Abstract
The mosquito microbiome significantly influences vector competence, including in Aedes albopictus, a globally invasive vector. Describing the microbiome and Wolbachia strains of Ae. albopictus from different regions can guide area-specific control strategies. Mosquito samples from Spain and São Tomé were analyzed using 16S rRNA gene sequencing and metagenomic sequencing. Wolbachia infection patterns were observed by sex and population. Female mosquitoes were blood-fed, a factor considered in analyzing their microbiota. Results revealed a dominance of dual Wolbachia infections, strains A and B, in the microbiome of both populations of Ae. albopictus, especially among females. Both populations shared a core microbiome, although 5 and 9 other genera were only present in Spain and São Tomé populations, respectively. Genera like Pelomonas and Nevskia were identified for the first time in Aedes mosquitoes. This study is the first to describe the Ae. albopictus bacteriome in Spain and São Tomé, offering insights for the development of targeted mosquito control strategies. Understanding the specific microbiome composition can help in designing more effective interventions, such as microbiome manipulation and Wolbachia-based approaches, to reduce vector competence and transmission potential of these mosquitoes.
Similar content being viewed by others
Introduction
Vector-borne diseases, which account for 20% of infectious diseases worldwide, pose a significant global health threat. Illnesses like dengue fever, yellow fever, chikungunya, and Zika put nearly 3.9 billion people1 at risk, and effective management and control of these disease vectors are crucial for reducing these risks of transmission.
Aedes albopictus, a mosquito species known for its ability to transmit diseases and for being a nuisance to humans2, has a worldwide distribution3 and is responsible for spreading dengue, chikungunya, and Zika viruses4,5,6. Its invasive nature7 and adaptability to different environments8 make it a significant threat to human health. Originally from Southeast Asia, Ae. albopictus has expanded its range to other continents, facilitated by global trade of used tires, ornamental plants, and road traffic4,9,10. Moreover, in recent decades, rapid urbanization has contributed to proliferation of Ae. albopictus populations, and this, along with inadequate or absent vector control measures, has further increased the risk of pathogen transmission by this species11. The rapid spread of Ae. albopictus mosquitoes in São Tomé and Príncipe12 and Spain13 underscores the need to understand and manage this species in these regions. São Tomé and Principe, an island nation in the Gulf of Guinea, is particularly vulnerable to vector-borne diseases due to its tropical climate and limited healthcare infrastructure but colonization events should be rare14. Aedes albopictus was first identified in São Tomé in 2016, although it is probable that the species had inhabited the island for a decade prior to this discovery12. In Spain, the first Ae. albopictus was detected in 2004, at Sant Cugat del Vallès15. Since then, the mosquito’s presence has proliferated across the country13,16,17,18 due to multiple introductions from abroad19. This expansion has escalated concerns among public health officials, emphasizing the need for increased surveillance and control measures20,21,22.
The host physiology of Aedes mosquitoes is heavily influenced by their microbiota, which has been shown to impact various aspects of reproduction, egg production, blood digestion23,24,25, regulation of immunity26, genetic diversity, vector competence, host–pathogen interactions and survival19,27,28. For instance, some bacteria can produce antimicrobial peptides that boost the mosquito’s immune system, while other might facilitate the digestion of blood meals. Additionally, the presence of certain microbial communities can influence the mosquito’s ability to transmit pathogens by either supporting or hindering the development of the pathogens within the mosquito. These factors collectively contribute to the vectorial capacity of mosquitoes and are key for pathogen transmission. Innovative vector control strategies have increasingly focused on manipulating the mosquito microbiome to reduce their capacity for disease transmission29 and control of populations. One promising approach involves introducing exogenous Wolbachia strains into Ae. albopictus populations, which has shown potential in disrupting pathogen transmission30. However, most of the Ae. albopictus populations are already naturally infected with this endosymbiont31 and different Wolbachia strains may exhibit various interactions when coexisting within a host30. Describing the native strains of our target-populations is the first step to assess possible interactions between the naturally occurring Wolbachia strains and the introduced type.
The genetic background of any given species plays a role in shaping its microbiome32. Different populations of mosquitoes may have genetic variations that affect their immune responses, susceptibility to certain microorganisms, and overall interactions with the microbiome. In the same way, different geographical locations may have unique microbial communities in soil, water, and vegetation, and the local climate and temperature can impact both the mosquito and the microorganisms it harbors33. Given the significance of understanding and managing vector-borne diseases, this study aimed to examine and to compare the microbiome composition and characterize the presence of Wolbachia strains in populations of Ae. albopictus from two countries with distinct climatic, habitat, epidemiological, and geographical conditions: São Tomé and Príncipe (Africa) and Spain (Europe). Investigation on the composition of the microbiome and Wolbachia strains in Ae. albopictus populations from these distinct geographical regions could provide valuable knowledge into potential control measures tailored to the specific problems of each area. By understanding the microbiome composition and the prevalence of different Wolbachia strains, researchers can develop targeted strategies, such as microbiome manipulation or Wolbachia-based interventions, to reduce the vector competence and transmission potential of these mosquitoes. This information could help develop new vector control strategies and contribute to reducing the global burden of vector-borne diseases.
Materials and methods
Mosquito collection and rearing
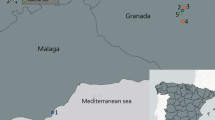
Eggs were collected using sixteen ovitraps installed in three distant regions of Spain: Valencia (Paterna), Huesca (Monzón), and Málaga (Rincón de La Victoria), as well as one region in São Tomé, Água Grande (São Tomé). This collection took place between late 2021 (September and October) and early 2022 (April), with the assistance of collaborators, as shown in Fig. 1. The ovitraps were designed as black plastic cups filled with 500 mL of dechlorinated water and lined with a strip of germination paper to provide a substrate for the mosquitoes to lay their eggs. The ovitraps were baited with a small amount of hay infusion (prepared by soaking 50 g of dried hay in 10 L of water for 3 days) to attract gravid female mosquitoes. The ovitraps were strategically placed in shaded and sheltered locations near human habitation and vegetation, ensuring optimal conditions for mosquito egg-laying. The germination papers were collected weekly, and new ones were placed in the ovitraps. The collected papers with eggs were transported to the laboratory for further processing.
Map indicating collection sites for Ae. albopictus. (A) Map of Spain showing the sampling locations in Valencia (Paterna), Huesca (Monzón), and Málaga (Rincón de La Victoria); (B) Regional map indicating the relative positions of Spain and São Tomé, per continent. (C) Map of São Tomé showing the sampling location in Água Grande. The map was generated with QGIS v3.34 software (https://qgis.org/en/site/).
Four Ae. albopictus colonies were subsequently established and maintained in the high-security insectary of the 'In Vivo Arthropod Security Facility' (VIASEF) available at IHMT, between March and May 2022. These colonies were initiated from a parental generation (F0) and maintained until the second generation (F2). Upon hatching in dechlorinated water, larvae were reared under controlled laboratory conditions (temperature: 26 ± 2 °C, relative humidity: 70 ± 5%, photoperiod: 12 h/12 h light/dark) and fed with Tetra Min flakes fish food (Tetra, Melle, Germany). The fish food flakes were processed into powder before being administered to the larvae. Adult mosquitoes were provided with a 10% glucose solution, and females were blood-fed on Mus musculus two to three times a week. The process of handling the animals used occurred under supervision and was carried out based on Community Council standards European Union of 24 November 1986 (86/609/EEC) and national legislation in force (Decree-Law 129/92 of June 2nd, Ordinance No. 100/92 of October 23rd). All animal experiments were based on protocols approved by the Direção-Geral de Veterinária, Ministério da Agricultura do Desenvolvimento Rural e das Pescas, Portugal (ID approvals: 023351 and 023355).
DNA extraction
DNA extractions from a total of 199 adult mosquitoes were carried out using two distinct methods, tailored to the specific requirements of our analyses. For the purpose of sequencing the 16S rRNA gene in a subset of 19 female samples, we employed the "NZY tissue gDNA isolation kit" provided by NZYtech, Portugal. Before DNA extraction, the 19 samples were treated with 10% bleach, followed by 70% alcohol, and finally distilled water to sterilize the mosquitoes. These selected samples originated from adult female mosquitoes belonging to the F0 generation, specifically targeted for our 16S rRNA gene sequencing study to provide a detailed microbiome analysis. The selection of these 19 samples was based on ensuring a representative subset that included mosquitoes from both geographic locations (Spain and São Tomé and Príncipe) and aimed to capture any potential differences in microbiota composition between these regions. The limited number of samples for 16S rRNA gene sequencing was due to resource constraints and the need to focus on a manageable subset for high-throughput sequencing and in-depth microbiome characterization.
For the remaining 180 samples, we utilized a ribosomal DNA extraction protocol that was adapted from the methodology described by Collins et al.34. This approach was applied to both male and female adult mosquitoes from the F0 and F1 generations to facilitate a comprehensive comparison of Wolbachia prevalence and strain distribution across the populations under study.
All DNA extractions were performed on individual mosquitoes, not pooled samples, to ensure accurate representation of each mosquito’s microbiome and Wolbachia infection status.
To ensure the integrity of our DNA extraction process and to eliminate the possibility of contamination, negative controls were systematically included in each batch of extractions.
16S metagenomic sequencing
The bacterial composition of Ae. albopictus microbiota was investigated by sequencing the V3 and V4 regions of the bacterial 16S rRNA gene in 19 samples extracted with the NZY tissue gDNA isolation kit. The sequencing of the amplified products was performed on the Illumina MiSeq platform using v3 chemistry and a 2 × 300 bp paired-end module. Library construction, sequencing, and bioinformatics analysis were performed by Eurofins Genomics Europe Sequencing GmbH (Konstanz, Germany). Briefly, bioinformatics pipeline began with the removal of sequences containing ambiguous bases, followed by the identification and exclusion of chimeric reads utilizing the UCHIME algorithm within the VSEARCH package35,36. The remaining high-quality sequences were then processed through Minimum Entropy Decomposition (MED), which efficiently partitions the marker gene datasets into Operational Taxonomic Units (OTUs)37,38. This method capitalizes on Shannon entropy, selectively considering only the informative nucleotide positions, thus enabling the delineation of sequences based on single nucleotide variances without the interference of random sequencing errors.
After OTU generation, taxonomic assignments were conducted through DC-MEGABLAST alignments against a curated reference database, ensuring the provision of the most specific taxonomic nomenclature to each OTU from the best-matching reference sequences. We applied the QIIME 1.9.1 software package for additional processing of OTUs and their taxonomic classifications, where the abundance data of bacterial taxa were normalized based on lineage-specific gene copy numbers to enhance accuracy39. A minimum sequence identity of 70% over at least 80% of the representative sequence was required to consider the sequence as a reference. Further processing of OTUs and taxonomic assignments was performed using QIIME software (version 1.9.1, http://qiime.org/). The abundances of bacterial taxonomic units were normalized using the 16S rRNA Gene Copy Number (GCN) correction. For this, the read numbers assigned to a species were divided by the known or assumed number of marker gene regions39. The analytical output comprised a comprehensive suite of files, elucidating the taxonomic structure and diversity within the Ae. albopictus microbiota. These outputs included summarized lists of identified taxonomic units per sample, representative sequences for each OTU, and matrices detailing the estimated abundances of OTUs and taxonomic units across samples. Alpha diversity was assessed using Shannon and Simpson diversity indices to evaluate the bacterial diversity within individual samples. Beta diversity was analyzed to compare the bacterial community composition between samples from Spain and São Tomé. These indices were calculated using the QIIME 1.91. software package36.
All sequences have been submitted to NCBI under the accession number for SRA data PRJNA1028981 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1028981).
Wolbachia genotyping
PCR genotyping of Wolbachia infection in 180 samples used Wolbachia-specific primers 328Fw and 691Rv for wAlbA, and 183F and 691R for wAlbB40. PCR mix and thermal cycling conditions were standardized. Universal primers wsp 81Fw and 691Rv were used to confirm Wolbachia-negative samples. Primers and PCR conditions details are provided in Supplementary Table S1.
Statistical analysis
Mixed and single infection rates were calculated by sex and population41. Comparisons between mixed and single infections in females and males were performed using the chi-square test. A p-value less than 0.05 was considered statistically significant.
Results
Microbiome composition
To analyze the bacterial communities within Ae. albopictus from Spain and São Tomé, we conducted 16S rRNA gene sequencing of the V3–V4 hypervariable regions across 19 individual adult female samples. After pre-processing and quality control, five samples from São Tomé yielded 606.801 clean sequences, and 14 samples from Spain yielded 798.002 clean sequences (Table 1). All these clean sequences were classified into OTUs (Operational Taxonomic Units): 344 from São Tomé and 517 from Spain (Table 1). The OTUs represented different taxonomic levels, and a correction was made to the species found39 (Supplementary Tables S2 and S3). The Fig. 2 shows shared and non-shared bacterial genera among the populations.
Compared to other studies on the microbiota of mosquitoes from the field, the number of taxa found per sample in our study is relatively low. To ensure that we captured the maximum diversity, rarefaction curves were generated for each sample. These curves, which plot the number of observed OTUs against the number of sequences sampled, indicated that the sequencing depth was sufficient to capture the majority of the bacterial diversity present in our samples (Supplementary Fig. S1). The rarefaction analysis confirmed that our sequencing effort was adequate, as the curves approached an asymptote, suggesting that additional sequencing would likely yield few additional OTUs.
Taxonomic units overview and microbiome diversity analysis
Tables 2 and 3 provide an overview of the taxonomic units identified in samples from São Tomé and Spain. Taxonomic units with readings below 0.1% were categorized as "other." OTU readings analysis showed a predominance of the genus Wolbachia in all samples, ranging between 92.4 and 98.8% in São Tomé samples and between 96.1 and 97.5% in Spanish samples.
Shannon and Simpson indices (Table 4) revealed similar bacterial diversity between Spanish and São Tomé populations, as well as among individual samples (Supplementary Table S4).
Wolbachia genotyping
Of the 180 adult mosquitoes analyzed (97 females and 83 males), 178 (98.89%) were infected with Wolbachia. Five (2.78%) were infected with the wAlbA strain and 34 (18.88%) with the wAlbB strain. Mixed infections with wAlbA and wAlbB strains occurred in 77.22% of the infected mosquitoes (Table 5). Single wAlbA infections were exclusive to females (p < 0.05), while single wAlbB infections occurred in both males and females but were more prevalent in males (p < 0.05). Mixed infections were more common in females than males (85.57% in females and 67.47% in males, of the total number of infected mosquitoes) (p < 0.05). A predominance of mixed infections was observed in all locations, with rates ranging from 62.50 to 82.76%. However, simple wAlbB infections were detected only in the three provinces of Spain (Valencia, Huesca, and Málaga) with rates ranging from 13.79 to 31.25% (Table 6) and simple wAlbA infections were found only in two provinces of Spain (Valencia and Huesca). In São Tomé, only mixed infections by wAlbAeB were found, all in females. Furthermore, it was the only place where negative samples (N = 2) were detected in males.
Discussion
Aedes albopictus microbiome
This study represents the inaugural attempt to provide a comprehensive insight into the composition of the Ae. albopictus bacterial microbiome in wild populations originating from Spain and São Tomé through the sequencing of the V3–V4 regions of the 16S rRNA gene. The main phyla identified in the Ae. albopictus bacterial microbiome were Proteobacteria and Firmicutes, consistent with previous studies42,43. Both populations exhibited unique bacterial genera (Spain: Aquabacterium, Methylophilus, Nevskia, Solimonas and Sphingomonas; São Tomé: Asaia, Dechloromonas, Aerococcus, Bacillus, Brevundimonas, Pseudomonas, Rhizobium and Stenotrophomonas), suggesting a geographic influence on the microbiome43. A shared bacterial microbiome was also observed, comprising Acinetobacter, Pelomonas, Ralstonia, and Wolbachia genera. In addition to Wolbachia, these results suggest the existence of a bacterial microbiome shared among populations of Ae. albopictus geographically very distant44. The presence of Pelomonas and Nevskia genera in the Aedes genus is a novel finding, and their role in the microbiome of hematophagous insects remains unknown, and further studies are needed to understand their interactions with hosts45,46,47,48. Pelomonas and Nevskia are often found in environmental samples, raising the question of whether these bacteria could be externally adhered to the mosquitoes. To address this, we ensured that the mosquitoes were thoroughly cleaned before DNA extraction. The cleaning process involved washing the mosquitoes with a solution of 0.1% bleach followed by 70% alcohol. This procedure is designed to remove external contaminants, thereby ensuring that the DNA extracted and analyzed represents the internal microbiome. Therefore, the presence of Pelomonas and Nevskia is likely indicative of their role within the mosquito microbiome rather than external contamination.
Consistent with prior research, the bacterial composition of Ae. albopictus was predominantly governed by the Wolbachia genus49. When Wolbachia infection is highly dominant, it can "mask" the DNA of other bacteria present42,50 leading to undetected and unsequenced sequences. Future research may consider individual tissue-based microbiome analysis51, to ensure that Wolbachia, which is naturally abundant in the ovaries, does not interfere with the sequencing data.
This highly dominant infection by Wolbachia in both populations may be related to interactions between microbiome components, such as interspecific competition for resources44,52 but also to laboratory rearing conditions49. Research into the microbiota of laboratory and wild Aedes mosquitoes has revealed that both are primarily characterized by a limited number of phyla44. Nonetheless, although the overall microbiota composition is comparable in laboratory-reared and wild mosquitoes, it was observed that the diversity of midgut bacterial communities was more extensive in mosquitoes collected from natural environments. In this study, female mosquitoes were blood-fed, which can substantially modify their microbiota. When compared to other studies on field-collected mosquitoes, the number of taxa found per sample in our study is relatively low. This discrepancy could be attributed to several factors. First, the laboratory rearing conditions, including the controlled diet and environment, may have influenced the microbial diversity, leading to a reduction in taxa compared to field-collected samples. Second, the dominance of Wolbachia in our samples might have overshadowed the detection of other bacterial taxa, potentially due to interspecific competition or the high abundance of Wolbachia masking other bacterial DNA. In fact, the diversity of female midgut bacteria in Ae. albopictus from the laboratory was reduced when compared to females from those from the field53. These disparities could be originated from variations in the origin of the blood meal, dietary factors, and habitat53,54. Furthermore, recent studies have indicated a more consistent and greater bacterial diversity in breeding water compared to larvae and adults, regardless of sample source, with a notable decrease in the microbial community diversity between the larvae and newly emerged adult mosquitoes53. The impact of blood meals and rearing within an insectary environment on the Ae. albopictus microbiome warrants thorough investigation and future studies should consider the analysis of larvae’s microbiome and the bacterial diversity of the breeding water.
Wolbachia genotyping
To better understand the Wolbachia populations naturally present in these Ae. albopictus, A and B strains31,55 were genotyped. Both strains were found to infect the Spanish and São Tomé populations in three different scenarios: mixed infection with both strains, simple infection with wAlbA, or simple infection with wAlbB. Mixed infection, which is predominant worldwide56,57,58,59,60 was also the predominant form in this study. Several factors could explain these results, including species susceptibility to infection, facilitation of secondary infections by an active Wolbachia infection, or the stable maintenance of dual infections in hosts61.
In line with previous studies31,62, the prevalence of mixed infections was significantly higher in females compared to males and this could be due to the maternal transmission31, ineffective transmission of one strain, or physiological mechanisms allowing Wolbachia infection in females but not in males63. The exact mechanisms of Wolbachia infection and dissemination in mosquito vectors remain unclear64. Maternal transmission is a key mechanism for the spread of Wolbachia, and high rates of maternal transmission have been documented for mixed infections31. However, some studies have identified simple infections in the progeny of females with mixed infections, indicating the possibility of inefficient transmission of one of the strains63. This phenomenon could also occur in the transmission from female progenitors to their male offspring, explaining the lower prevalence of mixed infections in males. Physiological differences between male and female mosquitoes also play a role. Wolbachia tends to infect the germline cells of the host, persisting in the ovaries of females but not infecting the sperm in males. This differential infection could be due to a tropism of Wolbachia for ovarian tissues, allowing stable transmission through the female line, while infection in males is not stable or necessary for the maintenance of the endosymbiont31,62.
The prevalence of wAlbB infection was higher than wAlbA, supporting prior findings for European populations55,57,62. This inequality is particularly pronounced in males and could be attributed to reduced wAlbA density over time57 or a lower reproduction rate63,65. Research has suggested that females may not efficiently transmit wAlbA to their progeny, resulting in a reduction in male fertility due to Cytoplasmic Incompatibility (CI)55.
In this study, males from São Tomé population were not infected by Wolbachia. In fact, low Wolbachia prevalence has been detected in natural Ae. albopictus infections in Cameroon, one of the possible origins of Ae. albopictus from São Tomé66. Therefore, is not possible to assume that males in this region do not host Wolbachia, as infected females were found. Thus, the absence of infected males in São Tomé may be related to the lower prevalence of endosymbiont infection in males and the small sample size (N = 2). This small sample size limits the ability to draw definitive conclusions and may not accurately represent the population. Cautious interpretation is required, and future studies with larger sample sizes are necessary to validate this finding and provide a more accurate representation of Wolbachia prevalence in the male mosquito population from São Tomé.
The number of Wolbachia-negative samples was minimal (1.11%), indicating that innate Wolbachia infection may provide selective advantages to hosts, such as higher fecundity or hatching rates67. Geographic variation in Wolbachia infection has been reported68, but this study did not find significant differences between samples from different locations in Spain, as well as other studies carried out in other locations of this country60. An uniform distribution pattern with a predominance of mixed infections has been suggested and supported by prior studies for Ae. albopictus populations across Europe62.
The rapid dissemination and uniformity of distribution in Europe could result from multiple introductions69,70 in different Mediterranean countries, leading to constant mixed infection of Ae. albopictus by Wolbachia. Maternal inheritance and cytoplasmic incompatibility may also play a role in promoting the spread of Wolbachia infection71.
Aedes albopictus vector control
To control Ae. albopictus populations, one approach involves creating a colony with a stable infection of specific exogenous Wolbachia strains that cause Cytoplasmic Incompatibility (CI) and non-viable offspring. The Incompatible Insect Technique (IIT) involves the release of males carrying these Wolbachia strains into the environment, leading to a reduction in the target population. This approach has been tested and implemented in Ae. albopictus, which naturally hosts two Wolbachia strains (wAlbA and wAlbB). Introducing a third strain, wPip (found in Culex pipiens molestus), resulted in a triple-infected colony that effectively decreased the number of Ae. albopictus females in the study area72. Recent strategies employed wPip transinfection in Ae. albopictus, accompanied by the removal of its native Wolbachia strains, yielding promising outcomes73. These findings underscore IIT's potential as a future tool for Ae. albopictus control.
To conclude, this study significantly enhances our understanding of the bacterial microbiome of Ae. albopictus populations from Spain and São Tomé. Our study provides valuable insights into the microbiota composition of Ae. albopictus populations from Spain and São Tomé, revealing the presence of Wolbachia and other bacterial endosymbionts, which establishes a foundational framework for future manipulations and the introduction of new strains for vector control. As the scientific community intensifies its search for innovative and effective methods to combat vector-borne diseases, the insights from this study become increasingly invaluable. Consistent with previous research, we found that the microbiota of Ae. albopictus is predominantly governed by the Wolbachia genus. The dominance of Wolbachia in both populations suggests a stable association, which could be considered a part of the core microbiota of Ae. albopictus. The concept of core microbiota refers to the set of microbial taxa that are consistently found across different populations of a host species, regardless of geographical location or environmental conditions. In our study, we identified several bacterial genera that were shared between the Spanish and São Tomé populations, such as Acinetobacter, Pelomonas, Ralstonia, and Wolbachia. This finding supports the idea that Ae. albopictus harbors a core microbiota, which may play crucial roles in the mosquito's physiology, including reproduction, immune regulation, and vector competence. Furthermore, the identification of novel genera like Pelomonas and Nevskia in Ae. albopictus highlights the dynamic nature of the mosquito microbiota and suggests potential new areas of research to explore their roles in mosquito biology and pathogen transmission. The presence of these core and unique bacterial taxa emphasizes the importance of understanding the microbiota composition for developing targeted vector control strategies.
Despite the valuable insights provided by this study, there are several limitations that should be acknowledged. The most significant limitation is the small sample size used for the microbiome analysis and the Wolbachia genotype study, particularly for mosquitoes from São Tomé. The limited number of samples may have restricted the detection of the full diversity of the microbiota and Wolbachia strains, potentially overlooking important taxa and variations. Additionally, the use of laboratory-reared mosquitoes, although necessary for controlled conditions, might not fully represent the natural microbiota found in field populations due to differences in environmental exposures and diet.
To ensure the robustness of our findings, future studies should aim to include larger sample sizes and consider a broader range of geographical locations. Furthermore, incorporating field-collected mosquitoes and longitudinal sampling could provide a more comprehensive understanding of the microbiota dynamics and their implications for vector competence and control strategies.
While the presence of Wolbachia in Ae. albopictus is well established, our study contributes to the understanding of Wolbachia strain distribution and prevalence in new geographical regions, specifically Spain and São Tomé. We observed a consistent dominance of Wolbachia across different populations, reinforcing the stability of this endosymbiont's association with its host. However, we also highlight the presence of dual infections with different Wolbachia strains, which could have implications for vector control strategies leveraging Wolbachia-induced pathogen interference.
Future research directions to expand upon our findings include examining the impact of the bacterial microbiome on Ae. albopictus vector competence, evaluating the potential of CRISPR/Cas9 gene editing in endosymbionts for vector control, investigating paratransgenic strategies, assessing the ecological consequences of microbiome manipulation, and using advanced sequencing technologies for a more detailed understanding of the mosquito microbiome.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI under the accession number for SRA data PRJNA1028981 (temporary submission ID: SUB13907152).SRA records will be accessible with the following link after the release date (2024-11-01): http://www.ncbi.nlm.nih.gov/bioproject/1028981.
References
WHO. Launch of the Global Arbovirus Initiative. https://www.who.int/news-room/events/detail/2022/03/31/default-calendar/global-arbovirus-initiative (2022).
Capinera, J. Encyclopedia of Entomology (Springer, 2008).
Manni, M. et al. Genetic evidence for a worldwide chaotic dispersion pattern of the arbovirus vector, Aedes albopictus. PLoS Negl. Trop. Dis. 11, e0005332 (2017).
Paupy, C., Delatte, H., Bagny, L., Corbel, V. & Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 11, 1177–1185 (2009).
Effler, P. V. et al. Dengue fever, Hawaii, 2001–2002. Emerg. Infect. Dis. https://doi.org/10.3201/eid1105.041063 (2005).
Ramchurn, S. K., Moheeput, K. & Goorah, S. S. An analysis of a short-lived outbreak of dengue fever in Mauritius. Euro Surveill. 14, (2009).
Medlock, J. M. et al. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. https://doi.org/10.1017/S0007485315000103 (2015).
Swan, T. et al. A literature review of dispersal pathways of Aedes albopictus across different spatial scales: implications for vector surveillance. Parasites Vectors 15, (2022).
Eritja, R., Palmer, J. R. B., Roiz, D., Sanpera-Calbet, I. & Bartumeus, F. Direct evidence of adult Aedes albopictus dispersal by Car. Sci. Rep. 7, 14399 (2017).
Gratz, N. G. Critical review of the vector status of Aedes albopictus. Med. Vet. Entomol. https://doi.org/10.1111/j.0269-283X.2004.00513.x (2004).
Hasan, S., Jamdar, S. F., Alalowi, M., Beaiji, A. A. A. & S. M.,. Dengue virus: A global human threat: Review of literature. J. Int. Soc. Prev. Commun. Dent. https://doi.org/10.4103/2231-0762.175416 (2016).
Reis, S., Cornel, A. J., Melo, M., Pereira, H. & Loiseau, C. First record of Aedes albopictus (Skuse 1894) on São tomé island. Acta Trop. 171, 86–89 (2017).
Goiri, F. et al. Progressive invasion of Aedes albopictus in Northern Spain in the period 2013–2018 and a possible association with the increase in insect bites. Int. J. Environ. Res. Public Health 17, 1678 (2020).
Loiseau, C. et al. High endemism of mosquitoes on São Tomé and Príncipe Islands: Evaluating the general dynamic model in a worldwide island comparison. Insect. Conserv. Divers. 12, 69–79 (2019).
Aranda, C., Eritja, R. & Roiz, D. First record and establishment of the mosquito Aedes albopictus in Spain. Med. Vet. Entomol. 20, 150–152 (2006).
European Centre for Disease Prevention and Control and European Food Safety Authority. Mosquito maps. European Centre for Disease Prevention and Control (ECDC). https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (2024).
Garrido, M. et al. Aedes albopictus in a recently invaded area in Spain: Effects of trap type, locality, and season on mosquito captures. Sci. Rep. 14, 2131 (2024).
Collantes, F. et al. Review of ten-years presence of Aedes albopictus in Spain 2004–2014: Known distribution and public health concerns. Parasit Vectors 8, 1–11 (2015).
Lucati, F. et al. Multiple invasions, Wolbachia and human-aided transport drive the genetic variability of Aedes albopictus in the Iberian Peninsula. Sci. Rep. 12, 20682 (2022).
ECDC. Autochthonous Cases of Dengue in Spain and France (2019).
Monge, S. et al. Characterization of the first autochthonous dengue outbreak in Spain (August–September 2018). Acta Trop. 205, 105402 (2020).
Navero-Castillejos, J. et al. Molecular characterization of imported and autochthonous dengue in northeastern spain. Viruses 13, 1910 (2021).
Gaio, A. D. O. et al. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (diptera: Culicidae) (L.). Parasit. Vectors 4, 1–10 (2011).
Coon, K. L., Brown, M. R. & Strand, M. R. Gut bacteria differentially affect egg production in the anautogenous mosquito Aedes aegypti and facultatively autogenous mosquito Aedes atropalpus (Diptera: Culicidae). Parasit. Vectors 9, 1–12 (2016).
Sicard, M., Bonneau, M. & Weill, M. Wolbachia prevalence, diversity, and ability to induce cytoplasmic incompatibility in mosquitoes. Curr. Opin. Insect Sci. https://doi.org/10.1016/j.cois.2019.02.005 (2019).
Xi, Z., Ramirez, J. L. & Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 4, e1000098 (2008).
Dickson, L. B. et al. Carryover effects of larval exposure to different environmental bacteria drive adult trait variation in a mosquito vector. Sci. Adv. 3, e1700585 (2017).
Souza-Neto, J. A., Powell, J. R. & Bonizzoni, M. Aedes aegypti vector competence studies: A review. Infect. Genet. Evol. https://doi.org/10.1016/j.meegid.2018.11.009 (2019).
Jupatanakul, N., Sim, S. & Dimopoulos, G. The insect microbiome modulates vector competence for arboviruses. Viruses. https://doi.org/10.3390/v6114294 (2014).
Blagrove, M. S. C., Arias-Goeta, C., Di Genua, C., Failloux, A. B. & Sinkins, S. P. A Wolbachia wMel transinfection in Aedes albopictus is not detrimental to host fitness and inhibits Chikungunya virus. PLoS Negl. Trop. Dis. 7, e2152 (2013).
Ahmad, N. A., Vythilingam, I., Lim, Y. A. L., Zabari, N. Z. A. M. & Lee, H. L. Detection of Wolbachia in Aedes albopictus and their effects on chikungunya virus. Am. J. Trop. Med. Hyg. 96, 148–156 (2017).
Novakova, E. et al. Mosquito microbiome dynamics, a background for prevalence and seasonality of West Nile virus. Front. Microbiol. 8, (2017).
Dada, N. et al. Considerations for mosquito microbiome research from the Mosquito Microbiome Consortium. Microbiome. https://doi.org/10.1186/s40168-020-00987-7 (2021).
Collins, F. H. et al. Comparison of DNA-probe and isoenzyme methods for differentiating Anopheles gambiae and Anopheles arabiensis (Diptera: Culicidae). J. Med. Entomol. 25, 116–120 (1988).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Rognes, T., Flouri, T., Nichols, B., Quince, C. & Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, (2016).
Eren, A. M. et al. Oligotyping: di erentiating between closely related microbial taxa using 16s rRNA gene data. Methods Ecol. Evol. 4, 1111–11119 (2013).
Eren, A. M. et al. Minimum entropy decomposition: Unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 9, 968–979 (2015).
Angly, F. E. et al. CopyRighter: A rapid tool for improving the accuracy of microbial community profiles through lineage-specific gene copy number correction. Microbiome 2, (2014).
Zhou, W., Rousset, F. & O’Neill, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. B Biol. Sci. 265, 209–515 (1998).
Vassarstats. Vassarstats: Website for Statistical Computation). http://vassarstats.net/newcs.html.
Bennett, K. L. et al. Dynamics and diversity of bacteria associated with the disease vectors Aedes aegypti and Aedes albopictus. Sci. Rep. 9, 12160 (2019).
Zhao, T. et al. Metagenome sequencing reveals the microbiome of Aedes albopictus and its possible relationship with dengue virus susceptibility. Front. Microbiol. 13, 891151 (2022).
Scolari, F., Casiraghi, M. & Bonizzoni, M. Aedes spp. and their microbiota: A review. Front. Microbiol. https://doi.org/10.3389/fmicb.2019.02036 (2019).
Vicente, C. S. L. et al. Characterization of bacterial communities associated with the pine sawyer beetle Monochamus galloprovincialis, the insect vector of the pinewood nematode Bursaphelenchus xylophilus. FEMS Microbiol. Lett. https://doi.org/10.1111/1574-6968.12232 (2013).
Zhukova, M., Sapountzis, P., Schiøtt, M. & Boomsma, J. J. Diversity and transmission of gut bacteria in Atta and Acromyrmex leaf-cutting ants during development. Front. Microbiol. 8, 1942 (2017).
Santos-Garcia, D., Mestre-Rincon, N., Zchori-Fein, E. & Morin, S. Inside out: Microbiota dynamics during host-plant adaptation of whiteflies. ISME J. 14, 847–856 (2020).
Tabbabi, A., Mizushima, D., Yamamoto, D. S. & Kato, H. Sand flies and their microbiota. Parasitologia 2, 71–87 (2022).
Lin, D. et al. Bacterial composition of midgut and entire body of laboratory colonies of Aedes aegypti and Aedes albopictus from Southern China. Parasit. Vectors 14, 1–13 (2021).
Minard, G. et al. Pyrosequencing 16S rRNA genes of bacteria associated with wild tiger mosquito Aedes albopictus: A pilot study. Front. Cell Infect. Microbiol. 4, 59 (2014).
Rosso, F. et al. Reduced diversity of gut microbiota in two Aedes mosquitoes species in areas of recent invasion. Sci. Rep. 8, 16091 (2018).
Brinker, P., Fontaine, M. C., Beukeboom, L. W. & Falcao Salles, J. Host, symbionts, and the microbiome: The missing tripartite interaction. Trends Microbiol. https://doi.org/10.1016/j.tim.2019.02.002 (2019).
Scolari, F. et al. Exploring changes in the microbiota of Aedes albopictus: Comparison among breeding site water, larvae, and adults. Front. Microbiol. 12, 624170 (2021).
Tuanudom, R., Yurayart, N., Rodkhum, C. & Tiawsirisup, S. Diversity of midgut microbiota in laboratory-colonized and field-collected Aedes albopictus (Diptera: Culicidae): A preliminary study. Heliyon 7, e08259 (2021).
Tortosa, P. et al. Wolbachia age-sex-specific density in Aedes albopictus: A host evolutionary response to Cytoplasmic Incompatibility?. PLoS One 5, e9700 (2010).
Lo, N., Casiraghi, M., Salati, E., Bazzocchi, C. & Bandi, C. How many Wolbachia supergroups exist?. Mol. Biol. Evol. https://doi.org/10.1093/oxfordjournals.molbev.a004087 (2002).
Shaikevich, E., Bogacheva, A. & Ganushkina, L. Dirofilaria and Wolbachia in mosquitoes (Diptera: Culicidae) in central European Russia and on the Black Sea coast. Parasite. 26, (2019).
Hu, Y. et al. Identification and molecular characterization of Wolbachia strains in natural populations of Aedes albopictus in China. Parasit. Vectors. 13, (2020).
Puerta-Guardo, H. et al. Wolbachia in native populations of Aedes albopictus (Diptera: Culicidae) from Yucatan Peninsula, Mexico. J. Insect Sci. 20, (2020).
Bueno-Marí, R. et al. Infecciones por Wolbachia pipientis en poblaciones de Aedes albopictus en la ciudad de València (España): Implicaciones para el control de mosquitos. Rev. Esp. Salud. Publica. 97, (2023).
Werren, J. H., Windsor, D. & Guo, L. Distribution of Wolbachia among neotropical arthropods. Proc. R. Soc. B Biol. Sci. 262, 197–204 (1995).
Belo, F. Wolbachia Infection in European Populations of Aedes albopictus (Universidade Nova de Lisboa Instituto de Higiene e Medicina Tropical, 2021).
Kittayapong, P., Baisley, K. J., Sharpe, R. G., Baimai, V. & O’Neill, S. L. Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am. J. Trop. Med. Hyg. 66, 103–107 (2002).
Bi, J. & Wang, Y. F. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci. https://doi.org/10.1111/1744-7917.12731 (2020).
Wiwatanaratanabutr, I. & Kittayapong, P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 102, 220–224 (2009).
Bamou, R. et al. Wolbachia detection in field-collected mosquitoes from Cameroon. Insects 12, 1133 (2021).
Dobson, S. L., Rattanadechakul, W. & Marsland, E. J. Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus. Heredity (Edinb) 93, 135–142 (2004).
Joanne, S. et al. Distribution and dynamics of Wolbachia infection in Malaysian Aedes albopictus. Acta Trop. 148, 38–45 (2015).
Pichler, V. et al. Complex interplay of evolutionary forces shaping population genomic structure of invasive Aedes albopictus in southern Europe. PLoS Negl. Trop. Dis. 13, e0007554 (2019).
Kotsakiozi, P. et al. Population genomics of the Asian tiger mosquito, Aedes albopictus: Insights into the recent worldwide invasion. Ecol. Evol. 7, 10143–10157 (2017).
Alphey, L. Genetic control of mosquitoes. Annu. Rev. Entomol. 59, 205–224 (2014).
Mains, J. W., Brelsfoard, C. L., Rose, R. I. & Dobson, S. L. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 6, 33846 (2016).
Caputo, B. et al. A bacterium against the tiger: Preliminary evidence of fertility reduction after release of Aedes albopictus males with manipulated Wolbachia infection in an Italian urban area. Pest Manag. Sci. 76, 1324–1332 (2020).
Acknowledgements
This work was initially conceived and designed during research stay of DBB at GHTM in 2019, as part of the Short-Term Scientific Missions (STSM) within the AIM-COST CA17108 Action. Subsequently, this study was funded by the Fundação para a Ciência e Tecnologia funding to GHTM-UID/04413/2020, LA-REAL-LA/P/0117/2020 and “healTh RIsk and social vulnerability to Arboviral Diseases in mainland Portugal” (TRIAD)—Ref. PTDC/GES-OUT/30210/2017.
Author information
Authors and Affiliations
Contributions
Research design: G.S. and C.A.S.; Mosquito collections: C.A.S., D.B.B., S.D.E.; Molecular genotyping: T.M., G.S.; Data analysis: G.S., T.M., C.A.S.; Manuscript writing: all authors; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Melo, T., Sousa, C.A., Delacour-Estrella, S. et al. Characterization of the microbiome of Aedes albopictus populations in different habitats from Spain and São Tomé. Sci Rep 14, 20545 (2024). https://doi.org/10.1038/s41598-024-71507-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71507-y
Keywords
This article is cited by
-
Effects of Wolbachia removal on microbial composition and diversity in Aedes albopictus: implication of using wAlbB for discriminating irradiation-based sterile and wild males
Infectious Diseases of Poverty (2025)
-
Virome profiling of Aedes albopictus across urban ecosystems in Guangdong reveals sex-specific diversity
Parasites & Vectors (2025)