Abstract
During their development, amphibians undergo various physiological processes that may affect their susceptibility to environmental pollutants. Naturally occurring fluctuations caused by developmental events are often overlooked in ecotoxicological studies. Our aim is to investigate how biomarkers of oxidative stress are modulated at different stages of larval development in the Amazonian amphibian species, Physalaemus ephippifer. The premetamorphosis, prometamorphosis and metamorphic climax stages were used to analyze total antioxidant capacity (ACAP), glutathione S-transferase (GST) activity, lipid peroxidation (LPO) levels and the expression of genes nrf2, gst, gsr (glutathione reductase) and gclc (glycine-cysteine ligase, catalytic subunit). Although there was no difference in ACAP and the genes expression among the studied stages, individuals from the premetamorphosis and prometamorphosis showed higher GST activity than ones under the climax. LPO levels were highest in individuals from the metamorphic climax. The present study suggests that the oxidative status changes during ontogeny of P. ephippifer tadpoles, especially during the metamorphic climax, the most demanding developmental phase. Variations in the redox balance at different developmental stages may lead to a divergent response to pollution. Therefore, we recommend that studies using anuran larvae as biomonitors consider possible physiological differences during ontogeny in their respective analyses.
Similar content being viewed by others
Introduction
The susceptibility of organisms to xenobiotics and environmental factors changes over the course of their lives. This can be especially pronounced in animals with a complex life cycle such as amphibians1. Amphibians have a unique lifestyle among tetrapods, encompassing the egg, tadpole, juvenile, subadult and adult stages2,3. During the transition from aquatic to terrestrial environment an anuran individual undergoes a biphasic developmental process that involves a series of biochemical, physiological, morphological and behavioral changes4.
Global changes (pollution, climate change, pathogens) are thought to be a major cause of the amphibians populations decline5, and has led to an increasing interest in the use of different amphibian species as bioindicators in ecotoxicological studies6,7,8,9. In this context, the use of amphibians in laboratory studies is important for understanding their basal physiology, and thus, supports the conscious choice of these organisms as environmental sentinels.
Amphibian ontogeny is a continuous and dynamic process that involves modifications in hormone levels (corticosterone and thyroid hormone)10, growth rate, cell proliferation and tissue specialization11,12. It can be divided into four well-defined periods: premetamorphosis, prometamorphosis, metamorphic climax and post-metamorphosis13,14. Each period is associated with different physiological requirements (metabolic rate and energy consumption), which can affect the oxidative status of individuals2,3,15,16.
Over the last three decades, a considerable number of studies have investigated the effects of pollutants on oxidative stress parameters in amphibians either in their premetamorphosis or post-metamorphic stages17. However, beyond the simple laboratory-based tools used to assess the effects of xenobiotics on amphibians, there is a need to obtain realistic results and to take into account the influence of potentially confounding factors (sex, developmental stage and time of sampling)18.
Stressors can accelerate the developmental process in larvae19,20, and with subacute or chronic exposure this can lead to a discrepancy between the ontogenetic stages of control and treated animals. Therefore, studies characterizing the physiological conditions of organisms in conjunction with natural variations of parameters are essential for future analyzes of impacts arising from human activities21,22.
Among the most used tools to environmental analysis, biomarkers stands out, which are defined as changes in structures or natural biochemical, molecular, cellular and physiological functions of an organism, resulting from exposure to environmental stressors or natural sources of stress23. Biomarkers allow the early diagnosis of possible impacts at low levels of biological organization, to try to avoid or mitigate possible damage at higher levels of organization, such as at the population level24.
Biochemical biomarkers can be classified as exposure, such as Total antioxidant capacity and Glutathione S-transferase, which indicate whether there has been contact with a stressor, and effect biomarkers, such as lipid peroxidation, which shows deleterious effects on molecules25. In turn, gene-expression biomarkers measure the amount of messenger RNA (mRNA) codified by genes related to oxidative stress and can provide information on how mechanisms related to synthesis and stability of RNA can be affected by stressors26.
The use of the species Physalaemus ephippifer (Steindachner, 1864) as a model for ecotoxicological studies in the Amazon comes from its wide distribution in Brazil27. P. ephippifer is also of great trophic importance in all life stages28,29. According to its ecological importance, it has characteristics that facilitate its taxonomic identification, field sampling and maintenance under laboratory conditions. The most part of the studies elucidates responses to exposure to environmental stressors7,9,30,31,32,33, generally using larvae at stage 25 Gosner Stages (GS)34, indicating a lack of analysis at later developmental stages.
To date, there are no studies analyzing the parameters of biochemical markers in P. ephippifer as a model species during its ontogeny. Moreover, little is known about the basal physiology of this species and how its biochemical responses related to the antioxidant system are modulated throughout ontogeny. Therefore, the aim of this work is to analyze the modulation of the antioxidant defense system and oxidative damage, parameters that are frequently used as biomarkers, at the different stages of anuran development.
Methods
Collection and acclimatization under laboratory conditions
P. ephippifer is a terrestrial anuran species that reproduces mainly during the rainy season and is found in temporary pools at forest edges35, in addition, around 410 eggs are deposited in foam nests in the vegetation of ponds36, making them easy to detect during collection. Thus, three nests were collected in different temporary pools in forest edge areas in the Gunma Ecological Park located in the municipality of Santa Bárbara, Pará (Fig. 1, 01º13′00.86''S and 48º17′41.18''W) in the early morning hours of the same day.
The nests were kept in containers with water from the puddles to prevent the eggs from drying out and transported to the laboratory. The nests were acclimatized for two days until hatching in the same container, with the aim of mixing larvae from different nests to maintain variability during the study. Acclimatization was carried out in plastic containers (36.5 × 23.5 × 7 cm) with a volume of 6 L, containing filtered water, constant aeration, in which the temperature (28 °C), pH (7.0), photoperiod (14/10 h) were controlled throughout the study. After hatching, the larvae were separated into four plastic containers under the same acclimatization conditions to avoid a high number of individuals in each container (120 individuals), and at each stage, larvae were collected randomly between containers. The animal collections were approved by the Biodiversity Authorization and Information System under protocol number 53433-1.
Experimental design
The tadpoles were fed once a day with commercial fish food (Novo Bits JBL—43% protein, 6% fat, 4% ether extract, 4% crude fiber, 10% mineral salts, 1.5% calcium) (0.250 mg), whereby food accumulation was avoided. The water was changed every second day, with removal of feces and possible food residues.
Larvae in three developmental stages were selected for the experiment: in premetamorphosis individuals between 28 and 29 Gosner stage (GS), in prometamorphosis between 38 and 39 GS and for the metamorphic climax all stages from 42 to 46.
The stages were determined on the basis of morphological parameters according to the Gosner table34 using a binocular stereomicroscope (model NSZ-606). The animals were then cryoanesthetized. No chemical reagent was used for anesthesia to avoid interference in oxidative stress parameters that are the focus of the study. All procedures were performed in accordance with the Brazilian legislation on the scientific use of animals (Law No. 11.794; October 8, 2008) and approved by the Ethics Committee for the Use of Animals (CEUA) of the Federal University of Pará, under the protocol 6371061016. All methods are reported in accordance with ARRIVE guidelines. The weight of the tadpoles was determined using an analytical balance (g), and the individuals were subsequently stored in 1.5 ml eppendorfs in an ultra-freezer at − 80 °C for further biochemical analysis.
The number of tadpoles collected varied at different stages according to the weight of the samples in order to quantify total proteins and to standardize and dosage of biochemical biomarkers. In the premetamorphosis, samples were pooled to achieve the required weight, with a total of 10 replicates of 20 larvae each. In all subsequent stages, samples were formed from only one individual. In prometamorphosis, 25 individuals were collected, and in metamorphic climax a total of 110 individuals were collected, covering stages 42–46.
Analysis of the biochemical biomarkers
Preparation of the samples
Samples were homogenized at a ratio of 1/4 (weight/volume) with a homogenization buffer and then centrifuged at 20,000 × for 20 min at 4 °C, according to Bainy et al.37. The obtained supernatant was aliquoted and stored at − 80 °C for later analysis.
Quantification of total proteins
Total proteins were analyzed using a commercial kit (Doles Ltda, Brazil) based on the Biuret assay (0.114 M trisodium citrate, 0.21 M sodium carbonate and 0.01 M copper sulfate). Measurements were performed in a multimodal microplate reader (Victor X3, Perkin Elmer) at 550 nm. The results are expressed in milligrams of protein/mL.
Glutathione S-transferase (GST)
Glutathione S-transferase activity was determined according to the method described by Habig, et al.38. In the analysis, the formation of the conjugate of 1-chloride-2,4-dinitrobenzene (CDNB) with reduced glutathione (GSH) catalyzed by Glutathione S-transferase in the sample is monitored for 1 min at 340 nm. Readings were performed in a spectrofluorimeter (Victor X3, Perkin Elmer) with a microplate reader. The results are expressed in UGST/ mg of protein, which corresponds to the amount of enzyme required to conjugate 1 μMol of CDNB/min/mg of protein at 25 °C and pH 7.0.
Total antioxidant capacity against peroxyl radicals (ACAP)
For the determination of ACAP, the method of Amado, et al.39 was used. This method is used to measure the antioxidant defense levels of organisms (enzymatic and non-enzymatic) exposed to radicals. It consists of the addition of ABAP (2′2′-azobis-2-methylpropiamidine dihydrochloride) in microplates together with biological samples and the fluorochrome H2DCF-DA (2',7'-dichlorofluorescein). When heated to 37 °C, ABAP generates peroxyl radicals which are scavenged by the antioxidants present in the sample. If they are not scavenged, the radicals promote the oxidation of the fluorochrome, which then begins to fluoresce in the chemical form DCF (oxidized H2DCF-DA). Measurements were performed in a fluorescence microplate reader (Victor X3, Perkin 108 Elmer) for 30 min. Samples diluted to 0.5 mg of protein were used for all developmental stages. Results are expressed as the inverse of the relative area.
Lipid peroxidation (LPO)
The degree of lipid peroxidation was determined according to the protocol of Oakes and Van Der Kraak40, in which a by-product of lipid peroxidation, malondialdehyde (MDA), is quantified. The method analyzes the product formed in the reaction between MDA and thiobarbituric acid (TBA 0.8%) in an acidic environment (acetic acid, 20%) at high temperature (95 °C), forming MDA-TBA2. Butylatedhydroxytoluene (BHT) was used as an antioxidant in the samples, while 1,1,3,3-tetramethoxypropane (TMP) served as a standard. Sodium dodecyl sulfate (SDS, 8.1%) was used as a surfactant and n-butanol was used to separate the organic from the inorganic phase. Readings were performed in a fluorescence reader (515 and 553 nm for excitation and emission, respectively). The results were expressed in nM MDA/g of wet tissue, which represents the MDA lipid concentration (nM) in weight (g) of tissue.
Analysis of the gene-expression biomarkers
To evaluate transcriptional levels, the reverse transcription technique was performed followed by quantitative PCR (RT-qPCR). Total RNA was extracted using the PureLink RNA Mini Kit (Thermo Fisher Scientific, 12183018A) following the manufacturer's instructions. RNA integrity (RIN–RNA Integrity Number) was assessed using the NanoVue spectrophotometer and 1% agarose gel. RNA was treated with DNase I (Thermo Fisher Scientific, EN0521) according to the manufacturer's instructions. Complementary DNA (cDNA) was synthesized using the High Capacity kit (Thermo Fisher Scientific, 4368814) following the manufacturer's instructions. PCR primers for genes related to oxidative stress (nrf2, gst, gsr, gclc) (Table 1). 1 µl of cDNA (30 ng/uL) was amplified using the RealQ Plus 2 × Master Mix Green, High ROX kit (Ampliqon, A323402) and 400 nM of each primer in a final volume of 20 uL. The cycling conditions were: 10 min at 95 °, 40 cycles of denaturation at 95 °C for 15 s and annealing and extension at 60 °C for 1 min. Expression levels were detected on the Bio-Rad CFX Maestro system. Actg gene transcriptional levels were used as a reference. The dissociation curve was evaluated to confirm specific amplification. Data were normalized using the Q-Gene program41,42.
Data analysis
A unidirectional analysis of covariance (ANCOVA) was performed to verify the influence of weight (covariate) on the response of the analyzed biomarkers (Glutathione S-transferase, Total antioxidant capacity and Lipid peroxidation). The independent variable corresponds to the stages of P. ephippifer and had three levels (premetamorphosis, prometamorphosis and metamorphic climax). The dependent variable was the results of the biomarkers expressed in each corresponding unit.
Initially, the assumption of homogeneity of the regression slope between the covariate and the variable was tested in all parameters. Subsequently, the normality of the data was tested using the Shapiro–Wilk test. If necessary, a Box-Cox transformation was applied to the data in order to meet normality assumptions. In addition, a Levene test was performed to check the homogeneity of the residual variances.
Differences in the responses of oxidative stress parameters (Glutathione S-transferase, Total antioxidant capacity and Lipid peroxidation) and genes expression were tested using a one-way analysis of variance (one-way ANOVA) followed by a post-hoc Tukey test. The data were analyzed in the program R, version 4.1.143 and for graphics with the package ggplot2.
For the comparison between the climax stages (42, 43, 44, 45, 46 GS), the assumptions of normality and homoscedasticity were tested using the Shapiro-Wilks and Levene tests. For Glutathione S-transferase and Lipid peroxidation data (parametric), a one-way ANOVA followed by a Tukey's post-hoc test was used for pairwise comparisons of means. Results were expressed as mean ± standard error. The significance level was set as p < 0.05. Total antioxidant capacity (non-parametric), analyzes were performed using the Kruskal–Wallis test followed by the Nemenyi post-hoc test and expressed as median ± 1st quartile. The assumed significance level was 5% in all cases44.
Results
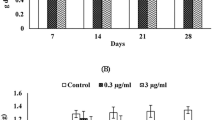
No statistically significant differences were observed between the groups regarding the expression level of the genes nfr2, gst, gsr and gclc (Fig. 2).
Genes of oxidative stress expression in Physalaemus ephippifer at different stages of larval development. (a) nfr2 expression. (b) gst expression. (c) gsr expression. (d) gclc expression. The absence of letters means that there are no statistical differences (p > 0.05). Different letters indicate statistical differences (p < 0.05). The values are given as mean ± standard error.
A unidirectional analysis of covariance (ANCOVA) was performed to verify the influence of weight (covariate) on the response of the biochemical biomarkers analyzed. The results of the biomarkers responses between the developmental stages of the species indicate that there was a non-significant effect of the initial covariate in the post-test (ACAP: F [1, 126] = 0.071, p = 0.791; GST: F [1, 126] = 0.071, p = 0.791; LPO: F [1, 136] = 1.773, p = 0.185). Although it was not significant, it was decided to control this variable in order to refine the analysis.For Total antioxidant capacity, there was no statistically significant effect on the developmental period of P. ephippifer (Table 2, Fig. 3a).
Biomarkers of oxidative stress in Physalaemus ephippifer at different stages of larval development. (a) Total antioxidant capacity. The absence of letters means that there are no statistical differences (p > 0.05), (premetamorphosis: n = 9; prometamorphosis: n = 13; metamorphic climax: n = 40). (b) Glutathione S-transferase activity, (premetamorphosis: n = 9; prometamorphosis: n = 21; metamorphic climax: n = 100). (c) Lipid peroxidation levels, (premetamorphosis: n = 9; prometamorphosis: n = 22; metamorphic climax: n = 109). Different letters indicate statistical differences (p < 0.05). The values are given as mean ± standard error.
The results for Glutathione S-transferase showed a statistically significant effect of developmental stage (Table 2). The Tukey post-hoc test also showed that there were significant differences between Glutathione S-transferase responses in the premetamorphosis and prometamorphosis stages compared to those in the metamorphic climax (p < 0.001). Individuals from the metamorphic climax had lower Glutathione S-transferase activity. No significant differences were found between individuals from the premetamorphosis and prometamorphosis stages (p = 0.790) (Fig. 3b).
ANOVA analyzes revealed significant effects on Lipid peroxidation depending on the stage of amphibian development (Table 2). The Tukey's post-hoc test indicated that there were no significant differences in the Lipid peroxidation values of tadpoles from prometamorphosis in relation to those from premetamorphosis and metamorphic climax. However, a significant difference was found between premetamorphosis and climax (Fig. 3c).
The values for Total antioxidant capacity, Glutathione S-transferase and Lipid peroxidation in individuals in the metamorphic climax are shown in Fig. 4a–c. Results did not reveal any significant differences between individuals at different stages of the metamorphic climax (42–46) for each analyzed oxidative stress parameter. Therefore, during further comparison among developmental periods (premetamorphic, prometamorphic and metamorphic climax), we grouped all stages (42–46 Gosner Stages) under the period of metamorphic climax.
Biomarkers of oxidative stress in Physalaemus ephippifer between the metamorphic climax stages from 42 to 46 Gosner stages. (a) Total antioxidant capacity (n = 5–12 per stage); (b) Glutathione S-transferase (n = 14–24 per stage); (c) levels of lipid peroxidation (n = 14–24 per stage). The absence of letters means that there are no statistical differences (n = 16–24 per stage). Total antioxidant capacity values are given as median and 1st quartile, Glutathione S-transferase and Lipid peroxidation as mean and standard error.
Discussion
This study investigated how the redox balance in the amazon population of P. ephippifer changes during three larval periods. Regarding the genetic expression is important to highlight that nrf2 is a transcription factor related to the cell's defense mechanisms against the effects of oxidative stress45, driving the regulation of antioxidant genes46 such as the gst, gsr (glutathione- disulfide reductase) and gclc (gcl catalytic subunit) genes. The absence of difference in the expression results of these genes suggests that the regulation is post-translational, in other words, the regulations occur in protein turnover47 involved in the antioxidant defense process. This becomes evident when we analyze the biochemical parameters where components of the antioxidant system and oxidative damage were examined to determine the oxidative status, focusing on parameters represented in most ecotoxicological studies. From the battery of antioxidant parameters, we determined the Total antioxidant capacity values, which represent the general antioxidant status of the organism. Glutathione S-transferase as an enzymatic component of the glutathione system and the main component of biotransformation phase II, which is involved in both detoxification and metabolic activation of xenobiotics. Finally, Lipid peroxidation as a marker for oxidative damage of lipids caused by a variety of free radicals and often triggered by the metabolism of xenobiotics48. Anuran larvae have different physiological needs during their development, which may affect their antioxidant response and the levels of oxidative damage. In their early stages, premetamorphosis these needs are mainly focused on survival and growth3,15,16,19. Although these processes require high energy expenditure and consequently may increase the formation of reactive oxygen species, we found lower Lipid peroxidation levels in P. ephippifer compared to the metamorphic climax. This can be corroborated by the higher Glutathione S-transferase activity, a parameter involved in the reduction of oxidative lipid damage (lipid hydroperoxides)48. Higher Glutathione S-transferase activity at earlier life stages may also be a mechanism for coping with exposure to external factors and xenobiotics that tadpoles encounter for the first time in their natural environment after hatching3. Enhanced antioxidant defenses (catalase and superoxide dismutase) and low Lipid peroxidation levels at early developmental stages compared to later ones was also observed in Xenopus laevis49.
Tadpoles undergoing prometamorphosis experience intense growth and increase in thyroid hormones and corticosterone as they approach their climax20. Changes in hormone status can be reflected on reactive oxygen species (ROS) levels and oxidative stress. Our results suggest that the level of oxidative damage in prometamorphic individuals is intermediate between the two sides of larval development (premetamorphic and metamorphic climax). Glutathione S-transferase activity remained similar to the premetamorphic stage, but was higher compared to climax. These data indicate that the increasing complexities of the organism and the changing developmental requirements have a slight effect on oxidative stress parameters in tadpoles in prometamorphosis.
It is noticed that the progressive increase in complexity throughout the development of the tadpoles was reflected in the increase in oxidative damage in the metamorphic climax. It has been suggested that ROS can play an important role in the tissue remodulation required for the transition of organisms from aquatic to terrestrial environments16,49,50. Some studies showed changes in redox balance during climax3,15,50.
In P. ephippifer, there was an increase in Lipid peroxidation during metamorphic climax compared to premetamorphosis, and a decrease in Glutathione S-transferase activity compared to the two previous stages. Higher ROS formation, which is not accompanied by Glutathione S-transferase activity, can lead to an increase in lipid damage and oxidative stress. Climax is also characterized by high levels of thyroid hormones, which can increase the metabolic rate51, along with an increase in corticosterone52.
An assessment of antioxidant responses between stages during climax was carried out to analyze whether there are differences between them, considering the changes that take place between 42 and 46 Gosner stages (lung development, changes in the pancreas, kidney, intestine, and tail regression)34. However, it was observed that there was no difference between the stages for any of the biomarkers analyzed. The significance of this result arises from the representativeness of this stage for biomonitoring studies. On the one hand, the responses of individuals in the climax can be represented in each of these Gosner stages, which would facilitate the logistics of field studies, since from this perspective it is not necessary to identify the most specific stages within the metamorphic climax. On the other hand, however, all internal changes that must take place during this sensitive life phase may limit the ability of organisms to respond to external factors.
In the literature, majority of studies in which anuran larvae are used as biomonitors are limited to acute exposure and establish the lethal and sublethal dose for various pollutants7,30,31,32,33. In addition, exposure occurs in premetamorphosis (usually at GS25). Few studies using biochemical biomarkers have been conducted in the other developmental stages of anurans, and none with anurans of the family Leptodactylidae. This suggests that in toxicity studies, concentrations of certain compounds that do not cause biochemical changes or oxidative stress in premetamorphosis, can lead to greater oxidative damage in anurans just prior to metamorphosis, when they normally have lower baseline antioxidant defenses, as shown in this study.
The results presented here suggest that the premetamorphosis stage is physiologically different from the other stages. Larvae in this stage have a significant level of antioxidant defenses that can keep up with metabolic demands, and it has greater responsiveness to stressors, as highlighted by Glennemeier and Denver53. This may differ in the subsequent stages, especially in the climax when the defenses naturally decline and oxidative damage occurs, as also described by Prokić et al.3. Finally, biomonitoring studies using anurans should take into account the ontogenetic differences in responses associated with the presence of environmental stressors, thus ensuring that toxicity parameters correspond to the physiological specificities of each developmental stage of anurans.
Conclusion
Anuran larvae exhibit remarkable characteristics during their developmental stages and it is important to understand how physiological parameters are modulated. The present study shows that the antioxidant response changes differently in the different developmental stages of Physalaemus ephippifer tadpoles. Moreover, the metamorphic climax was the most sensitive stage from a physiological point of view, as there was a decrease in antioxidant defenses and an increase in lipid peroxidation levels. Studies using anuran larvae as biomonitors of environmental quality should include analysis that account for differences between developmental stages. Further studies should also include other oxidative stress parameters to obtain a complete picture of the redox balance during ontogeny.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Lowe, W. H., Martin, T. E., Skelly, D. K. & Woods, H. A. Metamorphosis in an era of increasing climate variability. Trends Ecol. Evol. 36, 360–375. https://doi.org/10.1016/j.tree.2020.11.012 (2021).
Burraco, P., Rendón, M. A., Díaz-Paniagua, C. & Gomez-Mestre, I. Maintenance of phenotypic plasticity is linked to oxidative stress in spadefoot toad larvae. Oikos 2022, e09078. https://doi.org/10.1111/oik.09078 (2022).
Prokić, M. D. et al. Oxidative stress in Pelophylax esculentus complex frogs in the wild during transition from aquatic to terrestrial life. CBPA 234, 98–105. https://doi.org/10.1016/j.cbpa.2019.05.004 (2019).
Elinson, R. P. & del Pino, E. M. Developmental diversity of amphibians. Rev. Dev. Biol. 1, 345–369. https://doi.org/10.1002/wdev.23 (2012).
Pounds, J. A. Climate and amphibian declines. Nature 410, 639–640. https://doi.org/10.1038/35070683 (2001).
Yologlu, E. & Ozmen, M. Low concentrations of metal mixture exposures have adverse effects on selected biomarkers of Xenopus laevis tadpoles. Aquat. Toxicol. 168, 19–27. https://doi.org/10.1016/j.aquatox.2015.09.006 (2015).
Fanali, L. Z., Franco-Belussi, L., Bonini-Domingos, C. R. & de Oliveira, C. Effects of benzo[a]pyrene on the blood and liver of Physalaemus cuvieri and Leptodactylus fuscus (Anura: Leptodactylidae). Environ. Pollut. 237, 93–102. https://doi.org/10.1016/j.envpol.2018.02.030 (2018).
Shi, Q., Sun, N., Kou, H., Wang, H. & Zhao, H. Chronic effects of mercury on Bufo gargarizans larvae: Thyroid disruption, liver damage, oxidative stress and lipid metabolism disorder. Ecotoxicol. Environ. Saf. 164, 500–509. https://doi.org/10.1016/j.ecoenv.2018.08.058 (2018).
Freitas, J. S., Felício, A. A., Teresa, F. B. & Alves de Almeida, E. Combined effects of temperature and clomazone (Gamit®) on oxidative stress responses and B-esterase activity of Physalaemus nattereri (Leiuperidae) and Rhinella schneideri (Bufonidae). Chemosphere 185, 548–562. https://doi.org/10.1016/j.chemosphere.2017.07.061 (2017).
Cruz, J. C. & Fabrezi, M. Histology and microscopic anatomy of the thyroid gland during the larval development of Pseudis platensis (Anura, Hylidae). J. Morphol. 281, 122–134. https://doi.org/10.1002/jmor.21085 (2020).
Shi, Y. B., Wong, J., Puzianowska-Kuznicka, M. & Stolow, M. A. Tadpole competence and tissue-specific temporal regulation of amphibian metamorphosis: Roles of thyroid hormone and its receptors. Bioessays 18, 391–399 (1996).
Handrigan, G. R. & Wassersug, R. J. The anuran Bauplan: A review of the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol. Rev. 82, 1–25. https://doi.org/10.1111/j.1469-185X.2006.00001.x (2007).
Dodd, M. H. I. & Dodd, J. M. in Physiology of the amphibian Vol. 3 (ed B. Lofts) (Academic Press, 1976).
Etkin, W. Metamorphosis: A Problem in Development Biology (Appleton Century Crofts, 1968).
Petrović, T. G. et al. Effects of desiccation on metamorphic climax in bombina variegata: Changes in levels and patterns of oxidative stress parameters. Animals 11, 953 (2021).
Menon, J. & Rozman, R. Oxidative stress, tissue remodeling and regression during amphibian metamorphosis. CBPC 145, 625–631. https://doi.org/10.1016/j.cbpc.2007.02.011 (2007).
Park, J. K. & Do, Y. Current state of conservation physiology for amphibians: Major research topics and physiological parameters. Animals https://doi.org/10.3390/ani13203162 (2023).
Sparling, D. W., Linder, G., Bishop, C. A. & Krest, S. Ecotoxicology of Amphibians and Reptiles (CRC Press, 2010).
Burraco, P., Díaz-Paniagua, C. & Gomez-Mestre, I. Different effects of accelerated development and enhanced growth on oxidative stress and telomere shortening in amphibian larvae. Sci. Rep. 7, 7494. https://doi.org/10.1038/s41598-017-07201-z (2017).
Ruthsatz, K. et al. Altered thyroid hormone levels affect the capacity for temperature-induced developmental plasticity in larvae of Rana temporaria and Xenopus laevis. J. Therm. Biol. 90, 102599. https://doi.org/10.1016/j.jtherbio.2020.102599 (2020).
Cooke, S. J. et al. What is conservation physiology? Perspectives on an increasingly integrated and essential science†. Conserv. Physiol. https://doi.org/10.1093/conphys/cot001 (2013).
Depledge, M. H., Aagaard, A. & Györkös, P. Assessment of trace metal toxicity using molecular, physiological and behavioural biomarkers. Mar. Pollut. Bull. 31, 19–27. https://doi.org/10.1016/0025-326X(95)00006-9 (1995).
Lam, P. K. S. & Gray, J. S. The use of biomarkers in environmental monitoring programmes. Mar. Pollut. Bull. 46, 182–186. https://doi.org/10.1016/S0025-326X(02)00449-6 (2003).
Monserrat, J. M. et al. Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 146, 221–234. https://doi.org/10.1016/j.cbpc.2006.08.012 (2007).
Sogorb, M. A., Estévez, J. & Vilanova, E. in Biomarkers in Toxicology (ed Ramesh C. Gupta) 965–973 (Academic Press, 2014).
Piña, B., Casado, M. & Quirós, L. Analysis of gene expression as a new tool in ecotoxicology and environmental monitoring. Trends Analyt Chem. 26, 1145–1154. https://doi.org/10.1016/j.trac.2007.09.009 (2007).
Frost, D. R. (American Museum of Natural History, New York, 2023).
Bionda, C. et al. Diet of tadpoles of Physalaemus biligonigerus (Leiuperidae) from agricultural ponds in the central region of Argentina. Acta Herpetol. 8, 141–146. https://doi.org/10.13128/Acta_Herpetol-11433 (2013).
Rodrigues, L. C. & dos Santos-Costa, M. C. Trophic ecology of Physalaemus ephippifer (Anura, Leptodactylidae) in Eastern Amazonia. J. Herpetol. 48, 532–536 (2014).
Herek, J. S. et al. Genotoxic effects of glyphosate on Physalaemus tadpoles. Environ. Toxicol. Pharmacol. 81, 103516. https://doi.org/10.1016/j.etap.2020.103516 (2021).
Macagnan, N. et al. Toxicity of cypermethrin and deltamethrin insecticides on embryos and larvae of Physalaemus gracilis (Anura: Leptodactylidae). ESPR 24, 20699–20704. https://doi.org/10.1007/s11356-017-9727-5 (2017).
Rutkoski, C. F. et al. Morphological and biochemical traits and mortality in Physalaemus gracilis (Anura: Leptodactylidae) tadpoles exposed to the insecticide chlorpyrifos. Chemosphere 250, 126162. https://doi.org/10.1016/j.chemosphere.2020.126162 (2020).
Rutkoski, C. F. et al. Cypermethrin- and fipronil-based insecticides cause biochemical changes in Physalaemus gracilis tadpoles. ESPR 28, 4377–4387. https://doi.org/10.1007/s11356-020-10798-w (2021).
Gosner, K. L. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 (1960).
Rodrigues, L. C., Correa, F. S., Juen, L. & dos Santos-Costa, M. C. Effects of pond structural complexity on the reproduction of Physalaemus ephippifer (Anura, Leptodactylidae). Anim. Biol. 68, 405–415. https://doi.org/10.1163/15707563-17000152 (2018).
Hödl, W. An analysis of foam nest construction in the neotropical frog Physalaemus ephippifer (Leptodactylidae). Copeia 547–554, 1990. https://doi.org/10.2307/1446358 (1990).
Bainy, A. C. D., Saito, E., Carvalho, P. S. M. & Junqueira, V. B. C. Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat. Toxicol. 34, 151–162. https://doi.org/10.1016/0166-445X(95)00036-4 (1996).
Habig, W. H., Pabst, M. J. & Jakoby, W. B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8 (1974).
Amado, L. L. et al. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci. Total Environ. 407, 2115–2123. https://doi.org/10.1016/j.scitotenv.2008.11.038 (2009).
Oakes, K. D. & Van Der Kraak, G. J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 63, 447–463. https://doi.org/10.1016/S0166-445X(02)00204-7 (2003).
Muller, P. Y., Janovjak, H., Miserez, A. R. & Dobbie, Z. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques 32, 1372–1379 (2002).
Simon, P. Q-Gene: Processing quantitative real-time RT-PCR data. Bioinformatics (Oxford, England) 19, 1439–1440. https://doi.org/10.1093/bioinformatics/btg157 (2003).
R Core Team. (R Foundation for Statistical Computing, Vienna, 2023).
Zar, J. H. Biostatistical Analysis. 5th Edition edn, (Prentice Hall, 2010).
Clarke, J. L., Murray, J. B., Park, B. K. & Copple, I. M. Roles of Nrf2 in drug and chemical toxicity. Curr. Opin. Toxicol. 1, 104–110. https://doi.org/10.1016/j.cotox.2016.10.004 (2016).
Saha, S., Buttari, B., Panieri, E., Profumo, E. & Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules (Basel, Switzerland) https://doi.org/10.3390/molecules25225474 (2020).
Huang, D., Kosentka, P. Z. & Liu, W. Synthetic biology approaches in regulation of targeted gene expression. Curr. Opin. Plant Boil. 63, 102036. https://doi.org/10.1016/j.pbi.2021.102036 (2021).
Halliwell, B. & Gutteridge, J. M. Free Radicals in Biology and Medicine (Free Radicals in Biology and Medicine, 2015).
Rizzo, A. M., Adorni, L., Montorfano, G., Rossi, F. & Berra, B. Antioxidant metabolism of Xenopus laevis embryos during the first days of development. CBPB 146, 94–100. https://doi.org/10.1016/j.cbpb.2006.09.009 (2007).
Mahapatra, P. K., Mohanty-Hejmadi, P. & Chainy, G. B. N. Changes in oxidative stress parameters and acid phosphatase activity in the pre-regressing and regressing tail of Indian jumping frog Polypedates maculatus (Anura, Rhacophoridae). CBPC 130, 281–288. https://doi.org/10.1016/S1532-0456(01)00238-1 (2001).
van der Spek, A. H., Fliers, E. & Boelen, A. The classic pathways of thyroid hormone metabolism. Mol. Cell. Endocrinol. 458, 29–38. https://doi.org/10.1016/j.mce.2017.01.025 (2017).
Kulkarni, S. S., Denver, R. J., Gomez-Mestre, I. & Buchholz, D. R. Genetic accommodation via modified endocrine signalling explains phenotypic divergence among spadefoot toad species. Nat. Commun. 8, 993. https://doi.org/10.1038/s41467-017-00996-5 (2017).
Glennemeier, K. A. & Denver, R. J. Developmental changes in interrenal responsiveness in anuran amphibians. Integr. Comp. Biol. 42, 565–573. https://doi.org/10.1093/icb/42.3.565 (2002).
Acknowledgements
We thank Flávia Lopes for the translation of the manuscript and Johnata Azevedo for the design of the map. Lílian Lund Amado is a grantee of the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Process No. 309628/2022-9. We are also grateful to the UFPA Dean’s Office for Research and Postgraduate Studies, PROPESP (Pró Reitoria de Pesquisa e Pós-Graduação), which supported the study through the Qualified Publication Support Programme, PAPQ (Programa de Apoio à Publicação Qualificada).
Author information
Authors and Affiliations
Contributions
J.P.P.M., C.C.M.S., A.L.C, C.M., V.R.L.O.B. and L.L.A. made substantial contribution to the concept or design of the article; the acquisition, analysis, and interpretation of data for the article J.P.P.M., C.C.M.S., J.P.M.Q., R.A.C., M.L.C.S.F., A.R.N. and S.N.L. drafted the article, prepared figures and made analysis and interpretation of data for the article M.D.P, T.G.P, L.L.A, and V.R.L.O.B revised it critically for important intellectual content. All authours reviewed the manuscript and approved the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Monteiro, J.P.P., dos Santos, C.C.M., de Queiroz, J.P.M. et al. Natural modulation of redox status throughout the ontogeny of Amazon frog Physalaemus ephippifer (Anura, Leptodactylidae). Sci Rep 14, 20655 (2024). https://doi.org/10.1038/s41598-024-71022-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71022-0