAC4 can modify Osterix and regulate the differentiation process of OBs10. HDAC4 or 5 is involved in TGF-β/smad3-mediated Runx2 function inhibition, which further affects osteoblast differentiation15. Research has demonstrated that histone deacetylase inhibitors (HDACi) can diminish osteoclast generation and address osteolytic diseases. Furthermore, HDACi has the potential to upregulate the expression of pertinent genes during osteoblast induction by osteoblast precursors16,17. Previous studies have reported that LMK-235 can enhance dentin cell induction and tooth tissue regeneration. Notably, mRNA and protein expression levels of dentin sialophosphoprotein, dwarf-related transcription factor 2, alkaline phosphatase (ALP), and osteocalcin increased in dental pulp cells26. Our results indicate that under the influence of LMK-235, osteoblasts exhibit enhanced ALP staining and increased formation of mineralized nodules as shown by Alizarin Red staining. These observations are supported at the molecular level by polymerase chain reaction and Western blot analyses. In this study, we selected LMK-235, a relatively specific HDAC4/5 inhibitor, and found results consistent with previous findings involving HDAC inhibitors. This suggests that LMK-235 increases the expression of Runx2 by inhibiting HDAC4, thereby promoting the maturation and mineralization of osteoblasts.
To comprehend the role of LMK-235 in bone repair and reconstruction in vivo, we conducted a bone defect experiment with localized LMK-235 injection therapy. MicroCT results and H&E staining indicate that, compared to the control group, the treatment group exhibited closer-to-complete bone repair and a greater overall bone mass. Additionally, TRAP staining revealed an increase in osteoclasts in the treatment group. However, this can be explained as a sign of more vigorous bone repair activity in the treatment group. It shows that the treatment group entered the stage of bone remodeling early, when osteoclasts were responsible for absorbing aging bone and osteoblasts were responsible for synthesizing new bone. The control group is still in the initial stage of repair, indicating that drug treatment has accelerated the formation of bone. Overall, the comprehensive effect of LMK-235 on bone defect repair is indicative of its promotion of bone repair.
In this study, it is shows that LMK-235 can effectively inhibit the generation of osteoclasts by inhibiting HDAC4, and it also proves that LMK-235 can promote the generation of osteoblasts, and then play a therapeutic role in related bone diseases by affecting these two cells. Both in vitro and in vivo experiments confirmed that LMK-235 inhibited the formation of OCs induced by RANKL and promoted the formation of OBs. We hypothesized that LMK-235 inhibited the activation of NF-κB and p-smad2/3 signaling pathways in OCs by inhibiting the expression level of HDAC4. Promote the expression of Runx2 regulatory factor in OBs by inhibiting HDAC4. LMK-235 restored the degree of cranium osteolysis to some extent in vivo, and promoted the repair process of bone defect, which also strengthened the importance of exploring the molecular mechanism of LMK-235.
According to our research results at present, there are actually some shortcomings. For example, HDAC4, a histone deacetylase, is involved in the specific process of related signal pathways, and the downstream direct target has not been further studied in our system. Besides HDAC4, LMK-235 also has influence on HDAC5. Many studies show that HDAC5 has influence on osteoclasts and osteoblasts. As there is no specific inhibitor for HDAC4, LMK-235 was chosen to do related research in this study. Therefore, the effect of this drug on HDAC5 is worth further study. Moreover, due to the time factor, there is no fracture model caused by osteoporosis to explore the effect of drugs on OCs and OBs in systemic diseases. Therefore, more research is needed to explore the molecular mechanism of LMK-235 and use it as a better therapeutic drug choice.
The study is reported in accordance with ARRIVE guidelines.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Loi, F. et al. Inflammation, fracture and bone repair. Bone 86, 119–130. https://doi.org/10.1016/j.bone.2016.02.020 (2016).
Redlich, K. & Smolen, J. S. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discovery 11, 234–250. https://doi.org/10.1038/nrd3669 (2012).
Mohsin, S. et al. An update on therapies for the treatment of diabetes-induced osteoporosis. Expert Opin. Biol. Ther. 19, 937–948. https://doi.org/10.1080/14712598.2019.1618266 (2019).
Tsukamoto, M. et al. Elcatonin prevents bone loss caused by skeletal unloading by inhibiting preosteoclast fusion through the unloading-induced high expression of calcitonin receptors in bone marrow cells. Bone 85, 70–80. https://doi.org/10.1016/j.bone.2016.01.025 (2016).
Chen, Z. et al. The role of histone deacetylase 4 during chondrocyte hypertrophy and endochondral bone development. Bone Jt. Res. 9, 82–89. https://doi.org/10.1302/2046-3758.92.Bjr-2019-0172.R1 (2020).
Zhengke, W., Gangjian, Q. & Ting, C. Z. HDAC4: mechanism of regulation and biological functions. Epigenomics https://doi.org/10.2217/epi.13.73 (2014).
Jun, Y. et al. Insights into the function and clinical application of HDAC5 in cancer management. Front. Oncol. https://doi.org/10.3389/fonc.2021.661620 (2021).
Nakatani, T., Chen, T., Johnson, J., Westendorf, J. J. & Partridge, N. C. The deletion of Hdac4 in mouse osteoblasts influences both catabolic and anabolic effects in bone. J. Bone Mineral Res: Off. J. Am. Soci. Bone Mineral Res. 33, 1362–1375. https://doi.org/10.1002/jbmr.3422 (2018).
Teruyo, N., Tiffany, C. & Nicola, C. P. MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton. Bone https://doi.org/10.1016/j.bone.2016.06.010 (2016).
Lu, J. et al. Osterix acetylation at K307 and K312 enhances its transcriptional activity and is required for osteoblast differentiation. Oncotarget 7, 37471–37486. https://doi.org/10.18632/oncotarget.9650 (2016).
Zhang, D., Du, J., Yu, M. & Suo, L. Urine-derived stem cells-extracellular vesicles ameliorate diabetic osteoporosis through HDAC4/HIF-1α/VEGFA axis by delivering microRNA-26a-5p. Cell Biol. Toxicol. 39, 2243–2257. https://doi.org/10.1007/s10565-022-09713-5 (2023).
Chen, R. et al. MiRNA-19a-3p alleviates the progression of osteoporosis by targeting HDAC4 to promote the osteogenic differentiation of hMSCs. Biochem. Biophys. Res. Commun. 516, 666–672. https://doi.org/10.1016/j.bbrc.2019.06.083 (2019).
Lu, Z. et al. MiR-206 regulates the progression of osteoporosis via targeting HDAC4. Eur. J. Med. Res. 26, 8. https://doi.org/10.1186/s40001-021-00480-3 (2021).
Li, H. et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J. Clin. Investig. 119, 3666–3677. https://doi.org/10.1172/jci39832 (2009).
Kang, J. S., Alliston, T., Delston, R. & Derynck, R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. EMBO J. 24, 2543–2555. https://doi.org/10.1038/sj.emboj.7600729 (2005).
Sun, S. et al. HDAC inhibitor quisinostat prevents estrogen deficiency-induced bone loss by suppressing bone resorption and promoting bone formation in mice. Eur. J. Pharmacol. 927, 175073. https://doi.org/10.1016/j.ejphar.2022.175073 (2022).
Dudakovic, A. et al. Histone deacetylase inhibition promotes osteoblast maturation by altering the histone H4 epigenome and reduces Akt phosphorylation. J. Biol. Chem. 288, 28783–28791. https://doi.org/10.1074/jbc.M113.489732 (2013).
Roza, P., Divya, H. & Biswa Prasun, C. Recent histone deacetylase inhibitors in cancer therapy. Cancer https://doi.org/10.1002/cncr.34974 (2023).
Yixuan, L. & Edward, S. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harbor Perspect. Med. https://doi.org/10.1101/cshperspect.a026831 (2016).
Li, X. et al. Effect of BCLAF1 on HDAC inhibitor LMK-235-mediated apoptosis of diffuse large B cell lymphoma cells and its mechanism. Cancer Biol. Ther. 19, 825–834. https://doi.org/10.1080/15384047.2018.1472188 (2018).
Squarzoni, A., Scuteri, A. & Cavaletti, G. HDACi: the columbus’ egg in improving cancer treatment and reducing neurotoxicity?. Cancers https://doi.org/10.3390/cancers14215251 (2022).
Wu, B., Sodji, Q. H. & Oyelere, A. K. Inflammation, fibrosis and cancer: mechanisms, therapeutic options and challenges. Cancers https://doi.org/10.3390/cancers14030552 (2022).
Guo, Y. et al. Up-regulation of HO-1 promotes resistance of B-cell acute lymphocytic leukemia cells to HDAC4/5 inhibitor LMK-235 via the Smad7 pathway. Life Sci. 207, 386–394. https://doi.org/10.1016/j.lfs.2018.06.004 (2018).
Li, A. et al. Correction: HDAC5, a potential therapeutic target and prognostic biomarker, promotes proliferation, invasion and migration in human breast cancer. Oncotarget 8, 30619–30620. https://doi.org/10.18632/oncotarget.17542 (2017).
Trazzi, S. et al. HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Human Mol. Genet. 25, 3887–3907. https://doi.org/10.1093/hmg/ddw231 (2016).
Liu, Z. et al. HDAC inhibitor LMK-235 promotes the odontoblast differentiation of dental pulp cells. Mol. Med. Rep. 17, 1445–1452. https://doi.org/10.3892/mmr.2017.8055 (2018).
Gao, L. et al. Conditional deletion of HDAC4 from collagen type 2α1-expressing cells increases angiogenesis in vivo. Mol. Med. (Cambridge, Mass) 26, 36. https://doi.org/10.1186/s10020-020-00154-6 (2020).
Obri, A., Makinistoglu, M. P., Zhang, H. & Karsenty, G. HDAC4 integrates PTH and sympathetic signaling in osteoblasts. J. Cell Biol. 205, 771–780. https://doi.org/10.1083/jcb.201403138 (2014).
Takatsuna, H. et al. Inhibition of RANKL-induced osteoclastogenesis by (-)-DHMEQ, a novel NF-kappaB inhibitor, through downregulation of NFATc1. J. Bone Mineral Res.: Off. J. Am. Soc. Bone Mineral Res. 20, 653–662. https://doi.org/10.1359/jbmr.041213 (2005).
Xu, H., Wang, L., Chen, H. & Cai, H. HDAC4 depletion ameliorates IL-13-triggered inflammatory response and mucus production in nasal epithelial cells via activation of SIRT1/NF-κB signaling. Immunity, Inflamm. Dis. 10, e692. https://doi.org/10.1002/iid3.692 (2022).
Zou, M. L. et al. The smad dependent TGF-β and BMP signaling pathway in bone remodeling and therapies. Front. Mol. Biosci. 8, 593310. https://doi.org/10.3389/fmolb.2021.593310 (2021).
Shen, F. et al. Pharmacological and genetic inhibition of HDAC4 alleviates renal injury and fibrosis in mice. Front. Pharmacol. 13, 929334. https://doi.org/10.3389/fphar.2022.929334 (2022).
Abbas, S. S. et al. LCZ696 (sacubitril/valsartan) protects against cyclophosphamide-induced nephrotoxicity in adult male rats: up-regulation of Apelin-13/ACE2, miR-200, and down-regulation of TGF-β/SMAD 2/3 and miR-192. Life Sci. 306, 120850. https://doi.org/10.1016/j.lfs.2022.120850 (2022).
Mazzocchi, M. et al. LMK235, a small molecule inhibitor of HDAC4/5, protects dopaminergic neurons against neurotoxin- and α-synuclein-induced degeneration in cellular models of Parkinson’s disease. Mol. Cell. Neurosci. 115, 103642. https://doi.org/10.1016/j.mcn.2021.103642 (2021).
Aprile, P. & Kelly, D. J. Hydrostatic pressure regulates the volume, aggregation and chondrogenic differentiation of bone marrow derived stromal cells. Front. Bioeng. Biotechnol. 8, 619914. https://doi.org/10.3389/fbioe.2020.619914 (2020).
Iaquinta, M. R. et al. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics 11, 6573–6591. https://doi.org/10.7150/thno.55664 (2021).
Yu, X. et al. TGFβ-induced factor homeobox 2 blocks osteoblastic differentiation through targeting pSmad3/HDAC4/H4ac/Runx2 axis. J. Cell. Physiol. 234, 21284–21293. https://doi.org/10.1002/jcp.28733 (2019).
Li, J. et al. TMCO1-mediated Ca(2+) leak underlies osteoblast functions via CaMKII signaling. Nat. Commun. 10, 1589. https://doi.org/10.1038/s41467-019-09653-5 (2019).
Arnold, M. A. et al. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Develop. Cell 12, 377–389. https://doi.org/10.1016/j.devcel.2007.02.004 (2007).
Erickson, A. G. et al. A tunable, three-dimensional in vitro culture model of growth plate cartilage using alginate hydrogel scaffolds. Tissue Eng. Part A 24, 94–105. https://doi.org/10.1089/ten.tea.2017.0091 (2018).
Funding
This research was supported by National Natural Science Foundation of China (Grant No. 81871815), Research Project Supported by Shanxi Scholarship Council of China (Grant No. 2017-063), Natural Science Fund of Shanxi Province (Grant No. 201701D121170 and 20210302123284).
Author information
Authors and Affiliations
Contributions
Chongwei Chen and Yue Li contributed equally to this work. All authors contributed to the research concept. Chongwei Chen, Yue Li, Teng Feng, Xinping Chen, Chengwei Li and Yaqiong Chang performed experiments. Lu Li conducted statistical analysis. Chongwei Chen and Mengbo Zhu drafted the manuscript. Chongwei Chen, Yaqiong Chang and Shaowei Wang contributed to the conception and supervised the project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, C., Li, Y., Feng, T. et al. LMK-235 suppresses osteoclastogenesis and promotes osteoblastogenesis by inhibiting HDAC4. Sci Rep 14, 19973 (2024). https://doi.org/10.1038/s41598-024-70814-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70814-8