Abstract
This study was conducted to identify the characteristics and risk factors for early death in critically ill acute promyelocytic leukaemia (APL) patients in the Hemato-oncology ICU (HICU). A total of 44 APL patients from 2017 to 2023 were included. The mortality among APL patients in the HICU was high (27/44, 61.36%). Compared with patients who survived, nonsurvivors had a longer prothrombin time (P = 0.002), lower fibrinogen (P = 0.022), higher white blood cell count (P = 0.004) and higher creatinine (P = 0.037) on hosipital admission. Severe bleeding was the most frequent complication (34 cases, 77.27%), which occurred either preinduction or on Day 5 (IQR 3–7.5 days) of induction. Cerebral bleeding associated with consciousness disturbance was the main reason for HICU admission (18 cases, 40.9%). The leading cause of death was fatal haemorrhage (18/34, 52.94%), which occurred either preinduction or on Day 4 (IQR 3–7 days) of induction. Another common cause of death was sepsis (8/18, 44.44%), which occurred on Day 12 (IQR 9.5–24.75 days) during induction. In conclusion, the main cause of death in APL patients treated in the HICU was primary being attributed to fatal bleeding, followed by sepsis.
Similar content being viewed by others
Introduction
Acute Promyelocytic Leukaemia (APL) is a special subtype of acute myeloid leukaemia that is characterized by chromosomal translocation (t15; 17) (q22; q21), resulting in the production of the PML-RARAα fusion protein in 95% of cases, which prevents both apoptosis and differentiation at the promyelocyte stage1.
The remarkable complete remission (CR) rates reported in more than 90% of APL patients and the improved long-term survival of APL patients are largely attributable to the introduction of arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) in induced differentiation therapy2,3. However, in APL cohorts, early death (ED), defined as death between 0 and 30 days after diagnosis, continues to be significant, accounting for 8.2–32.6% of the total mortality4,5,6.
APL frequently involves severe complications such as differentiation syndrome (DS), bleeding, and infection, among others, before, during or after induction. ED due to severe complications of initial APL continues to be a major contributor to treatment failure7,8,9. The presenting coagulopathy is often linked to ED for APL, Most typically, complications associated with the condition, like bleeding, cause deaths within the first week of hospital admission. In contrast, severe infection might also be the cause of fatalities that occur later than that of bleeding10. In addition, venous and arterial thrombosis occurs in up to 20% of APL patients, and frequent thrombotic events include deep vein thrombosis (DVT), pulmonary embolism, myocardial infarction and ischaemic cerebrovascular events. However, thrombotic complications rarely result in death11,12,13.
Frequently, severe complications result in admission to the HICU, and aggressive supportive measures for severe complications are essential for improving CR and long-term survival. Induction differentiation treatment is frequently administered concurrently to APL patients in the HICU. Induction-related DS, leucocytosis, respiratory failure, aggravated haemorrhage and secondary infection worsen the patient’s state. Therefore, the severe complications and causes of death of APL patients in the HICU have certain characteristics14.
Consequently, it is crucial for clinicians, especially those working in the ICU, to be knowledgeable about the special complications and management concerns faced by critically ill APL patients. Furthermore, understanding of the factors affecting lethality during induction is essential for critical care providers.
Results
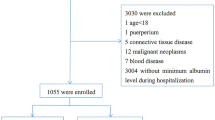
In this study, a total of 52 APL patients were screened in the HICU (approximately 15% of all APL patients during the study period). However, 8 patients were excluded: 1 patient with chronic renal failure who received regular dialysis and was admitted to the HICU, 4 patients who declined therapy, and 3 patients who did not receive ATO treatment. Overall, 44 patients were included in this study (Fig. 1).
Twenty-seven (61.36%) of the 44 cases in total died, and 17 (38.63%) cases survived. Among the 44 patients, 28 (63.63%) were female, and 16 (36.36%) were male. There was no significant difference in the lethality between female and male patients (Table 1).
Table 1 shows the clinical data of the patients, including age, underlying disease, creatinine, prothrombin time (PT), fibrinogen (FIB), D-dimer (DD), white blood cell count (WBC), Haemoglobin (HGB) and Platelet (PLT), as well as details about the APL induction scheme, Sanz score for risk stratification of patients undergoing induction treatment and a comparison of continuous renal replacement therapy (CRRT) and mechanical ventilation during HICU treatment. Compared with patients who survived, nonsurvivors had a longer PT (P = 0.002), a lower FIB (P = 0.022), a higher WBC (P = 0.004) and a higher creatinine (P = 0.037) upon hospital admission. Three patients passed away before induction, and the remaining 41 patients received ATO-based induction, 24 (24/41, 58.53%) of whom died during induction. Compared with ATO alone, ATO plus Chemotherapy (CMT) resulted in a higher lethality (14/17, 82.35% vs. 10/24, 41.66%, P = 0.012). The median time of induction for nonsurvivors was 7 days (IQR 4–32 days), which was lower than that for survivors (31 days, IQR 28–32 days). There were 7 (7/24, 29.16%) high-risk patients in the ATO group, and 13 (13/17, 76.47%) high-risk patients in the ATO + CMT group. No significant difference was found between the surviving and nonsurviving patients in terms of the risk stratification based on Sanz score, in both ATO group and ATO + CMT group.
The median HICU treatment duration for all patients was 2.5 days (IQR 1–7 days), and neither the underlying disease nor the HICU treatment duration significantly differed between the nonsurvivors and survivors. Compared with survivors, most nonsurvivors received mechanical ventilation treatment due to respiratory failure (20/27, 74% vs. 0/17, 0%, P < 0.001) (Table 1).
Temporal distribution of severe complications in APL patients
In this study, the most prevalent severe complication was severe bleeding, which occurred in 34 patients (77.27%) during their hospital stay. The median duration of severe bleeding during induction was 5 days (IQR 3–7.5 days). Cerebral bleeding was the most common type of severe bleeding (20 cases, 45.45%), occurring before induction (13 cases, 29.45%) and during the first week of induction (7 cases, 15.9%). The median time of cerebral bleeding during induction was 4 days (IQR 3–5 days). The second most common type of severe bleeding was gastrointestinal bleeding (17 cases, 38.63%), which occurred before induction (9 cases, 20.45%) and during the first week (6 cases, 13.63%), second week (1 case, 2.27%) and third week (1 case, 2.27%) of induction. The median duration of gastrointestinal bleeding during induction was 4 days (IQR 3–7.75 days). The third most common type of severe bleeding was pulmonary bleeding (12 cases, 27.27%), which occurred before induction (6 cases, 13.63%), and during the first week (3 cases, 6.81%) and second week of induction (3 cases, 6.81%). The median time of pulmonary bleeding during induction was 7 days (IQR 5–11.5 days), and there were no additional cases of severe bleeding postinduction. In this study, 5 patients continued anti-infection therapy, and the remaining 12 patients were discharged after induction therapy.
Sepsis is another prevalent severe complication of APL patients in HICU. Eighteen patients (40.90%) developed sepsis which occurred before induction (2 patients, 4.54%) and during the first week (4 patients, 9.09%), second week (9 patients, 20.45%), third week (2 patients, 4.54%), and fourth week (1 patient, 2.27%) of induction. Sepsis developed later than severe bleeding (P = 0.011), and the median duration of sepsis during induction was 8 days (IQR 7.25–11.75 days). There were no additional cases of sepsis postinduction.
Severe DS development was common during induction (8 cases, 18.18%) and during the first week (2 cases, 4.54%), second week (4 cases, 9.09%) and third week (2 cases, 4.54%). The median duration of severe DS during induction was 12 days (IQR 7.25–17 days).
There were few cases of severe thrombotic complications, with one case each of pulmonary embolism, multiple cerebral infarctions, and lower extremity deep venous thrombosis preinduction. An additional 2 patients (4.54%) experienced acute renal insufficiency before induction and on Day 8 of induction respectively, and 1 patient (2.27%) experienced ventricular tachycardia on Day 23 of induction (Fig. 2 and Table 2).
In summary, severe bleeding was the most common complication in the early stages of APL. After two weeks of induction, the incidence of severe bleeding decreased gradually, whereas the incidence of sepsis and severe DS increased. Severe complications became much less common after the third week of induction.
Reasons for HICU admission
In this study, cerebral bleeding associated with conscious disturbance was the most common reason for HICU admission (18 patients, 40.9%). Among these patients, 11 (25%) were admitted to the HICU before induction, and the remaining 7 (15.9%) were admitted during the first week of induction. For cerebral bleeding with conscious disturbance during induction, the median time of HICU admission was 3 days (IQR 3–5 days). Two patients (4.54%) entered the HICU due to subarachnoid bleeding complicated with serious headache preinduction.
Thirteen patients (29.54%) were admitted to the HICU for respiratory failure. Among these patients, 4 (9.09%) were complicated by respiratory failure before induction due to pulmonary bleeding (3 patients) and pulmonary embolism (1 patient); another 9 (20.45%) experienced respiratory failure during induction due to pulmonary bleeding (4 patients), serious DS (1 patient) and pulmonary infection (4 patients). The median time of HICU admission for respiratory failure during induction was 8 days (IQR 7–12 days).
Seven patients (15.9%) were admitted to HICU for sepsis or septic shock. Among these patients, 2 (4.54%) experienced septic shock before induction, whereas 5 (11.36%) experienced sepsis or septic shock during induction. During induction, the median time of HICU admission for sepsis or septic shock was 8 days (IQR 7–11 days). There were no cases of sepsis postinduction.
Furthermore, one patient was admitted to the HICU because of ventricle tachycardia on Day 23 of induction, and the other patient was admitted because of gastrointestinal bleeding accompanied by hypovolemic shock on Day 7 of induction. Owing to acute renal insufficiency, one patient with anuria preinduction and another with oliguria on Day 8 of induction were admitted to the HICU for CRRT (Table 3).
Reasons for death
A total of 27 patients (61.36%) died, 3 (6.81%) died before induction and 24 (54.54%) died during induction. During induction, the median time to death was 7 days (IQR 3–11 days). Sepsis, fatal bleeding and pulmonary embolism were the causes of death. After induction treatment, no patients passed away while in the HICU.
Fatal bleeding was the leading cause of death, demonstrating the highest lethality (18/34, 52.94%), and the median time to death during induction was 4 days (IQR 3–7 days). Three patients (3/12, 25%) passed away due to pulmonary bleeding, and 15 patients (15/20, 75%) passed away due to cerebral bleeding. Among the patients who died of cerebral bleeding, 3 patients (3/20, 15%) died prior to induction, whereas 12 patients (12/20, 60%) died during the first week of induction. During induction, the median time to death from cerebral bleeding was 2 days (IQR 1–3.75 days). The median time to death due to pulmonary bleeding during induction was on Day 9 (IQR 3–12 days), and the lethality due to pulmonary bleeding (25%) was lower than that resulting from cerebral bleeding (75%). During the third week of induction and until the end of the treatment, no fatal bleeding deaths occurred.
Sepsis is another important cause of death in APL patients in the HICU. The lethality from sepsis (8/18, 44.44%) was slightly lower than that from fatal bleeding (18/34, 52.94%). Sepsis is more common in the second week of induction, and cases of sepsis death are still occasionally observed in the later period of induction. During induction, the median time to death from sepsis was 12 days (IQR 9–24.75 days).
Death due to thrombotic complications was rare, and only one patient experienced pulmonary embolism before induction and passed away on Day 7 of induction. There were no deaths due to DS in our study (Table 4) (Fig. 3).
Only 5 of the 20 patients who experienced cerebral bleeding survived, 15 patients (15/20, 75%) died. The basal ganglia region was the most frequent (11/20, 55%) and most fatal (all died) site of cerebral bleeding. Subarachnoid bleeding was also a frequent site of cerebral bleeding (7/20, 35%); 3 patients (3/20, 15%) died and 4 patients (4/20, 20%) survived. One patient (1/20, 5%) experienced a small amount of thalamic haemorrhage and recovered completely. One patient (1/20, 5%) died of multiple-site cerebral bleeding. There was a significant difference (P = 0.013) in the cerebral bleeding site between the surviving and dying patients (Table 5).
The infection sites among the 18 APL patients with sepsis were pulmonary infection (12/18, 66.66%), bloodstream infection (4/18, 22.22%) and skin and soft tissue infection (2/18, 11.11%).
Among the 18 patients with sepsis, bacteria were the most common pathogens (6/18, 33.33%), followed by fungi (1/18, 5.55%) and viruses (1/18, 5.55%). The infection site or aetiology of sepsis was not significantly different between survivors and nonsurvivors (Table 5).
Discussion
Serious complications in the early stages of APL are frequently fatal and account for the majority of deaths, making APL a particularly concerning condition. APL patients who survive the early stages of the illness have high rates of both CR and long-term survival. In the HICU, there is a special group of critically ill APL patients. The clinical features and causes of death were analysed. The results suggested that fatal bleeding was the most prevalent severe complication and the main cause of death in the early stage of APL.
Only one study published in 2018 has detailed the clinical characteristics of initial APL patients in the ICU17. The study included 18 APL patients and revealed that the most common reason for ICU admission was respiratory failure (39%, n = 7) followed by septic shock (22%, n = 4). Multiple organ failure and cerebral bleeding are the most common causes of death among APL patients.
Our study indicated that cerebral bleeding was the most significant consequence for APL patients, accounting for the majority of HICU admissions, due to its frequent presentation of consciousness alteration, coma, or severe headache. There was a high incidence of cerebral bleeding in the early stage of APL, as evidenced by the majority of cases occurring before and within the first week of induction. However, no incidence of cerebral bleeding occurred from the second week of induction, indicating that the risk of cerebral bleeding in APL patients was significantly reduced after one week of aggressive treatment, including PLT infusion, coagulation factor supplementation and symptomatic supportive treatment (Supplementary Information 1).
Pulmonary bleeding was relatively uncommon compared with cerebral bleeding. It is possible that pulmonary haemorrhage may have been underdiagnosed. Analysis of its causes revealed that pulmonary haemorrhage was diagnosed based on the symptom of haemoptysis; however, haemoptysis was not obvious in patients with minor pulmonary haemorrhage, and CT and MRI scans do not provide a confirmatory diagnosis of pulmonary hemorrhage. When haemoptysis occurred, the patients experienced relatively more pulmonary haemorrhage and were more likely to develop respiratory failure and be admitted to HICU for respiratory support.
Sepsis-related HICU admissions were also frequent; patients in this study often presented with shock or respiratory failure. Sepsis frequently occurs during the second week of induction. This is related to APL patients’ compromised immune system, the use of glucocorticoids in DS, and subtle signs of infection that can easily be missed by doctors and can lead to sepsis or septic shock.
APL patients admitted to the HICU due to thrombotic complications were rare in our study. The majority of the clinical signs of thrombotic complications are modest, or the symptoms may be masked by the presence of more significant accompanying complications, causing doctors to overlook them.
Some APL patients were treated with CRRT after admission to the HICU due to acute renal failure, which was associated with microvascular thrombosis and leukaemic cell infiltration. Only one patient was brought to the HICU because of severe DS complicated with respiratory failure, and the remaining cases of severe DS occurred during induction in the HICU.
In this study, the mortality among APL patients reached as high as 61.36%, and fatal bleeding, sepsis and pulmonary embolism were the causes of death. Fatal bleeding was the leading cause of lethality before induction and within two weeks of induction.
Intracranial bleeding, particularly in the subarachnoid region and basal ganglia, is the most frequent cause of death. Basal ganglia bleeding was the most common and fatal type of severe bleeding, and all patients with basal ganglia bleeding died with rapid deterioration of their condition. This complication is the primary reason for the high lethality. In addition, multiple cerebral bleeding and massive subarachnoid bleeding deaths were also observed. A tiny quantity of subarachnoid or thalamic haemorrhage is characteristic of survivors. These findings suggest that the location and volume of intracranial bleeding are closely related to patient prognosis. Pulmonary bleeding is a comparatively uncommon cause of death in APL patients in the HICU.
In immunocompromised APL patients, sepsis is also a common cause of death in HICU. A few patients with pulmonary or cerebral bleeding died due to serious pulmonary infection or sepsis because of an insufficient ability to expectorate, or aspirate, or due to ventilator-associated pneumonia. Additionally, according to our data, sepsis primarily occurred in Week 2 of induction, which was mainly due to the onset of leucopoenia in the patients during induction. A previous study revealed that the median day of onset of febrile neutropenia was Day 10 (range, Day 3–24) of induction and 13.7% of patients died from sepsis14.
The results from our study demonstrated that the lethality among patients in the ATO + CMT group and ATO group was 82.35% and 41.66%, respectively. The patients in the ATO + CMT group were sicker than those in the ATO group: There were 13 high-risk patients in ATO + CML group, and 7 high-risk patients in ATO group. Therefore, lethality was higher in the ATO + CMT group than in the ATO group in our study.
This study has several limitations. Due to the limited sample size, further multicentred studies should be conducted. Additional studies are needed to elucidate whether certain subgroups of APL patients would benefit from early intensive care management before the development of severe bleeding or sepsis.
Conclusion
In summary, APL patients in the HICU have significant high mortality. Fatal bleeding is the leading cause of death in these patients, predominantly in the early phase of the disease. Meanwhile, greater attention has to be paid to sepsis, which is another significant cause of death in APL patients in the HICU.
Methods
Patients
A retrospective study with medical chart review was performed. Data were collected between October 2017 and May 2023. Fifty-two patients with APL with t (15; 17) translocation and/or a PML-RARα fusion protein who were admitted to the HICU were considered at the First Affiliated Hospital of Harbin Medical University, China. The Ethics Committee of First Affiliated Hospital of Harbin Medical University approved this study. Written informed consent was obtained from all the patients upon hospital admission. All study procedures were performed in accordance with the relevant guidelines and regulations.
HICU admission and discharge criteria
The criteria for HICU admission were as follows: severe complications, such as significant bleeding, including gastrointestinal, pulmonary, and cerebral bleeding; sepsis or septic shock; serious thrombotic complications, such as pulmonary embolism; and severe DS. The aforementioned problems can result in life-threatening symptoms such as shock, respiratory failure, disturbance of consciousness, multiple organ failure, and malignant arrhythmia.
The criteria for HICU discharge were as follows: the patient’s vital signs remained stable; additionally, the patient’s condition gradually improved, and the patient required no critical care, was conscious and capable of communicating clearly, had infection under control, and had no continuous bleeding.
Inclusion and exclusion criteria
The inclusion criterion was newly diagnosed critically ill APL patients admitted to the HICU for aggressive supportive measures.
The exclusion criteria were as follows: patients who declined therapy in the HICU; patients with chronic renal failure receiving regular dialysis in the HICU; and patients whoes induction regimen did not contain ATO.
In this study, patients were divided into nonsurvivor group and survival group according to the their survival status during HICU treatment.
Treatment strategies for critically Ill APL patients in the HICU
After morphological confirmation of the APL diagnosis, ATO 0.16 mg × kg−1 × d−1 for 18 h, with a daily maximum dose of 10 mg, was administered as an induction regimen immediately (generally no later than 48 h after hospital admission) for a minimum of 28 days until CR.
During induction therapy, hydroxyurea (2–4 g/day) was administered when the WBC count was higher than 20 × 109/L. Cytarabine (100 mg/day) was administered when the WBC count was greater than 50 × 109/L. PLT transfusion was administered when the PLT count was lower than 30 × 109/L. FIB or cryoprecipitate transfusion was used to maintain the FIB above 100–150 mg/dL. Fresh frozen plasma (400 ml/day) was administered when coagulation disorder occurred. Dexamethasone (10–20 mg/day) was administered when DS was present.
Patients who experienced respiratory failure were provided with ventilator management or high-flow nasal cannula oxygen therapy immediately. The International Guidelines for the Management of Sepsis and Septic Shock were followed while sepsis occurred15. CRRT was given to patients with acute renal failure and volume overload.
Clinical data collection
To obtain the general clinical information about APL patients, including sex, age, and underlying disease status, laboratory results, such as WBC, HGB, PLT, PT, FIB, DD, and creatinine levels, were obtained at the time of hospital admission.
Information was gathered about the APL patients’ induction schedules. Data were collected regarding the incidence and temporal distribution of sereve complications during three time periods: preinduction, during induction, and postinduction until hospital discharge; the reasons for and timing of HICU admission; the causes and temporal distribution of death while in the HICU, and the need for CRRT and mechanical ventilation treatment during HICU therapy.
Definitions and criteria
Sepsis-3.0 was used to confirm the diagnosis of sepsis or septic shock16. Severe complications were defined as a life-threatening complications requiring intensive care unit (ICU) care and treatment.
Sanz score categorizes APL patients into two groups: standard-risk patients (with WBC count < 10 × 109/L) and high-risk patients (with WBC count ≥ 10 × 109/L)18.
Statistical analysis
The measurement data are represented by the median (interquartile range (IQR)). The categorical variables are represented as the frequency (percentage) and were compared via the Chi-square test. Variables with a nonnormal distribution were compared via the Mann‒Whitney U nonparametric test. In all the statistical analyses, a p < 0.05 was considered statistically significant. IBM SPSS Statistics Version 25.0 was used to analyse the data.
Ethical approval
Ethics approval was obtained from the ethics committee of the participant hospital (the number of ethic approval: The First Affiliated Hospital of Harbin Medical University/article/ethical review 2,024,234. Date: 2024/01), and the study was conducted in accordance with the Declaration of Helsinki.
Data availability
The data used to support the finding of this study are included within the article and its supplementary information files.
Abbreviations
- APL:
-
Acute promyelocytic leukaemia
- APTT:
-
Activated partial thromboplastin time
- ATO:
-
Arsenic trioxide
- ATRA:
-
All-trans retinoic acid
- BSI:
-
Bloodstream infection
- CMT:
-
Chemotherapy
- CR:
-
Complete remission
- CRRT:
-
Continuous renal replacement therapy
- DD:
-
D dimer
- DIC:
-
Disseminated intravascular coagulation
- DS:
-
Differentiation syndrome
- DVT:
-
Deep vein thrombosis
- FIB:
-
Fibrinogen
- HGB:
-
Haemoglobin
- IMV:
-
Invasive mechanical ventilation
- MCI:
-
Multicerebral infarction
- PE:
-
Pulmonary embolism
- PT:
-
Prothrombin time
- PLT:
-
Platelet
- SSTIS:
-
Skin and soft tissue infection
- VT:
-
Ventricular tachycardia
- WBC:
-
White blood cell count
References
Jimenez, J. J., Chale, R. S., Abad, A. C. & Schally, A. V. Acute promyelocytic leukemia (APL): A review of the literature. Oncotarget 11, 992–1003. https://doi.org/10.18632/oncotarget.27513 (2020).
Stahl, M. & Tallman, M. S. Acute promyelocytic leukemia (APL): Remaining challenges towards a cure for all. Leuk. Lymphoma 60, 3107–3115. https://doi.org/10.1080/10428194.2019.1613540 (2019).
Ghiaur, A. et al. Acute promyelocytic leukemia: Review of complications related to all-trans retinoic acid and arsenic trioxide therapy. Cancers 16, 1160. https://doi.org/10.3390/cancers16061160 (2024).
Shein, R. et al. Outcomes for patients with acute promyelocytic Leukemia in South Africa. Clin. Lymphoma Myeloma Leuk. 21, e348–e352. https://doi.org/10.1016/j.clml.2020.12.006 (2021).
Ciftciler, R. et al. The factors affecting early death in newly diagnosed APL patients. Open Med. 14, 647–652. https://doi.org/10.1515/med-2019-0074 (2019).
Zhu, H. H. et al. Early death and survival of patients with acute promyelocytic Leukemia in ATRA plus Arsenic Era: A population-based study. Front Oncol. 11, 762653. https://doi.org/10.3389/fonc.2021.762653 (2021).
Lehmann, S. et al. Continuing high early death rate in acute promyelocytic leukemia: A population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia 25, 1128–1134. https://doi.org/10.1038/leu.2011.78 (2011).
Altman, J. K. et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk. Res. 37, 1004–1009. https://doi.org/10.1016/j.leukres.2013.05.007 (2013).
Rego, E. M. et al. Improving acute promyelocytic leukemia (APL) outcome in developing countries through networking, results of the International Consortium on APL. Blood 121, 1935–1943. https://doi.org/10.1182/blood-2012-08-449918 (2013).
Hassan, I. B. et al. Characteristics features and factors influencing early death in Acute promyelocytic leukemia; Experience from United Arab Emirates (UAE). Int J Hematol. 106, 90–98. https://doi.org/10.1007/s12185-017-2211-7 (2017).
Park, J. H. et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 118, 1248–1254. https://doi.org/10.1182/blood-2011-04-346437 (2011).
Lehmann, S. et al. Early death rates remain high in high-risk APL: Update from the Swedish Acute Leukemia Registry 1997–2013. Leukemia 31, 1457–1459. https://doi.org/10.1038/leu.2017.71 (2017).
Xu, F. et al. Analysis of early death in newly diagnosed acute promyelocytic leukemia patients. Medicine 96, e9324. https://doi.org/10.1097/MD.0000000000009324 (2017).
Yedla, R. P. et al. Complications during Induction chemotherapy in acute promyelocytic leukemia: An institutional experience. South Asian J. Cancer 12, 274–279. https://doi.org/10.1055/s-0042-1757303 (2022).
Evans, L. et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock. Crit. Care Med. 49, e1063–e1143. https://doi.org/10.1097/CCM.0000000000005337(2021) (2021).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810. https://doi.org/10.1001/jama.2016.0287 (2016).
Ferreyro, B. L. et al. Acute promyelocytic leukemia in the intensive care unit: A retrospective analysis. Leuk. Res. 73, 41–43. https://doi.org/10.1016/j.leukres.2018.08.004 (2018).
Sanz, M. A. et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood 96, 1247–1253 (2000).
Author information
Authors and Affiliations
Contributions
H.L. and Y.Z. conceived and designed the study. H.L. collected the data. H.L. and Y.Z. analysed the data, prepared the tables and figures, and wrote the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Zhang, Y., Fan, S. et al. Analysis of early death in critically ill patients with acute promyelocytic leukaemia in the HICU. Sci Rep 14, 19987 (2024). https://doi.org/10.1038/s41598-024-71082-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71082-2
Keywords
This article is cited by
-
Evaluating Outcomes in Acute Promyelocytic Leukemia Patients Treated with All-Trans-Retinoic Acid and Arsenic Trioxide
Indian Journal of Hematology and Blood Transfusion (2025)
-
Induction treatments with and without addition of one dose anthracycline to all-trans retinoid acid and arsenic in pediatric non-high-risk acute promyelocytic leukemia: study protocol for a randomized controlled trial
Trials (2024)