Abstract
The non-conventional manufacturing technologies are notorious when it comes to productivity and processing time in production-related industries. However, the aerospace and other high-end sectors enjoy another quality matrix of these processes and compromise on the processing time. For instance, the machinability of hard-to-cut materials such as Ti6Al4V aerospace alloy for micro-impressions is challenging and commonly carried out through non-conventional processes. Among these processes, the electric discharge machining (EDM) is famous for machining Ti6Al4V. In the current study, the EDM process is enhanced through modified dielectrics such as kerosene with non-ionic liquids (span 20, 60, and 80) and cryogenically treated tool electrodes (aluminum and graphite), and is compared to the conventional kerosene-based process. A three-phase experimental campaign is deployed to explore parametric effects including modified dielectric conditions (non-ionic liquid type and concentration), tool material, and machine parameter pulse ON:OFF time. A total of 60 experiments (54 modified dielectrics and 6 as baseline) were performed to explore process physics. The statistical analyses show that the unmodified process (kerosene dielectric-based) results in the least favorable results 0.58 mm3/min against cryo-graphite and 1.2 mm3/min against cryo-aluminum electrodes. However, the modified dielectrics outperform and improve process dynamics by altering dielectric conditions through hydrophilic-lipophilic balance. Surface morphological analysis shows significantly shallow craters on the machined surface showing evidence of effective flushing through a modified dielectric (S-20) as compared to a kerosene-based dielectric. A thorough microscopical, statistical, and scanning electron-based analysis is carried out to explain the process and correlate significant improvements.
Similar content being viewed by others
Introduction
In the class of high-performance materials titanium and its alloy have gained popularity in aeronautics, automotive, bio-medical, chemical plants, and power generation industries1. This surge in demand for alloys especially Ti6Al4V is because of its remarkable properties that include high toughness, high strength-to-weight ratio, high stability in a rigorous oxidizing environment, high thermal resistance, corrosion resistance, and high biocompatibility2,3. The remarkable attributes come with a processability challenge in the industry. The machinability of the alloy is difficult because of its hard-to-cut mechanical properties. Therefore, a comprehensive list of opportunities is being explored to bring the processability of hard-to-cut alloys into the economic range. Conventionally, industries heavily rely on traditional processes such as milling, turning, drilling, etc. because of their ease of availability in the market, efficient machining, and labor costs. This domain requires a two-step methodology, firstly processing the material, and secondly surface treatment. However, the alloy possesses high hardness with low thermal conductivity4, which limits the options. Therefore, additional support through advanced cooling systems, novel tooling solutions, and high-energy machining strategies are required to process the material. These solutions come with additional costs and are difficult to integrate into production systems. In addition, the need for post-processing remains in its place. Moreover, advanced applications require intricate shapes and high control over dimensional integrity which is an inherent limitation of traditional processes. Conclusively, these conventional machining processes are uneconomical due to the high primary and secondary processing costs with limited surface quality and dimensional accuracy5,6.
For advanced applications, non-conventional processes are given preference because of the aforementioned challenges. Among these processes, electric discharge machining offers an attractive one-step solution for machining hard-to-cut materials and their surface modification to improve engineering applications7. The process uses an electro-thermal phenomenon to erode the material from the workpiece with successive electric sparks in a dielectric medium. It incorporates material vaporization and the erosion process takes place through ignition, plasma generation, discharging, and ejection phases8. In the ignition phase, a high potential difference for breakdown voltage settled between electrodes which drives the plasma phase where current increases gradually with ionized dielectric. In the discharge phase, a continuous flow of current melts the material which vaporizes rapidly. Lastly, in the ejection phase, the flushing of the plasma is carried out which results in an impression on the workpiece9. A servo control mechanism is utilized that maintains an inter-electrode gap after every attempt of machining to maintain a plasma channel with a specific energy. Ramya et al. have evaluated the performance of Brass and copper tools to EDM the Mild steel as a workpiece against the Tool wear Rate (TWR), material removal rate (MRR), and surface roughness (SR) as response parameters by using current, time and voltage as a constant electric parameter10. Nevertheless, electric discharge machining technology shows an underrated performance in achieving high material removal which is a limitation for production systems. Therefore, several solution methodologies are being evaluated to improve the machining rate without compromising on the quality matrix. In addition, superior control over a surface is required which contains defects such as the formation of micro-cracks, recast layer, and thermal residual stresses11.
During the electric discharge machining process, dielectric plays a crucial role in regulating the discharging process, flushing away the debris from the inter-electrode gap, and cooling down the surface12. Different dielectrics such as kerosene oil, deionized water, water oil emulsion, mineral oil, and dielectrics with additives are employed to increase the process efficiency in terms of material removal rate and surface quality13. Sana et al.14 added metallic powder in dielectric to enhance process yield. The powder particles enlarge the interelectrode gap through a bridging effect by stimulating discharge energy with an intense plasma channel9. However, the challenge with powder-mixed dielectric is the electrostatic force of attraction in between powder particles which causes the agglomeration. The agglomeration damps the spark intensity and decreases the process rate which ultimately affects surface integrity15. Mughal et al.2 resulted in the formation of a thick recast layer on the Ti6Al4V due to localized agglomeration of SiC powder particles. To overcome this problem, various surfactants are introduced to enhance the useability with further benefits. The word surfactant is subdivided into three words as “surface active agent”16. Surfactants can be classified as ionic, non-ionic, and zwitterionic. Among these classes, non-ionic surfactants are famous because of their wide useability. These surfactants have an amphiphilic structure which contains two subgroups such as hydrophilic (water-loving) and lipophilic (oil-loving)17.
One of the attractive properties of non-ionic surfactants is that they do not break down into cations and anions but retain their bonding capacity and keep the solution concentration constant throughout18. Furthermore, non-ionic surfactants depend on hydrophilic and lipophilic balance-HLB values which make them hydrophilic or lipophilic. Hydrophilic groups in non-ionic surfactants can contain polyoxyethylene, glyceryl, or sorbitol groups19. The application of these surfactants includes wetting, cleaning, solubilizing agents, and lowering the surface tension of liquids. Because of the properties related to surface tension, they can prevent the accumulation of powder particles, facilitate the bridging effect, disperse plasma channels, and improve the cutting efficiency of the process20,21,22. Kolli et al.23 reported the use of surfactant Span-20 for the machining of titanium alloy with graphite powder. The study resulted in a higher material removal rate with a lower recast layer and lower powder agglomeration. Wu et al24 evaluated the role of surfactant for the machining of SKD 61 against quality matric containing material removal rate and surface roughness being controlled by discharge current and gap voltage. Dong and Liu et al.25 evaluated two non-ionic surfactants Span-80 and Brij L23 in water/oil nano-emulsion for the machining of SKD61. The authors showed that MRR increased by 44% and tool wear reduced by 40.33% by employing non-ionic surfactants. In addition to efficient control over process parameters and changing dielectric properties, an effective selection of tool electrodes is required to achieve desired results. In this regard, different electrodes are tested in the literature.
A recent tool-treatment technology has been introduced in electric discharge machining which is at an early stage of testing. Cryogenic treatment is a supplementary process to the conventional heat treatment process that is utilized to convert the austenite structure of grains into martensite structure26, formation of fine ŋ-carbide to fill up the voids and cavities and distribution of uniform carbides that homogenize the µ-structure27. In this regard, cryogenic treatment is carried out on a tool or workpiece to induce hardness and modify the grain structure. The improved physical properties of the metal play a crucial role in determining thermal, and electrical properties and wear resistance of the material28. Paper et al.29, investigated the impact of the cryogenic treated copper tool on the SS 304 stainless steel which resulted in lower surface roughness and relative tool wear in comparison to non-cryogenic tools. Grewal et al.30 reported minimum deviation dimensional requirements by employing a deep cryogenic copper tool to machine EN24 steel. Singh and Singh et al.31 explained that cryogenic treatment is a complementary process to improve the physical and mechanical properties of a material with a high hardness which has the potential to result in dimensional stability and limited tool wear. Kumar and Kumar32 analyzed electrode temperature, electrode wear, and machining rate. The cryogenically treated tool reduced the wear rate by 18%. The novel points which must be addressed critically are as follows:
-
1.
There is no comprehensive comparison presented in literature which compares conventional kerosene dielectric with different surfactants to evaluate and optimize machining performance.
-
2.
Considering the functionality of surfactants in altering dielectric properties, it is pertinent to explore interaction of different concentrations with cryogenically enhanced tool electrodes.
-
3.
The application of points 1 and 2 is not comprehensively available in literature, which indicates a knowledge gap.
-
4.
Considering the prime limitation of electric discharge machining process, which is slow speed, different researchers have altered tool electrode properties, added powders or liquids to dielectrics, and introduced additional systems such as magnetic stirring or ultra sonification. However, the possibility of tool electrodes of different materials which are thermally treated in parallel with altered dielectric properties employing various surfactant types and concentrations is not studied.
-
5.
To improve the machine speed, a thorough investigation is needed to evaluate different interactions of physical variables along with machine variables. This will lead towards knowledge development on the critical issue.
From the above-cited literature, it has been shown that a very scarce literature available on the comparative analysis for kerosene, kerosene with Span 20 (S-20), Kerosene with Span 60 (S-60), and Kerosene with Span 80 (S-80) dielectrics to promise the cutting efficiency of Titanium alloy. Whereas, the cryogenically treated tools Al and Gr have not been investigated so far for the machining of Titanium alloy (Ti-6Al-4V) for these surfactant-based dielectrics at various concentrations which is a novelty of this study. Experimentation was carried out to show the comparative analysis between dielectric without surfactant and dielectric with surfactants (Span-20, 60, 80). The design of the experiment was full factorial L-64. The results were analyzed by using box plots and a Scanning electron microscope. The most suitable electrode material, surfactant, and concentration have been proposed and validated.
Materials and methods
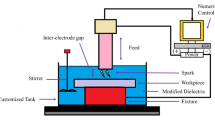
In current research, titanium alloy Ti-6Al-4V was used because of its wide applications in industry. The selection of the material was derived because of excellent mechanical properties, superior corrosion resistance, ability to perform at higher temperature conditions, and related engineering attributes. Along with the benefits, the material is hard to cut which brings difficulty in processing. Because of the challenges in the machinability of the alloy, the main objective is to explore the physical science behind the influence of treated electrodes under modified dielectrics for the improvement of cutting efficiency. The cutting efficiency is explored through the material removal rate during electric discharge machining. The machine tool used to carry out the experimentation was the die sinking model Electric Discharge Machine (Table 1).
Since the process is stochastic, therefore, different variables influence the control over results. However, input parameters such as pulse on off time are utilized to enhance the machining efficiency with higher yield considering its significant synergistic role in melting and removal of unwanted material. The process utilizes thermo-electric energy for processing; therefore, conductive electrodes are required. Therefore, the electrode used as the workpiece is Ti6Al4V having remarkable engineering attributes as physical properties mentioned in (Table 2).
Considering the requirements of production systems where the alloy is used, the electric discharge machining is a relatively slow process. Therefore, a three-step methodology is opted to improve the cutting efficiency. In the first step, the tools Aluminum (Al) and Graphite (Gr) were selected because of their supremacy established in the literature for better cutting efficiency. The workpiece was of length x width x thickness (300 × 100 × 2.5 mm3) dimensions and the tools had a diameter of 15 mm. Secondly, to further enhance the quality of the process, the tool electrodes were cryogenically treated which significantly alters the microstructure to favorable conditions. The cryogenic treatment was done in a chamber at −185 °C in liquid nitrogen for 24 h inducing specific hardness with a modified grain structure (as per manufacturers’ sheet). The treatment was carried out as per the recommended conditions in the literature. Thirdly, the dielectric medium which is used during the cutting process was modified to facilitate melting and debris removal. For this purpose, three different hydrocarbon-based surfactants were selected as per their ability to facilitate the established applications. The concentration of the surfactant additives was taken as a variable with an optimization point of view. The impact of these cryogenic tools has been investigated in the presence of kerosene (for baseline to measure improvement KPI), kerosene with Span-20 (S-20), kerosene with Span-60 (S-60), kerosene with Span-80 (S-80) at 12.5, 25, and 37.5 g/L concentrations. The selection of surfactant was carried out to improve dielectric properties. The important attributes of selected surfactants are mentioned in (Table 3).
However, for the selection of the process parameters’ levels, a thorough trial experimentation was carried out. The selection of the parametric settings was based on the criteria where the complete impression is achieved, significant balanced depth is acquired, and there are no burn marks because of an improper flushing system. To ensure adequate flushing, a customized setup was designed where a separate dielectric tank was made to ensure proper mixing and focused flushing. After completion of the preliminary experimental phase, parametric settings were selected which are tabulated in (Table 4).
Similarly, the surfactant concentration was defined based on guidelines in the literature and the ability to investigate the reasonable influence of variables. An extensive mature experimental plan was made using the full factorial design of experiments approach where 60 experiments were performed under two repetitions. Among these experiments, 6 experiments were executed without any additives (surfactants) and 54 experiments were done with additives (surfactants). The combined influence of process variables was recorded on material removal rate which was calculated by measuring the achieved depth of the workpiece and time. The Coordinate Measuring Machine was used to calculate the removed volume as methodology is shown in (Fig. 1). The obtained results are discussed in detail considering the physical phenomenon involved during EDM of the Titanium Ti-6Al-4V alloy. The optimal electrode material, with appropriate surfactant at a specified concentration, has been proposed to improve process capabilities.
A summary of the three-phase experimental campaign is implemented to explore process capability. The details are as follows:
-
1.
Phase 1: Pilot experimentation without standardized design of experiments is carried out. The criteria of success for this campaign were a selection of process parameters which does not result in extensive spark interruption, carbon marks, deep crates, and burn marks. The issues are commonly faced due to unmatched process physics and material’s engineering properties. The result of this campaign was the identification of variable and constant parameters and their selected ranges as shown in (Table 4).
-
2.
Phase 2: A full factorial design of experiments campaign was run to evaluate process sensitivity. Individual experimental runs were examined concerning their surface quality and cutting performance. The mechanism of modified dielectrics is shown in (Fig. 2) where the agglomeration of debris is avoided through monomers.
-
3.
Phase 3: The confirmatory experimentation is carried out on the Desirability-Function recommended parametric settings.
The working mechanism of non-ionic liquids affects differently to the agglomerated debris. The physical structure of the monomer is shown in the image which alters dielectric properties based on their Hydrophilic and Lipophilic balance. The lipophilic tail bonds with oil droplets to reduce surface tension and increase flow rate and the hydrophilic head surround bond with debris to reduce their surface tension. Conclusively hydrophilic-lipophilic balance alters the working mechanism of dielectric and improves functionality.
Results and discussions
Statistical analysis
A statistical analysis of variance is used to examine the influence of electric discharge machining parameters, tool electrode materials, and surface surfactant characteristics on the machining process. Data were analyzed quantitatively using constant variance results, independence, and normality assumptions. A normal probability plot was used to evaluate the linearity of the data. Figure 3 illustrates the normality of the residuals. Additionally, the coefficient of correlation was estimated at a 95% confidence level by Looney and Gulledge (1985)33 and led to R2 = 96.37% for 54 experiments with two repetitions. According to the correlation coefficient (Table 5), the residuals satisfy the normal distribution of residuals at a significant level above the critical confidence level.
An illustration of the residuals in Fig. 3 is provided to demonstrate the randomness of the procedure as well as the parametric effects of the experimental design. A residual independence assumption is also evaluated using this method, which provides a baseline for the analysis of variance. To identify bias in data, the experimenter needs to be able to visualize the data to identify patterns that may be subjective to his/her experiences. Consequently, irregularity supports the independence of the data and confirms the existence of the condition (as was demonstrated by the Durbin-Watson test against 54 residuals).
Data variance is typically examined with plots to identify the validity of the assumption that variance is constant. The residual plot must be structureless to satisfy the assumption. It appears that the assumption of constant variance can be supported by both the 95% confidence interval of the brown-forsythe test and the visual representation of the plot.
Analysis of variance is a commonly used statistical method for determining the significance of process variables about response characteristics. A 95% confidence interval is used for the analysis of variance (α = 0.05). There is a direct correlation between variables with greater confidence than 95% (lower p-value than 0.05) and weak variables, followed by variables with 90% and 85% confidence levels. Variables with less than 85% confidence are not significant. It is necessary to evaluate the F-value in addition to the P-value to determine the control variables. The relative significance of each process variable is also determined by calculating its percentage contribution (Eq. 1).
In Table 6, the variances of surface roughness are compared between tools surfaced using dielectric EDM based on surfactants and tools treated cryogenically. P-values, F-values, and percentage contributions (PCR) are used to determine significant values. The high F-value indicates that the variable is sensitive to the characteristics of the response.
Surfactant type (p-value 0.565), pulse ON: OFF time (p-value 0.015), and electrode material (p-value 0.001) contributed 0.56, 6.82, and 38.46% percentage contributions, respectively. The response of the process variables is affected by some interactions between the variables as shown in (Fig. 4). A significant portion of the response is controlled by surfactant type and concentration (p-value 0.231), pulse ON: OFF time (p-value 0.140), and electrode material (p-value 0.062). The combined effect of surfactant concentration and pulse ON: OFF time (p-value 0.017) significantly affects the MRR. The combined effect of surfactant and electrode material controls and pulse ON: OFF time is 3.66 and 10.48%, respectively. There is also the possibility of analyzing the three-way interaction of surfactant type and its concentration with other variables, although their significance is not high. The interaction between the process variables and the responses, however, confirms that the effects of the process variables are combined. In addition to surfactant type, concentration, and electrode material, there are a variety of other factors that contribute to the cumulative effect. As a result, it is essential to make an optimized selection when designing the system.
EDM in pure kerosene
The potentiality of cryogenically treated aluminum and graphite tools is evaluated against material removing mechanism under modified dielectrics such as kerosene, and kerosene mixed S-20, S-60, and S80. The machining efficiency in terms of material removal rate is shown against pulse ON: OFF time and tool materials. As shown in Fig. 5, at a pulse ratio of 50:25 in pure kerosene Al showed higher MRR as compared to Gr tool because of the Aluminum expressed a high packing density (2.75 g cm−3) than that of graphite (1.77 g cm−3 ) . The energy transfer was higher at the frontal area of tool in which atoms were more compacted than loose structure of atoms as they were in graphite tool. Therefore, at high discharge energy Al showed better MRR than Gr27. With an increase of pulse ratio 50:50, machining got stable due to proper flushing and balance in pulse on: off time that promoted a high energy spark that directed properly on workpiece and MRR increase 25.80 and 190% respectively in case of both electrodes. Whereas, further increase in the pulse ratio 50:100 causes to increase the pulse off time that shorter the spark erosion time that was ultimately decreased the MRR about 10% and 31.03% in case of Al and Gr electrodes respectively due to unstable machining and large inter-sparking time30. 51.66% better cutting efficiency was observed for Al tool than Gr tool due to aforementioned reasons in pure kerosene.
Figure 6 showed the surface morphology of workpiece that was machined in pure kerosene oil, it can be depicted that machining under kerosene produced the more carbon dregs on the surface due to the breakage of hydrocarbon dielectric with high energy plasma generation that would increase the thermal stresses and a concentration of cracks. These cracks are stretched throughout the machined surface with negative consequences in terms of lower fatigue life of work part31. Due to moderate cooling rate of kerosene oil more time required to cool the surface and the propagation of gas bubbles made the surface porous with the nucleation of microporosity19. In addition to this, poor dispersion of debris restricted their motion in the dielectric that will ultimately form the cluster of globules on machined surface with some redeposited debris and the surface finishing became poor32. Formation of craters on machined surface is a function of discharge energy, due to the concentrated electric spark that was directed on workpiece erodes the more material with wide range craters are observed in (Fig. 6) Ref.34.
EDM in modified dielectrics
MRR in Surfactant S-20, S-60, S-80
In order to overcome aforementioned problems during the machining in pure kerosene oil, surfactant molecules were introduced into dielectric to get highest cutting efficiency with superior surface properties. From Fig. 7 it can be explained that the highest MRR 1.73 mm3/min was achieved for surfactant Span-20 then it showed decreasing trend for the rest of two surfactants like the highest value of MRR attained for Span-60 was 1.4 mm3/min and 1.38 mm3/min was achieved for Span-80. Hydrophilic-lipophilic balance (HLB) value plays a crucial role in this regard, as greater HLB value refers the condition in which the polarity of surfactant molecule increases facilitates the ionization of surfactant molecules in the dielectric with high conductivity working medium. Span-20 has HLB value 8.6 which increased the conductivity of dielectric with shorter bridging time and relay time, these factors uplifted the discharge energy per spark and intense plasma channel generated with higher MRR ensured24. Furthermore Span-60 showed HLB value 4.7, and Span-80 had HLB value 4.3 therefore their conductivity reduced in dielectric and lower MRR resulted. Span-20 exhibited 16.47% higher MRR than Span-60 and 17.05% higher MRR than Span-80. Whereas, the Span-60 expressed 0.70% higher MRR than Span-80. Surface integrity was analyzed in terms of cracks density, craters, redeposited debris globular shaped appendages and microporosity. From scanning electron microscopic analysis presented in later sections, surface that was machined under Span-20 shows high influence of higher polarity of surfactant molecules and higher conductivity which resulted proper machining. Small interconnected large number of shallow craters increased MRR with better surface quality. As compared to surface machined in pure kerosene lesser microporosities, crack density and redeposited debris appeared on machined surface due to lesser surface tension of dielectric molecules and debris. Surface machined in Span-60 higher redeposited debris and globules appendages owing to higher flash point of Span-60 (185.3°C) that required higher discharge energy to melt the material that caused the melt solidification on workpiece surface with higher globules formation. Figure 7 exhibited a box plot showing the excellent performance of Span-80, due to the lower HLB value of Span-80 as compared to other two surfactants (the conductivity of dielectric media fell down that hampered the MRR). Higher flash point 186°C generated the high intense plasma with concentrated spark that formed deeper craters and due to high discharge energy crack density and melted drops concentration increased on machined surface35. Although surface machined under Span-80 showed lower surface integrity, S-20 generated surface is still better than a surface machined in dielectric without surfactant due to higher dispersion rate with lower molecular surface tension. Kanlayasiri and Boonmung33 investigated that EDM of AISI DC53 hardened steel reduced the surface crack density, redeposited melts, debris and globules in heat affected layer due to capability of Span-20 of lowering the surface tension and uniform distribution of discharge energy.
Effect of concentration on MRR
The effect of concentration of surfactant was very crucial and need to be validated. From Fig. 8 it can be predicted that the mean of MRR on 12.5 g/L was 0.74 mm3/min and at 25 g/L was around 0.75 mm3/min. However, the further increase in surfactant concentration up to 37.5 g/L dropped the MRR and it became 0.72 mm3/min. At lower concentration of surfactant, the adsorption phenomenon was prominent that reduced the surface tension of a dielectric molecules and debris produced during machining due to a proper balance in Van der Waals forces between dielectric and surfactant molecules. The described phenomenon prompted the generation of high energy plasma channel with higher MRR resulted. Further increase in surfactant concentration induced critical micelle concentration (CMC) and the adsorption of surfactant on dielectric molecules surface and on debris got saturated that escalated the surface tension and MRR declined consequently36. Therefore, 1.3% rise in MRR observed when concentration changed from 12.5 to 25 g/L despite that an increase of 4% was observed with increase in surfactant concentration from 25 to 37.5 g/L due to critical micelle concentration.
Combined effect of surfactants and their Conc
Figure 9 displayed a combined effect of both input parameters i.e., surfactants and their concentration on a response measure MRR. It can be elaborated that Span-20 exhibited a mode MRR 0.88 mm3/min at 25 g/L which was 12 and 6.25% higher than MRR of workpiece that was recorded at 12.5 and 37.5 g/L respectively for the same surfactant. Whereas Span-60 showed a higher MRR mean value 0.98 mm3/min at same concentration 25 g/L which was 15.29 and 17.34% greater than MRR at 12.5 and 37.5 g/L respectively.
In addition to this, Span-80 also expressed the mode MRR 0.97 mm3/min at same level of surfactant concentration that was 25 g/L. This erosion rate was observed 46% greater than MRR that was recorded at 12.5 g/L and 36.08% larger at 37.5 g/L. Therefore, Span-60 showed a mode MRR 0.98 mm3/min at 25 g/L which was higher 11.36% than Span-20 and 1.02% than Span-80 in all three categories of surfactants at different levels of concertation. A dedicated reason for this trend was the size of alky chain of Span-60 (C24H46O6) that was longer as compared to other two surfactants. Rosen Milton et al.37 investigated that longer alkyl chain of lipophilic group in surfactant molecules induced the more adsorption of surfactant on the dielectric molecules and the surface tension reduced due to lowering interfacial molecular tension and energy balance in between the molecules. Then this alkyl chain structured shrank down to Span-20 surfactant up to (C18H34O6) 18 carbon atoms that ultimately reduced their effect and MRR decreased by employing Span-20. A prime concentration in combination with surfactant was 25 g/L that produced maximum cutting efficiency with Span-60 molecule. Increasing concentration more than 25g/L of surfactant created a saturation point and critical micelle concentration (CMC) which dropped the adsorption of surfactant on dielectric molecules and debris surface and MRR decreased consequently36,38. Surface machined in Span-60 showed the higher MRR with large number of deeper craters that enhanced the MRR with lower surface integrity. Prolonged cracks and melted drops appeared more due to high concentrated energy sparks. Span-80 has lower HLB value, smaller alkyl chain structure indicated better surface properties in comparison with Span-60. Whereas, Span-20 has highest HLB value that expressed higher dispersion rate by reducing surface tension with significant increase in conductivity so at 25 g/L in Span 20 SEM image in (Fig. 10) exhibited best surface morphology with smaller, shallower interconnected craters that promoted MRR however with better surface topography, defects free surface with less microporosity resulted due to the uniform distribution of discharge energy.
Combined effect surfactant and pulse ratio
Pulse ratio is a combined ratio of pulse on time and pulse off time. From Fig. 11 it can be inferred that Span-20 exhibited maximum MRR mean value 1.08 mm3 / min at pulse ratio 50:25 this MRR fell down when pulse ratio increased up to 50:100. MRR at Pr 50:50 decreased down by 30.55% and at 50:100 dropped by 21.29% with respect to MRR at 50:25 respectively.
Though the same trend was observed for the Span-80 surfactant. At Pr 50:25 MRR was 0.8 mm3 / min and when pulse ratio increased up to 50:100 it declined around 0.55 mm3 / min that showed the approximately 31.25% reduction in MRR. A proposed reason for above mentioned decreasing trend was the large intermediate time between two pulses. Pulse-off (pulse pause) time is defined as a time on which no spark was generated and the debris between the electrodes flushed away in spite of this a large gap between two sparks extended a time in which dielectric restored its insulating capability and for the next pulse generation more discharge energy is required to erode the material and resultantly material erosion reduce in the very next pulse-on time. Therefore, in both case Span-20 and in Span-80 increasing pulse off time made dielectric to regain its insulation and lesser ionization decreased the MRR9. Span-60 showed the maximal MRR 0.9 mm3/min that was 11.11% higher when pr 50:50 and 5.55% when Pr was 50:25. A relative increase in pulse pause time with respect to pulse on time with lowered the surface tension of dielectric molecules and proper flushing promoted the cleaning of surface with a little increase in MRR (Fig. 12). A significant higher MRR was observed with a mean value 1.08 mm3/min when Span 20 surfactants employed at pr 50:25. Parveen et al39 evaluated the impact of pulse on time and pulse off time on MRR with Cu and Gr tool for the machining of Titanium alloy, they found that the increase in pulse off time increase the MRR significantly for Cu tool and for Gr tool increase in pulse off time decreased the MRR because it depended on the nature of tool material, workpiece material, electric and non-electric input parameters. From the SEM in Fig. 10 it can be shown that due to high pulse energy and high conductivity of Span-20 surfactant generated a defect free surface due to more dispersion, lower surface tension of carbon dregs debris and melted drops because they were overlapped by hydrophilic group and promoted to higher material removal with better surface fining.
A smaller number of globules are appeared because less surface tension of melted drops showed a higher flow rate and clean surface generated (Fig. 13). Whereas, if a comparison was made between different surfaces under varied surfactants, then it can be predicted that a surface machined in Span-80 showed a poorer surface as compared to Span-60 even at same pulse ratio due to the difference in HLB value, flash point and molecular structure inferiority of Span-80 surfactant. Surface machined under Span-80 showed deeper craters and more melted drops and globules in Fig. 13 easily observed this occurred due to lower dispersion rate, higher surface tension and less conductivity of dielectric media. Span-60 exhibited a surface with high cracks and craters as compared to Span-20 however this surface was observed much better than machined in pure kerosene and in Span-80.
Pulse ratio & surfactant concentration
Pulse ratio (Pr) is a combination of pulse on time and pulse off time. Pulse on time shows a direct relation to the discharge energy and cutting efficiency irrespective to other selected parameters. A balance between pulse ON: OFF time dedicated to achieving higher MRR with excellent surface characteristics. From Fig. 14 it can be inferred that the highest MRR has been achieved at Pr 50:25 a further increased in pulse ratio lessened the MRR around 0.52 mm3/min and at a Pr 50:100 a fall of 65.90% observed. A dedicated reason for the observed descending trend was the unbalancing between pulse on: off ratio, a large pulse off time extended the inter-sparking time that allowed the dielectric to retain its insulating strength and cutting efficiency reduced subsequently. Adnan et al.40 investigated the effect of pulse energy to explore the cutting efficiency of steel 304, he asserted that an increase in pulse off time (pulse ratio) a decrease in the intensity of discharge observed which was given in term of pulse on time. Nevertheless, increased in pulse off time decreased the cutting efficiency after some specific value. When a Pr 50:50 a maximum mean MRR 0.92 mm3/min achieved at 12.5 g/L in addition to this when Pr set at 50:25 and 50: 100 a maximum MRR 1.15 and 0.92 mm3/min were attained at 25 g/L. further, increased in surfactant concentration promoted the self-aggregation and MRR reduced at 37.5 g/L. Therefore, Pr 50:25 and Sc 25 g/L showed best mean MRR 1.2 mm3/min.
EDM with cryogenic treated tools
Surfactant and tools material effect on MRR
Selection of tool material plays an important to determine the cutting efficiency. Referring to Fig. 15 it can be explained that two tool materials Aluminum and Graphite were utilized to evaluate cutting efficiency. Deep cryogenic treatment of these tools improved their grain structure by filling up the cavities with carbides and tool structure get homogenize to reduce tool wear rate with higher cutting efficiency. These two deep cryogenic treated (DCT) electrodes such as Al showed MRR 1.1 mm3/min and DCT Gr 0.73 mm3/min a greater difference between their cutting efficiency was recorded due to the density, electrical conductivity and electrical resistivity of these two electrodes. Al showed greater density (2.75 g cm−3), electrical conductivity (35 × 106 S m−1) and lower electrical resistivity (2.7 µΩ cm) as compared to Gr electrode that promoted proper flow of electrons through the tool body which excited the plasma channel and concentrated the spark that melted more material as compared to Gr electrode27,41.
It has been observed that the addition of surfactant Span-20 in the kerosene dielectric raised the MRR that was achieved by both electrodes (Figs. 15, 16). The performance of Al and Gr electrodes increased by 41.66 and 50% respectively due to the addition of surfactant. A dedicated reason was a lower surface tension of the dielectric and debris molecules. A surfactant molecule contained on long alkyl chain of lipophilic group that adsorbed on oil molecule and lowered its surface tension whereas, carbon dregs were surrounded by hydrophilic head as explained above in (Fig. 2). Therefore, dispersion rate enhanced, and performance of both electrodes increased14. Further the addition of Span-60 lowered MRR up to 1.025 mm3/min for Al (6.81%) and 0.7 mm3/min (4.10%) for Gr due to the lower HLB value and higher flash point of Span-60 as compared to Span-20. However, the addition of Span-60 still showed the higher MRR for Al and for Gr as compared to MRR observed in pure kerosene. Addition of Span-80 decreased MRR for Al by 0.20% and a little decrease in mean value of MRR for Gr observed around 10%. From the micrograph in Fig. 18 (comparing to Fig. 17) a surface machined by graphite electrode it can be predicted that due to the porous nature of graphite electrode those sintered powdered particles generated bridging effect at irregular points in tool-workpiece interfaces that uplifted the MRR for Gr electrode however these reasons enhanced surface roughness due to the formation of arcing phenomenon41. Due to high melting point of graphite electrode 3300 °C it utilized more thermal energy that increased the thermal stress and concentration of deeper and large craters increased substantially due to the formation of graphite dust non uniform surface with high microporosity generated. As compared to graphite, surface generated by Al electrode in Fig. 17 showed shallow interconnected craters that promoted the high surface integrity. At Pr 50:25 and Span-20 Cryogenic treated Al showed more aligned grain structure that increased current flow from workpiece and reduced the sideway sparking and enhanced surface uniformity and smoothness with nano porosity resulted.
Surfactant conc. and electrode material
A combined impact was studied that was depicting in Fig. 16 that DCT Al and Gr electrodes were utilized to measure cutting efficiency at vary surfactant concentration such as 12.5, 25 and 37.5 g/L simultaneously. For DCT Gr electrode it can be observed that at 12.5 g/L mean MRR was recorded 0.3 mm3/min further increase in surfactant concentration from 25 to 37.5 g/L maximum increase observed was 0.78 mm3/min. it was researched that the increase in surfactant concentration (Sc) reduced the surface tension with an increased dispersion rate that raised the MRR due to higher conductivity of dielectric24. Therefore, the trend of high MRR with concentration can be explained from micrograph 16 that showed the greater and deeper craters on machined surface which acted as evidence that increased surfactant concentration increased the MRR up to certain limit. For Al tool best recorded MRR was 1.15 mm3/min at 25 g/L. So, the increased concentration of surfactant from 12.5 to 25 g/L improved the cutting efficiency however the further increase in surfactant concentration minimized the cutting efficiency approximately 28.26%. Referring to surface machined by Al tool in Fig. 17 it can be predicted at 25 g/L small craters are formed with less crack density due to the uniform distribution of plasma channel and high inter-electrode gap.
Tool material and pulse ratio
A relative performance of both DCT electrodes were evaluated in comparison with pulse ratio to plot the results for MRR. Aluminum electrode has better electrical conductivity (35 × 106 S m−1) and density (2.75 g cm−3)than graphite (0.3 × 106 S m−1, 1.77 g cm−3)28. These two features made Al electrode to give proper flow of electrons through its surface so Al electrode exhibited 43.08% better performance than Gr electrode. Figure 17 the smooth surface machined via Al electrode due to high energy flow and surface machined by Gr from Fig. 18 showed the surface with large crater density and porosity due to the unstable machining that was caused by the interruption of graphite particles between inter-electrode gap which caused arcing and lead the poor surface generation.
Al electrode showed maximum MRR at Pr 50:25 because at this state pulse on time promoted high discharge energy and generated high impulsive force and pulse off time which was set at 25 µs in comparison to Pulse on time proper balance of flushing attained (Fig. 19). Therefore, this value of MRR was recorded 12.5 and 25% better than at Pr 50:50 and 50:100 respectively. Whereas, DCT Gr electrode showed lower MRR by 43.08% if it was compared with DCT Al electrode at Pr 50:25 due to smaller grain size nature that made the EDM machining unstable with a generation unstable sparking between tool and workpiece electrode as graphite particles interfered during machining17. At same selected condition such as Pr 50:25 for Gr electrode exhibited 21.42% better MRR than measured at 50:100 pulse ratio due to higher spark intensity and balancing of spark generation and pulse pause time. Therefore, the highest MRR resulted at Pr 50:25 for Al electrode due to aforementioned reasons.
Process optimization and validation
The Desirability Function is used to carry out optimization of the different experimental conditions. The individual experiment shows different physics-based processes introducing multi-physics interactions. Therefore, analytical modelling of the problem for parametric optimization is not suitable here. The Desirability Function ranks the individual desirability intents and predicts the response characteristics1,2.
The desirability (di) is potentially targeted to achieve higher quality of response which often is the case of "larger the better" intent. The individual di are calculated for each experiment keeping the objective in the front. The experimental value-based desirability index is calculated using (Eq. 2). Considering the material removal rate, the higher it is the better it will be.
where:
Individual desirability di;
Expected response xi; Lower value xmin; Upper value xmax;
Individual weighting r.
The expected outcome xi exceeds the set value then it is given di as 1 and 0 in the other case which is certainly not interesting case. Similarly, if the target is other way round such as "smaller the better" the desirability index could be computed through (Eq. 3).
The expected outcome xi oversteps the threshold corresponds to 0 desirability, which is not an interesting result. The other case is interesting and given desirability of 1. Considering the study, the key target here is to reduce processing time through Eq. 3 which is considered an objective function.
However, there could be a case with nominal value being interesting to stakeholders then “nominal the best” objective is pursued through (Eq. 4).
where:
Target T;
Weightings t and S.
The experimental results are compared in between the conventional process and the modified dielectrics as shown in (Fig. 20).
The Table 7 and Fig. 20 shows the results of optimization experiments and recommendations for parameter selection. The statistical analyses show that unmodified process (kerosene dielectric based) result least favorable results MRR 0.58 mm3/min against cryo-graphite and MRR 1.2 mm3/min against cryo-aluminum electrodes. However, the modified dielectrics outperform and improve process dynamics through altering dielectric conditions. The non-ionic liquid-based dielectrics resulted in the highest material removal rate of MRR 1.7 mm3/min against cryo-aluminum and Span-20 dielectric. The cryo-aluminum electrode performs similarly in the case of S-60 and S-80. Whereas cryo-graphite showed an overall lower material removal rate than cryo-aluminum in all conditions.
The desirability function showed 100% attainment of interesting outcome of the highest material removal rate. The surface characteristics on the optimized settings are shown in (Fig. 21) where shallow craters are achieved. The highest material removal rate of 1.7 mm3/min is achieved through S-20 dielectric 25 g/L concentration, 50:25 µSec pulse ON:OFF time, and aluminum electrode. The standard deviation of 0.047 and average experimental value of 1.67 is shown in (Table 7).
Conclusions
The potentiality of various surfactants with different concentrations is comprehensively evaluated with cryogenic-treated electrodes during the EDM of difficult-to-cut aeronautical alloy Ti6Al4V. The findings are examined through process physics and related evidence to improve process efficiency in terms of surface integrity. Based on the results, the following conclusions are drawn:
-
Based on the analysis of variance, surfactant type (p-value 0.565), pulse ON: OFF time (p-value 0.015), and electrode material (p-value 0.001) contributed 0.56, 6.82, and 38.46% percentage contributions, respectively.
-
At pulse ratio 50:25 in pure kerosene Al showed higher MRR as compared to Gr tool because of the Aluminum expressed a higher packing density (2.75 g cm−3) than that of graphite (1.77 g cm−3). The energy transfer was higher at the frontal area of tool in which atoms were more compacted than loose structure of atoms as they were in graphite tool.
-
It has been observed that the addition of surfactant Span-20 in the kerosene dielectric raised the MRR that was achieved by both electrodes. The performance of Al and Gr electrodes increased by 41.66 and 50% respectively due to the addition of surfactant. A dedicated reason was a lower surface tension of the dielectric and debris molecules. A surfactant molecule contained on long alkyl chain of lipophilic group that adsorbed on oil molecule and lowered its surface tension whereas, carbon dregs were surrounded by hydrophilic head.
-
The statistical analyses show that unmodified process (kerosene dielectric based) result least favorable results 0.58 mm3/min against cryo-graphite and 1.2 mm3/min against cryo-aluminum electrodes. However, the modified dielectrics outperform and improve process dynamics through altering dielectric conditions.
-
Surface morphological analysis shows significantly shallow craters on the machined surface showing evidence of effective flushing through modified dielectric (S-20) as compared to kerosene-based dielectric.
-
The highest material removal rate of 1.7 mm3/min is achieved through S-20 dielectric 25 g/L concentration, 50:25 µSec pulse ON:OFF time, and aluminum electrode. Span-20 has HLB value 8.6 that increased the conductivity of dielectric with shorter bridging time and relay time, these factors uplifted the discharge energy per spark and intense plasma channel generated with higher MRR ensured.
The current work involves multi-physics interactions for example thermo-electrical process mechanism, altered material properties of tool electrode through cryogenic treatment, and improved dielectric attributes through mixing non-ionic liquids to enrich debris removal. The theoretical tools available are limited to incorporate these many multi-physics interactions which led authors to explore experimental results first. Therefore, this study primarily focuses on experimental results and correlates the physical mechanisms with evidence from scanning electron microscopy and statistical analyses.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
References
Farooq, M. U., Anwar, S., Ali, M. A., Hassan, A. & Mushtaq, R. T. Exploring wide-parametric range for tool electrode selection based on surface characterization and machining rate employing powder-mixed electric discharge machining process for Ti6Al4V ELI. Int. J. Adv. Manuf. Technol. 129, 2823–2841 (2023).
Mughal, M. P. et al. Surface modification for osseointegration of Ti6Al4V ELI using powder mixed sinking EDM. J. Mech. Behav. Biomed. Mater. 113, 104145 (2021).
Li, G. et al. High cycle fatigue behavior of additively manufactured Ti-6Al-4V alloy with HIP treatment at elevated temperatures. Int. J. Fatigue, 184, 108287 (2024).
Al-Amin, M. et al. Analysis of hybrid HA/CNT suspended-EDM process and multiple-objectives optimization to improve machining responses of 316L steel. J. Mater. Res. Technol. 15, 2557–2574 (2021).
Ji, R. et al. Study on high wear resistance surface texture of electrical discharge machining based on a new water-in-oil working fluid. Tribol. Int. 180, 108218 (2023).
Challapalli, A. & Li, G. Machine learning assisted design of new lattice core for sandwich structures with superior load carrying capacity. Sci. Rep. 11, 18552 (2021).
Xie, Z. J., Mai, Y. J., Lian, W. Q., He, S. L. & Jie, X. H. Titanium carbide coating with enhanced tribological properties obtained by EDC using partially sintered titanium electrodes and graphite powder mixed dielectric. Surf. Coatings Technol. 300, 50–57 (2016).
Chen, Y., Sun, S., Zhang, T., Zhou, X. & Li, S. Effects of post-weld heat treatment on the microstructure and mechanical properties of laser-welded NiTi/304SS joint with Ni filler. Mater. Sci. Eng. A 771, 138545 (2020).
Wu, X. et al. Sustainable and green sinking electrical discharge machining utilizing foam water as working medium. J. Cleaner Prod. 45, 142150 (2024).
Ramya, C. R., Girisha, L., Koushik, P. K., Harish, G. V & Ravi, C. C. Comparison of Performance Parameters of Mild Steel in EDM Process using Copper and Brass Tool. 1–4 (2019).
Yuhua, C., Yuqing, M., Weiwei, L. & Peng, H. Investigation of welding crack in micro laser welded NiTiNb shape memory alloy and Ti6Al4V alloy dissimilar metals joints. Opt. Laser Technol. 91, 197–202 (2017).
Sultan, T., Kumar, A. & Gupta, R. D. Material removal rate, electrode wear rate, and surface roughness evaluation in die sinking EDM with hollow tool through response surface methodology. Int. J. Manuf. Eng. 2014, 1–16 (2014).
Sanghani, C. R. & Acharya, G. D. Effect of various dielectric fluids on performance of EDM: A review. Tmet 55–71 (2016).
Sana, M., Asad, M., Farooq, M. U., Anwar, S. & Talha, M. Machine learning for multi-dimensional performance optimization and predictive modelling of nanopowder-mixed electric discharge machining (EDM). Int. J. Adv. Manuf. Technol. 130, 5641–5664 (2024).
Kalaman, S., Yaşar, H., Ekmekci, N., Öpöz, T. T. & Ekmekci, B. Powder mixed electrical discharge machining and biocompatibility : A state of the art review. The 18th International Conference on Machine Design and Production 803–830 (2018).
Dukhin, A. S. & Goetz, P. J. Ionic properties of so-called “non-ionic” surfactants in non-polar liquids. Dispers. Technol. Inc Bedford Hills N. Y. 15, 151–157 (2005).
Farooq, M. U. & Anwar, S. Investigations on the Surface Integrity of Ti6Al4V under modified dielectric(s)-based electric discharge machining using cryogenically treated electrodes. Processes 11, 877 (2023).
Zhao, Y. Integrated unified phase-field modeling (UPFM). Mater. Genome Engin. Adv. 2(2), e44 (2024).
Shikhmurzaev, Y. D. Capillary Flows with Forming Interfaces (Chapman and Hall/CRC, 2007).
Bart, J. C. J., Gucciardi, E. & Cavallaro, S. Formulating lubricating oils. In Biolubricants: Science and Technology (eds Bart, J. C. J. et al.) (Elsevier, 2013).
Nyberg, E., Schneidhofer, C., Pisarova, L., Dörr, N. & Minami, I. Ionic liquids as performance ingredients in space lubricants. Molecules 26, 1013 (2021).
Song, Z., Liang, Y., Fan, M., Zhou, F. & Liu, W. Ionic liquids from amino acids: Fully green fluid lubricants for various surface contacts. RSC Adv. 4, 19396–19402 (2014).
Kolli, M. Influence of span 20 surfactant and graphite powder added in dielectric fluid on EDM of titanium alloy. Bonfring Int. J. Ind. Eng. Manag. Sci. 4, 62–67 (2014).
Wu, K. L., Yan, B. H., Lee, J. W. & Ding, C. G. Study on the characteristics of electrical discharge machining using dielectric with surfactant. J. Mater. Process. Technol. 209, 3783–3789 (2009).
Dong, H. et al. Sustainable electrical discharge machining using water in oil nanoemulsion. J. Manuf. Process. 46, 118–128 (2019).
Wang, J. et al. Effects of high temperature and cryogenic treatment on the microstructure and abrasion resistance of a high chromium cast iron. J. Mater. Process. Technol. 209, 3236–3240 (2009).
Singh, A. & Grover, N. K. Wear properties of cryogenic treated electrodes on machining of En-31. Mater, Today Proc. 2, 1406–1413 (2015).
Virdi, R. L., Chawla, P. & Singh, S. Comparing and optimization of material removal rate of cryogenically treated and untreated copper tools using EDM. Asian J. Eng. Appl. Technol. 5, 18–22 (2016).
Paper, C. Study of the effect of cryogenic treatment of tool electrodes during electro discharge machining. International Conference on Precision, Meso, Micro and Nano Engineering 8–13 (2013).
Singh Grewal, G. & Parkash Dhiman, D. Effect of deep cryogenic treatment on copper electrode for non-traditional electric discharge machining (EDM). Mechan. Sci. 10, 413–427 (2019).
Singh, R. & Singh, K. Enhancement of tool material machining characteristics with cryogenic treatment: A Review. In: Proc. 2010 International Conference on Industrial Engineering and Operations Management Dhaka, Bangladesh 9–10 (2010).
Kumar, S. V. & Kumar, M. P. Machining process parameter and surface integrity in conventional EDM and cryogenic EDM of Al–SiCp MMC. J. Manuf. Process. 20, 70–78 (2015).
Kanlayasiri, K. & Boonmung, S. Effects of wire-EDM machining variables on surface roughness of newly developed DC 53 die steel: Design of experiments and regression model. J. Mater. Process. Technol. 192, 459–464 (2007).
Farooq, M. U. et al. Curved profiles machining of Ti6Al4V alloy through WEDM: Investigations on geometrical errors. J. Mater. Res. Technol. 9, 16186–16201 (2020).
Croda Europe Ltd. Span and Tween. www.croda.com/Europe 44, 6–11 (2009).
Liu, Y., Ji, R., Zhang, Y. & Zhang, H. Investigation of emulsion for die sinking EDM. Int. J. Adv. Manuf. Technol. 47, 403–409 (2010).
Rosen Milton, J. Surfactants and Interfacial Phenomena (Wiley, 2004).
Wu, Y. et al. Metastable structures with composition fluctuation in cuprate superconducting films grown by transient liquid-phase assisted ultra-fast heteroepitaxy. Mater. Today Nano 24, 100429 (2023).
Praveen, L., Geeta Krishna, P., Venugopal, L. & Prasad, N. E. C. Effects of pulse on and off time and electrode types on the material removal rate and tool wear rate of the Ti-6Al-4V Alloy using EDM machining with reverse polarity. IOP Conf. Ser. Mater. Sci. Eng. 330, 012083 (2018).
Shao, L. et al. Why do cracks occur in the weld joint of Ti-22Al-25Nb alloy during post-weld heat treatment? Front. Mater. 10, 1135407 (2023).
Poljanec, D., Kalin, M. & Kumar, L. Influence of contact parameters on the tribological behaviour of various graphite/graphite sliding electrical contacts. Wear 406, 75–83 (2018).
Acknowledgements
The authors appreciate the support from Researchers Supporting Project Number (RSPD2024R685), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Muhammad Umar Farooq: Conceptualization Visualization, Formal analysis, Writing – original draft, Writing – review & editing. Mehdi Tlija: Validation, Funding Acquisition, Writing – review & editing. Shafahat Ali: Conceptualization, Methodology, Software, Validation, Visualization, Formal analysis, Investigation, Writing – review & editing. Anamta Khan: Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. Adediran Adeolu Adesoji: Visualization, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farooq, M.U., Tlija, M., Ali, S. et al. Application of non-ionic liquids-based modified dielectrics during electric discharge machining (EDM) of Ti6Al4V alloy to enhance machining efficiency and process optimization. Sci Rep 14, 20797 (2024). https://doi.org/10.1038/s41598-024-71447-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71447-7
Keywords
This article is cited by
-
Performance evaluation and multi-objective optimization of EDM parameters for Ti6Al4V using different tool electrodes
Scientific Reports (2025)
-
EDM-based analysis of Fe-based shape memory alloys using Cu-W electrodes with multiple output optimization and microstructural validation
Scientific Reports (2025)
-
A comprehensive review on powder mixed electrical discharge machining: advances in dielectric enhancement and machining efficiency
Discover Applied Sciences (2025)
-
Emerging frontiers in electro-discharge machining: A comprehensive review of research trends, challenges, and innovative solutions
Forschung im Ingenieurwesen (2025)
-
Feasibility study of printing chromium on the surface of AISI 4340 steel using the electrical discharge process
Progress in Additive Manufacturing (2025)