Abstract
Cytokine storm (CS) emerges as an exacerbated inflammatory response triggered by various factors such as pathogens and excessive immunotherapy, posing a significant threat to life if left unchecked. Quercetin, a monomer found in traditional Chinese medicine, exhibits notable anti-inflammatory and antiviral properties. This study endeavors to explore whether quercetin intervention could mitigate CS through a combination of network pharmacology analysis and experimental validation. First, common target genes and potential mechanisms affected by quercetin and CS were identified through network pharmacology, and molecular docking experiments confirmed quercetin and core targets. Subsequently, in vitro experiments of Raw264.7 cells stimulated by lipopolysaccharide (LPS) showed that quercetin could effectively inhibit the overexpression of pro-inflammatory mediators and regulate the AKT1-FoxO1 signaling pathway. At the same time, quercetin can reduce ROS through the Keap1-Nrf2 signaling pathway. In addition, in vivo studies of C57BL/6 mice injected with LPS further confirmed quercetin's inhibitory effect on CS. In conclusion, this investigation elucidated novel target genes and signaling pathways implicated in the therapeutic effects of quercetin on CS. Moreover, it provided compelling evidence supporting the efficacy of quercetin in reversing LPS-induced CS, primarily through the regulation of the AKT1-FoxO1 and Keap1-Nrf2 signaling pathways.
Similar content being viewed by others
Introduction
Cytokine storm (CS) is a systemic inflammatory syndrome with excessive hyperactivation of immune cells characterized by increased cytokine release, including interleukin-6 (IL-6), tumor necrosis factor α (TNF-α) and monocyte chemotactic protein 1 (MCP-1), which causes severe pathologic complications, such as sepsis, tissue damage, multiple organ failure, and ultimately, death1. CS might be stimulated by multiple factors such as pathogens, auto-inflammation, monogenic, or therapeutic intervention and the lungs are the main organ to be affected by CS2.
Macrophages play a pivotal role in infection and inflammation as the principal innate immune cells, exerting crucial regulatory functions in pathological inflammation. Within the tissue microenvironment, they exhibit polarization into either the classically activated M1 phenotype, characterized by pro-inflammatory properties, or the alternatively activated M2 phenotype, which demonstrates anti-inflammatory characteristics. Dysregulation in macrophage phenotypes can result in unchecked inflammatory responses, thereby precipitating CS and subsequent tissue damage3. Considering the pivotal role of macrophages in CS progression, modulating macrophage overactivation emerges as a promising strategy for CS intervention.
Quercetin, a flavonoid compound, possesses a spectrum of biological properties, including antioxidant, anti-inflammatory, antiviral, and neuroprotective effects4,5,6,7. Research indicated that quercetin exerted its anti-inflammatory effects by targeting Syk/Src/IRAK-1 to inhibit LPS-induced macrophage activation, while also preventing LPS-induced oxidative stress and inflammation through pathways NOX2/ROS/NF-κB8,9. However, the specific targets and signaling pathways through which quercetin regulates CS remain elusive. Therefore, we embarked on an exploration of new targets and potential mechanisms of quercetin for CS treatment, employing network pharmacology, molecular docking, and experimental validation techniques.
The Forkhead box O (FoxO) family of transcription factors assumes pivotal roles in diverse cellular processes encompassing cell growth, metabolism, survival, and inflammation10,11,12. Nonetheless, FoxO1’s nuclear export or phosphorylation culminates in its inactivation, abrogating its capacity to engage with target regulatory elements13. Notably, studies have underscored that elevated FoxO1 expression post-inflammatory injury prompts macrophages to unleash an array of inflammatory mediators, thereby exacerbating inflammatory damage14,15. In LPS-treated mice, macrophages exhibited heightened FoxO1 levels; transfection of FoxO1 into Raw264.7 cells markedly upregulated interleukin-1β (IL-1β) and concurrently downregulated interleukin-10 (IL-10) expression16. Furthermore, FoxO1 serves as a direct substrate of AKT, and its activity hinges on AKT phosphorylation. Notably, AKT inhibition in macrophages abolishes FoxO1 phosphorylation and nuclear exclusion, signifying AKT phosphorylation as a pivotal regulatory event governing FoxO1 activity17. Conversely, the Keap1-Nrf2 pathway constitutes a principal defense mechanism safeguarding cells and tissues against oxidative stress while upholding homeostasis. Kelch-like ECH-associated protein 1 (Keap1) serves as an electrophilic reagent and sensor of redox damage, whereas Nuclear factor erythroid 2-related factor (Nrf2) acts as a transcription factor modulating various cytoprotective genes. Oxidative stress prompts Keap1 modification, resulting in its inactivation and disassociation from Nrf2. Consequently, stabilized Nrf2 translocates to the nucleus, where it acts as a transcription factor, activating oxidative stress-responsive genes, thereby exerting antioxidant effects18.
In this study, we found that quercetin could effectively inhibit inflammatory responses and oxidative stress in vitro and exhibited anti-inflammatory activity in mice model. Likewise, the AKT1-FoxO1 and Keap1-Nrf2 signaling pathways may be involved in quercetin-mediated anti-inflammatory and antioxidant activities.
Materials and methods
Screening for target genes of quercetin and CS
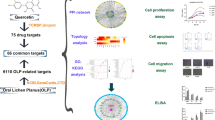
The two-dimensional (2D) molecular structure and SMILES of quercetin were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/), the world's largest database of chemical information. To predict potential quercetin targets, 2D structures or SMILES were imported into the Swiss Target Prediction Database (http://swisstargetprediction.ch/). The target genes associated with CS were acquired from the OMIM (https://www.omim.org), GeneCards (https://www.genecards.org), and PharmGKB (https://www.pharmgkb.org) databases by using the following keywords; “cytokine storm” and “cytokine release syndrome.” Subsequently, to analyze and screen common target genes of CS and quercetin, the Venny.2.1.0 e-mapping tool (https://bioinfogp.cnb.csic.es/tools/venny/) was used, and then a Venn diagram was drawn.
To acquire a protein–protein interaction network (PPI), the overlapping genes were submitted to the STRING database (https://cn.string-db.org/); the species restriction was “Homo sapiens,” and the confidence level was > 4.0 for exploring their relationship.
The acquired data were imported to Cytoscape 3.9.1 software for visualization. The core target genes were screened via CytoNCA plug-in using the closeness centrality, betweenness centrality, degree centrality, eigenvector centrality, network centrality and local average connectivity.
GO function and KEGG pathway enrichment analyses
The CS and quercetin target genes intersection were converted to the corresponding gene IDs for gene ontology (GO) functional and Kyoto Protocol Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses in DAVID database (https://david.ncifcrf.gov/). GO comprises biological processes (BP), molecular function (MF), and cellular components (CC). KEGG enrichment analysis can forecast some potential signaling pathways involved in biological processes19,20,21. Subsequently, for analyzing the GO and KEGG data, the Bioinformatics (http://www.bioinformatics.com.cn/) platform was employed.
Molecular docking
Molecular docking can predict the binding potential between drugs and targets. Quercetin and the eight core targets were subjected to molecular docking via SYBYL-X2.1.1 software. First, the crystal structures of the protease were retrieved in the RCSB Protein Data Bank (PDB, http://www.rcsb.org/) database as the receptors; then the ligand was docked with the receptor by first extraction the ligand, removing the water molecules, modifying the terminal residues, and hydrogenating to generate the active pocket. Lastly, the total score was used to record the strength of the interaction between the small molecule and the target.
Cell viability assay
Murine macrophage cell line Raw264.7 (SC-6005, ATCC) was cultured in 96-well plates (1 × 104 cells/well) in DMEM medium with 10% fetal bovine serum and 1% antibiotics (100 unit/ml penicillin and 100 μg/ml streptomycin) at 37 °C and 5% CO2 overnight. The next day, cells were treated with different concentrations (2.5, 5, 10, 20, and 40 μg/ml) of quercetin for 24 h. Subsequently, cell viability was tested by CCK-8 kit assay (MA0218, meilunbio), per the kits’ instructions.
Enzyme-linked immunosorbent assay (ELISA)
Raw264.7 cells were seeded in 48-well plates at a density of 5 × 104 cells overnight; then, in the Control and LPS groups, the media was replaced with 500 μl fresh complete medium, whereas in the drug groups, 500 μl medium containing the corresponding drug concentrations was added. The dexamethasone (Dex) group represented a positive control. Furthermore, LPS was added to each group except the Control to achieve a final concentration of 1 μg/ml. After 24 h of co-culture, the cell supernatant was collected, and several inflammatory mediators, including IL-6, TNF-α, IL-1β, and MCP-1, were measured using ELISA (Dakowei Biotechnology Ltd.), per the manufacturers’ instructions.
Flow cytometry analysis
Raw264.7 cells were propagated and treated as mentioned above, collected after 24 h, and co-stained after probing with CD11b (101207, BioLegend), CD40 (124612, BioLegend), CD80 (104733, BioLegend) antibodies for 20 min at room temperature, while blank and positive controls (single stained tubes for each antibody) were prepared. The cells were then washed with PBS, resuspended in 500 μl PBS, gated, and then analyzed using a flow cytometer.
Detection of nitric oxide (NO)
Raw264.7 was propagated overnight at the density 1 × 105 in 24-well plates before receiving the corresponding treatment based on the experimental groups. After 24 and 48 h of co-incubation with the drug and LPS, the cell supernatant was obtained, and the expression level of NO was detected by the Griess method using NO kit (S0021S, Beyotime).
Detection of intracellular reactive oxygen species (ROS)
Raw264.7 (1 × 106/well) were inoculated in 6-well plates and co-culture with LPS and drug for 24 and 48 h before collecting their supernatants. ROS kit (CA1420, Solarbio) detected intracellular ROS levels. The probe DCFH-DA was added to the cell precipitates and incubated at 37 °C for 20 min. DCFH-DA is a non-fluorescent substance, and the kit uses the principle that the probe can enter cells, where it will be subsequently hydrolyzed into DCFH by esterase, and the intracellular ROS will then oxidize non-fluorescent DCFH to produce fluorescent DCF. Flow cytometry and fluorescence microscopy measured intracellular ROS's fluorescence intensity.
Immunofluorescence assay
Raw264.7 cells were propagated and treated as mentioned above, Raw264.7 cells were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.5% Triton X-100 for 20 min, and after being closed with 5% BSA for 2 h at room temperature, the cells were incubated with anti-FoxO1 antibody (1:100) at 4 °C overnight. After incubation with Alexa Fluor 488-labeled secondary antibody (1:800 dilution,HA1121, HUABIO), the cellular localization of the cells to FoxO1 was assessed using a confocal microscope (Leica, German).
RNA extraction and quantitative Real-time PCR (qRT-PCR)
The total RNA of treated Raw264.7 cells was extracted using a total RNA extraction kit (RE-03111, FOREGENE). The cDNA was synthesized using the RT Easy™II (With gDNase) kit (RT-01032, FOREGENE), which was then amplified via a Real-Time PCR Easy™-SYBR Green kit (QP-01014, FOREGENE). The relative expression levels of mRNA were calculated by the 2−△△ct method.
Western blot
Cellular proteins were extracted using the RIPA lysis buffer (E-BC-R327, Elabscience), a protease inhibitor (GRF101, epizyme), and a phosphatase inhibitor (GRF102, epizyme). The proteins were quantified using the BCA protein assay kit (P0010, Beyotime), then mixed with sample loading buffer (P0295, Beyotime) and boiled for 10 min. Proteins were separated on 10% SDS–polyacrylamide gels (PG112, epizyme), transferred to PVDF membranes (IPVH00010, Millipore), which were then blocked with 5% skimmed milk at room temperature, and incubated overnight with primary antibodies at 4 °C. The primary antibodies were as follows: anti-TLR2 (ab209216, Abcam), anti-TLR4 (14358, CST), anti-MyD88 (4283, CST), anti-AKT1 (ET1609-51, HUABIO), anti-phospho-AKT1 (ET1607-73, HUABIO), anti-FoxO1 (ET1608-25, HUABIO), anti-Keap1 (10503-2-AP, Proteintech), anti-Nrf2 (16396-1-AP, Proteintech). Subsequently, the membranes were washed and then incubated with a secondary antibody (RS0002, Immunoway) for 1 h. The proteins were visualized by a supersensitive ECL kit (PD203, Oriscience) and a chemiluminescent imaging system.
Anti-CS in vivo
All procedures were conducted following ARRIVE guidelines. The Ethics Committee of West China Hospital has approved this study and confirmed the statement that all methods were performed in accordance with relevant guidelines and regulations. Male C57BL/6J mice (8 weeks old, 23 ± 2 g) were purchased from Beijing Huafukang Biotechnology Co., Ltd. The mice were first acclimatized with the environment for a week and then categorized into six groups: control, LPS, high quercetin dose (100 mg/kg), medium quercetin dose (50 mg/kg), low quercetin dose (25 mg/kg) and Dex (5 mg/kg). The mice were in 12 h fast condition before the experiment; then, four drug groups were treated with quercetin and Dex at corresponding concentrations by gavage and intraperitoneal injection, respectively. The Control and LPS groups received the same volume of normal saline. After 2 h, mice were anesthetized with an intraperitoneal injection of 0.3% pentobarbital (55 mg/kg), then 50 μl of 5 mg/kg LPS was intratracheally administered in each group (except the Control) to establish the CS model. 4 h after LPS treatment, the mice were killed, the skin on the front of the neck was cut open, the trachea was separated and exposed, the indentation needle was inserted into the trachea and fixed, and the irrigation solution was irrigated with normal saline three times, 0.6 ml each time, with a recovery rate of 80–90%. The BALF was collected for the detection of cytokines.
Histological analysis
To observe the lung's histopathological alterations, mice were sacrificed 24 h after LPS stimulation; lung tissues were dissected, fixed with 4% formaldehyde, embedded in paraffin, sectionalized, and finally stained with hematoxylin–eosin (HE) for visual analysis.
Statistical analysis
All the statistical measurements were performed using GraphPad Prism 9.0, and the acquired data are expressed as means ± standard deviation (SD). Differences between the two groups were assessed using One-way ANOVA analysis and were considered significant at P < 0.05.
Results
Acquisition of quercetin targets against CS
The 2D molecular structure (Fig. 1A) and SMILES [C1=CC(=C(C=C1C2=C(C(=O) C3=C(C=C(C=C3O2) O) O) O) O) O)] of quercetin were downloaded from PubChem. The PubChem CID of quercetin is 5280343, and the molecular formula and weight are C15H10O7 and 302.23. Subsequently, this structure was uploaded to the Swiss Targets Prediction Database, and 100 genes were identified as quercetin targets. Furthermore, 8390 CS target genes were obtained from three disease databases, including GeneCards, OMIM, and PharmGKB, after screening for disease and removing the duplication. Venn’s diagram (Fig. 1B) indicated the potential 90 CS target genes selected after matching drugs to target genes.
The PPI network (Fig. 1C), constructed using STRING, consisted of 90 nodes and 375 edges, with PPI enrichment p-value < 1.0e−16. The nodes represent target proteins, and the edges represent predicted and confirmed interactions between proteins. This network was visualized with Cytoscape 9.0 to identify core targets, which were then screened using the CytoNCA plug-in. Based on six parameters-betweenness centrality, closeness centrality, degree centrality, rigenvector centrality, network centrality, and local average connectivity, 8 core target genes, including AKT1, EGFR, SRC, MMP9, KDR, PIK3R1, CDK1, and MMP2 were filtered. These were significant genes associated with the quercetin mechanism, which regulates the occurrence and development of CS (Fig. 1D).
Potential mechanism and signal pathways of quercetin in regulating CS
GO and KEGG enrichment analyses were performed through the DAVID database to further explore the BP and potential mechanisms of 90 target genes involved in CS. The result of the GO enrichment analysis (Fig. 2A) displayed 262 BP, 54 CC, and 112 MF. The major BP included protein phosphorylation, negative regulation of the apoptotic process, and protein autophosphorylation. The target genes were mainly associated with the following determined CC: cytosol, plasma membrane, cytoplasm, nucleus, etc. Moreover, MF included ATP binding, protein serine/threonine/tyrosine kinase activity, protein kinase activity, protein serine/threonine kinase activity, etc.
The KEGG enrichment assay indicated the possible signaling pathways via which quercetin improves CS, revealing the therapeutic mechanisms of CS (Fig. 2B). It identified the CS-related key signal pathways involved in the PI3K-AKT, FoxO and ErbB. The specific signal pathways of PI3K-AKT are listed in Fig. 2C.
Molecular docking results
The interaction of eight core targets-AKT1, KDR, CDK1, EFGR, MMP2, MMP9, SRC, and PIK3R1 with quercetin in the generated active pocket regions was assessed. The higher the total score, the more stable the binding activity. The binding activity is extremely high when the total score is > 7. The docking results revealed that among the 8 core targets, the interaction between the quercetin and AKT1 was the strongest, with a total score of 8.35 (Fig. 3A–E).
Quercetin attenuated LPS-induced expression of proinflammatory factors in Raw264.7 cells
The effects of quercetin and drug solvent dimethyl sulphoxide (DMSO) on cell viability were assessed by CCK8 assay, which indicated that 2.5, 5, and 10 μg/ml concentrations do not affect cell survival (Fig. 4A), therefore, these concentrations were selected as low, medium and high doses for subsequent experiments. LPS is an essential component of the Gram-negative bacterial cell wall and induces inflammation, allowing its application in various in vivo and in vitro experiments22. Under inflammatory conditions, LPS exposure activates macrophages to produce diverse cytokines and also promotes oxidative stress23,24. Consequently, a CS model was established utilizing 1 μg/ml LPS(Fig. 4B). As shown in Fig. 4C, quercetin significantly inhibited LPS-induced proinflammatory cytokines (IL-6, TNF-α, IL-1β) and MCP-1 in a dose-dependent manner.
Quercetin inhibited LPS-induced inflammatory factors in vitro. (A) Survival of Raw264.7 cells after 24 h intervention with different concentrations of quercetin. (B) A scheme for quercetin intervention in LPS-induced macrophage activation. (C) Quercetin reduced the concentration of IL-6, TNF-α, IL-1β and MCP-1 released by LPS-activated macrophages in a concentration-dependent manner. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
Upon exposure to LPS, activated M1 macrophages secrete a plethora of cytokines25. In addition, the surface markers CD40 and CD80, indicative of M1 macrophage activation, undergo alterations (Fig. 5A). To scrutinize these changes, we conducted flow cytometry analysis. As illustrated in Fig. 5B, C, the proportion of CD40+ CD80+ cells constituted approximately 70% in the LPS-untreated group, which exhibited a reduction following quercetin treatment. This observation finds validation in quantitative polymerase chain reaction (qPCR) results (Fig. 5D), wherein mRNA levels of CD40 and CD80 were augmented upon LPS stimulation, a response mitigated by quercetin intervention in a dose-dependent manner. These findings suggested that quercetin could hold promise in ameliorating LPS-induced macrophage polarization.
The ability of quercetin to modulate macrophage polarization in vitro. (A) Schematic representation of LPS-stimulated macrophage polarization. (B) Flow cytometry results indicated that quercetin treatment decreased the expression of CD40, CD80, surface markers of M1 phenotype macrophages. (C) Statistical analysis of flow cytometry results in (B). (D) Quercetin treatment also decreased mRNA expression of CD40 and CD80. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
Quercetin inhibited LPS-induced NO production in Raw264.7 cells
Nitric oxide (NO) has been implicated in various cellular responses to external stimuli such as ischemia and LPS stimulation26. Our NO detection assays unveiled an absence of NO in the resting state at 24 and 48 h, but its significant induction following LPS exposure. Notably, quercetin exhibited a dose-dependent reduction in NO release in the cell supernatant, with the most pronounced effect observed at 10 μg/ml (Fig. 6A). Inducible nitric oxide synthase (iNOS) is exclusively present under inflammatory conditions and is responsible for sustained NO production. Therefore, we delved deeper into the expression of NOS2 mRNA (encoding iNOS protein) and iNOS protein. Both quantitative polymerase chain reaction (qPCR) and western blot analyses demonstrated that quercetin downregulated the expression of both in a dose-dependent manner compared to the LPS group at 24 and 48 h (Fig. 6B–F, Supplementary Fig. 2A, B).
Effects of quercetin on LPS-induced NOS2 mRNA, iNOS protein expression and NO release in Raw264.7 cells. (A) At both 24 and 48 h, quercetin reduced LPS-induced NO release in a concentration-dependent manner. (B) NOS2 mRNA levels also decreased with increasing drug concentrations after 24 and 48 h of quercetin treatment. (C, D) After 24 and 48 h of LPS attack, iNOS expression at the protein level was down-regulated in a concentration-dependent manner in response to quercetin intervention. (E, F) The iNOS expression levels at 24 and 48 h were normalized to GAPDH. Original blots are presented in Supplementary Fig. 2A, B. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
Quercetin regulated the AKT1-FoxO1 signaling pathway
The molecular docking results revealed a high binding score of quercetin to AKT1, suggesting its potential to regulate AKT1 activity. In comparison to the LPS-treated group, quercetin administration led to an increase in AKT1 phosphorylation without altering its total protein levels (Fig. 7A, B, Supplementary Fig. 2C). Following LPS stimulation, the expression of FoxO1, a transcription factor, and its nuclear translocation are augmented. However, FoxO1 is subject to negative regulation by AKT, as AKT phosphorylation prompts its exclusion from the nucleus, thereby mitigating inflammation16,27. Immunofluorescence experiments depicted an intensified nuclear fluorescence upon LPS treatment, which significantly decreased following quercetin intervention (Fig. 7C, Supplementary Fig. 1A). Correspondingly, western blot analysis illustrated that LPS augmented total FoxO1 expression, a trend reversed by quercetin treatment (Fig. 7D, E, Supplementary Fig. 2D). Additionally, quercetin exhibited a dose-dependent downregulation of Toll-like receptor 2 (TLR2), Toll-like receptor 4 (TLR4), and MyD88 expression following LPS stimulation (Supplementary Fig. 1B–G, Supplementary Fig. 2G–I). In summary, our findings suggested that quercetin could induce AKT1 activation, leading to subsequent FoxO1 inactivation.
Effects of quercetin on AKT1 and FoxO1 expression levels in response to LPS stimulation. (A) The level of phosphorylated AKT1 gradually up-regulated after quercetin intervention. (B) p-AKT1 levels were normalized to total AKT1 levels. (C) Immunofluorescence assessment of FoxO1 intranuclear expression levels in Raw264.7 cells after LPS and quercetin treatment. The FoxO1 was stained as green granular dots, while the nucleus was stained with blue. The highest expression of FoxO1 in the nucleus was observed in the LPS group, and the fluorescence of FoxO1 in the nucleus gradually decreased with the increase of quercetin concentration. (D) Western blotting analysis similarly confirmed that quercetin down-regulated FoxO1 expression in Raw264.7 cells. (E) FoxO1 levels were normalized to GAPDH. Original blots are presented in Supplementary Fig. 2C, D. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
Quercetin activated Keap1-Nrf2 signaling pathway to mediate antioxidant response
Oxidative stress, emblematic of the imbalance between reactive oxygen species (ROS) and antioxidant defenses, can potentiate inflammatory responses and exacerbate tissue damage28. To confirm whether the quercetin-induced antioxidative effect involves ROS inhibition, intracellular ROS levels were assessed through flow cytometry analysis. Results revealed a gradual increase in cellular ROS levels over time in the untreated LPS cells compared to the control group, reaching approximately 70% and 90% at 24 and 48 h, respectively. Moreover, quercetin demonstrated an augmented ability to scavenge ROS with increasing time and drug dosage (Fig. 8A, B). Green fluorescence, indicative of intracellular ROS content, exhibited a progressive decline with escalating drug concentrations (Fig. 8C, D). Both flow cytometry and fluorescence microscopy analyses indicated that quercetin alleviated the high ROS levels induced by LPS. Subsequently, we investigated the expression of key factors in the Keap1-Nrf2 axis. Our results showed low or negligible expression of Nrf2 in the control group, with Nrf2 accumulation increasing with higher quercetin doses following LPS stimulation and quercetin intervention. Conversely, Keap1 expression exhibited an inverse trend compared to Nrf2 (Fig. 8E–G, Supplementary Fig. 2E, F). Hence, our findings suggested that quercetin could mitigate oxidative stress by activating the Keap1-Nrf2 signaling pathway.
Effects of quercetin on intracellular ROS levels under LPS induction at 24 and 48 h. (A, B) Flow cytometry results showed that quercetin enhanced the scavenging of intracellular ROS with increasing concentration and time under LPS induction. (C, D) The intracellular ROS content was observed by fluorescence microscopy, and green fluorescence represents ROS. Magnification is 20×. (E) Western blotting analysis revealed that Keap1 protein expression was down-regulated and Nrf2 protein expression was up-regulated in Raw264.7 cells treated with LPS and quercetin. (F, G) The expression levels of Keap1 and Nrf2 were normalized to their respective GAPDH at 24 and 48 h. Original blots are presented in Supplementary Fig. 2E, F. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
Quercetin prevented LPS-induced CS in mice
To assess the in vivo anti-inflammatory activity of quercetin, four inflammatory factors in BALF, including IL-6, TNF-α, IL-1β, and MCP-1, were measured by ELISA, which indicated their rapid upregulation after LPS stimulation and quercetin reversed this effect in a concentration-dependent manner under preconditioning (Fig. 9A, B).
Quercetin inhibited CS in vivo studies. (A) Scheme for in vivo induction and intervention of CS. (B) Quercetin suppressed the LPS-induced elevation of IL-6, TNF-α, IL-1β and MCP-1 in BALF. (C) Histological study of the protective effect of quercetin against LPS-induced CS lung injury. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with the LPS group.
As H&E-staining indicates (Fig. 9C), the control group indicated normal lung structure and clear alveolar morphology, while LPS exposure distinctly caused lung tissue congestion, edema, and extensive inflammatory cell infiltration, destroying the lung structure and preventing normal lung function. Pretreated quercetin mice had improved histopathologic changes induced by LPS in a concentration-dependent manner. The examination of pathological changes in lung tissue and BALF inflammatory factors demonstrated that quercetin could effectively protect mice from LPS attacks.
Discussion
CS is a systemic immune overreaction. Under normal circumstances, pro-inflammatory and anti-inflammatory factors are in a state of mutual balance, which is disrupted when pathogens invade or medical intervention, resulting in the excessive emission of the cytokines and inducing CS. If not treated properly, it can lead to systemic damage, multi-organ failure, or even death29. In CS caused by immune-related pneumonitis and viral infection, activated macrophages produce excessive amounts of IL-6, TNF-α, and IL-1β accompanied by elevated chemokines. IL-6 is a crucial target for CS treatment and is a risk factor for assessing the severity of COVID-19 as it is associated with a high mortality rate30,31,32,33,34. Corticosteroids and cytokine monoclonal antibodies are essential for CS treatments. However, the optimal dose and duration of corticosteroids in immune-related pneumonia remains undetermined and could exacerbate the risk of opportunistic infections35,36,37. In COVID-19, despite the benefits of corticosteroids, there is some variation in different patients38. Furthermore, it has been studied that they are associated with high mortality, hyperglycemia, and infection39. Monoclonal cytokines antibodies, such as IL-6R monoclonal antibodies, TNF inhibitors, and IL-1 antagonists, are specific for specific cytokines, and unfortunately, CS comprises multiple cytokines. Some clinical trials have shown that monoclonal antibodies are only effective in some people40,41.
LPS used to model pneumonia is a classical approach that activates macrophages and monocytes to produce high levels of inflammatory cytokines (IL-6, TNF-α, IL-1β, etc.) while eliciting oxidative stress, with literature suggesting that it can also mimic the CS that occurs in the lungs42,43,44,45,46. Pneumonia frequently ensues as a consequence of CS and often stems from viral infections. In our investigation, we successfully established in vitro and in vivo CS models utilizing LPS, with in vivo modeling achieved through tracheal infusion of LPS. The groups treated solely with LPS exhibited elevated levels of inflammatory factors (IL-6, TNF-α, IL-1β, MCP-1) alongside inflammatory cell infiltration in lung tissue. Our findings highlighted quercetin's capacity to modulate the inflammatory response induced by LPS-activated macrophages, effectively suppressing the release of inflammatory factors and thereby exerting an anti-inflammatory effect.
The findings from network pharmacology unveiled quercetin's capacity to modulate CS primarily by targeting AKT1, EGFR, SRC, MMP9, KDR, PIK3R1, CDK1, and MMP2, with AKT1 being the most significantly regulated. Moreover, KEGG enrichment analysis indicated a potential association between quercetin's mechanism of action against CS and the PI3K-AKT signaling pathway. AKT1, an intracellular kinase, governs various biological processes such as cell growth, survival, and metabolism, serving as a pivotal signaling node in various tissues and cellular inflammatory responses47,56,49. Within macrophages, AKT1 represents the sole subtype. Macrophages lacking AKT1 exhibit heightened responsiveness to LPS and display a robust pro-inflammatory reaction, while AKT1 ablation induces the production of M1-type macrophages50. Furthermore, AKT1 phosphorylates and deactivates downstream GSK3β, thereby diminishing NF-κB activation and fostering the expression of the anti-inflammatory cytokine IL-1051. These findings suggest that AKT1 may play a pivotal anti-inflammatory role in inflammation. The PI3K-AKT signaling pathway indicates that AKT1 might mediate the inflammatory response through the downstream FoxO1 signaling pathway. Multiple studies have implicated FoxO1 in promoting inflammatory signaling16,17, 52. It has been observed that the TLR4/MyD88/MD2-NF-κB signaling pathway is markedly activated following FoxO1 overexpression, whereas silencing of FoxO1 downregulates levels of inflammatory pathway proteins15. However, AKT-mediated phosphorylation of FoxO1 leads to its nuclear exclusion and inhibition of its activity. Sun et al.53 demonstrated that Schisandrin substantially reversed the high expression of total FoxO1 protein in the nucleus and upregulated AKT phosphorylation following LPS stimulation. Consistent with these findings, our study revealed that LPS stimulation increased FoxO1 protein levels in Raw264.7 cells. Quercetin targeted AKT1 and significantly phosphorylated it, thereby inhibiting the entry of FoxO1 into the nucleus and reducing the expression of pro-inflammatory genes. This inhibitory effect of quercetin on FoxO1 was further confirmed using immunofluorescence assays.
CS can induce severe oxidative stress, leading to heightened production of ROS and subsequent damage to crucial organs. The Keap1-Nrf2 pathway serves as a primary defense mechanism within cells, safeguarding against oxidative stress and preserving homeostasis. Upon encountering ROS, Keap1 undergoes modification, dissociating from Nrf2. This allows Nrf2 to translocate to the nucleus, where it accumulates and counteracts oxidative stress, thereby protecting cells18,54. Curcumin, a bioactive compound found in turmeric, has been shown to scavenge ROS generated in macrophages, shielding them from oxidative stress by activating the Keap1-Nrf2 pathway55. Similarly, studies by Liu et al.56 demonstrated that Mollugin activated the Keap1-Nrf2 pathway, mitigating oxidative stress in Raw264.7 cells. Additionally, astaxanthin has been found to safeguard against LPS-induced cellular inflammation and acute lung injury in mice by suppressing iron-induced cell death through the Keap1-Nrf2 pathway57. In our investigation, we observed a quercetin dose-dependent decrease in Keap1 levels and an increase in Nrf2 protein levels, significantly inhibiting LPS-induced ROS production. This finding suggests a potential contribution of quercetin to antioxidant stress effects.
In summary, the present study demonstrated that quercetin could inhibit LPS-induced inflammation and alleviate cytokine storm in vitro and in vivo. Mechanistically, quercetin exerted its protective effects by regulating AKT-FoxO1 and Keap1-Nrf2 pathway.
Conclusion
This study employed network pharmacology and molecular docking technology to identify the effective target genes of quercetin against CS. It also preliminarily revealed that quercetin might act against CS through signaling pathways such as PI3K-AKT and FoxO. In addition, The in vitro and in vivo experiments confirmed that quercetin could play an anti-inflammatory role. Collectively, it could regulate AKT1-FoxO1 and Keap1-Nrf2 signaling pathways.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Change history
12 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-78855-9
Abbreviations
- BALF:
-
Bronchoalveolar lavage fluid
- BP:
-
Biological process
- CC:
-
Cellular composition
- COVID-19:
-
Coronavirus disease 2019
- CS:
-
Cytokine storm
- DEX:
-
Dexamethasone
- DMSO:
-
Dimethyl sulphoxide
- FoxO:
-
Forkhead box proteins O
- GO:
-
Gene ontology
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- Keap1:
-
Kelch-like ECH-associated protein 1
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LPS:
-
Lipopolysaccharides
- MCP-1:
-
Monocyte chemotactic protein 1
- MF:
-
Molecular function
- NO:
-
Nitric oxide
- Nrf2:
-
Nuclear factor erythroid 2-related factor
- PPI:
-
Protein–protein interaction network
- ROS:
-
Reactive oxygen species
- TCM:
-
Traditional Chinese medicine
- TNF-α:
-
Tumor necrosis factor α
References
Fajgenbaum, D. C., Longo, D. L. & June, C. H. Cytokine storm. N. Engl. J. Med. 383(23), 2255–2273 (2020).
Karki, R. & Kanneganti, T.-D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 42(8), 681–705 (2021).
Shapouri-Moghaddam, A. et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 233(9), 6425–6440 (2018).
Ansari, M. A., Abdul, H. M., Joshi, G., Opii, W. O. & Butterfield, D. A. Protective effect of quercetin in primary neurons against Aβ(1–42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 20(4), 269–275 (2009).
Das, N. et al. Quercetin alleviates inflammation after short-term treatment in high-fat-fed mice. Food Funct. 4, 6 (2013).
Granato, M. et al. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 41, 124–136 (2017).
Wu, W. et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 8, 1 (2015).
Yang, W. S. et al. Myrsine seguinii ethanolic extract and its active component quercetin inhibit macrophage activation and peritonitis induced by LPS by targeting to Syk/Src/IRAK-1. J. Ethnopharmacol. 15, 1165–1174 (2015).
Sul, O.A.-O. & Ra, S.A.-O. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 26, 6949. https://doi.org/10.3390/molecules26226949 (2021).
Sullivan, I. O. et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 6, 1 (2015).
Savai, R. et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat. Med. 20(11), 1289–1300 (2014).
Poojary, V. K., Penberthy, K. K., Buckley, M. W., Arandjelovic, S. & Ravichandran, K. Ex vivo modulation of the Foxo1 phosphorylation state does not lead to dysfunction of T regulatory cells. Plos One 12, 3 (2017).
Webb, A. E. & Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 39, 159–169 (2014).
Riol-Blanco, L. et al. Immunological synapse formation inhibits, via NF-κB and FOXO1, the apoptosis of dendritic cells. Nat. Immunol. 10(7), 753–760 (2009).
Han, C. et al. FoxO1 regulates TLR4/MyD88/MD2-NF-κB inflammatory signalling in mucosal barrier injury of inflammatory bowel disease. J. Cell Mol. Med. 24(6), 3712–3723 (2020).
Su, D. et al. FoxO1 links insulin resistance to proinflammatory cytokine IL-1β production in macrophages. Diabetes 58(11), 2624–2633 (2009).
Fan, W. et al. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J. 29(24), 4223–4236 (2010).
Yamamoto, M.A.-O., Kensler, T. W. & Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 98(3), 1169–1203 (2018).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M.A.-O.X. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M.A.-O.X., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Pooladanda, V., Thatikonda, S., Muvvala, S. P., Devabattula, G. & Godugu, C. BRD4 targeting nanotherapy prevents lipopolysaccharide induced acute respiratory distress syndrome. Int. J. Pharmaceut. 2021, 601 (2021).
Chan, E. L. & Murphy, J. T. Reactive oxygen species mediate endotoxin-induced human dermal endothelial NF-κB Activation. J. Surg. Res. 111(1), 120–126 (2003).
Simon, F. & Fernández, R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J. Hypertens. 27(6), 1202–1216 (2009).
Tan, H.-Y. et al. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxid. Med. Cell. Longevity 2016, 1–16 (2016).
Zhu, F. et al. Brd4 inhibition ameliorates Pyocyanin-mediated macrophage dysfunction via transcriptional repression of reactive oxygen and nitrogen free radical pathways. Cell Death Dis. 11, 459 (2020).
Liu, P., Cheng, H., Roberts, T. M. & Zhao, J. J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8(8), 627–644 (2009).
Yang, C.-S. et al. TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J. Immunol. 190(12), 6368–6377 (2013).
Jarczak, D. & Nierhaus, A. Cytokine storm—definition, causes, and implications. Int. J. Mol. Sci. 23, 19 (2022).
Addeo, A., Obeid, M. & Friedlaender, A. COVID-19 and lung cancer: Risks, mechanisms and treatment interactions. J. ImmunoTherapy Cancer 8, 1 (2020).
Knoll, R., Schultze, J. L. & Schulte-Schrepping, J. Monocytes and Macrophages in COVID-19. Front. Immunol. 2021, 12 (2021).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124(2), 188–195 (2014).
Tay, S. H. et al. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: A case series of 25 patients and review of the literature. Front. Immunol. 2022, 13 (2022).
Zhu, Z. et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 95, 332–339 (2020).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 36(17), 1714–1768 (2018).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. ImmunoTherapy Cancer 5, 1 (2017).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 1 (2020).
Prescott, H. C. & Rice, T. W. Corticosteroids in COVID-19 ARDS: Evidence and hope during the pandemic. Jama 324, 1292–1295 (2020).
Cai, J. et al. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metabol. 33(2), 258-269.e3 (2021).
Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 384(16), 1503–1516 (2021).
Ip, A. et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study. Plos One 15, 8 (2020).
Kang, J.-Y. et al. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109 (2022).
Kim, J. H. et al. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-κB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem. Pharmacol. 71(8), 1198–1205 (2006).
Suryavanshi, S. V., Zaiachuk, M., Pryimak, N., Kovalchuk, I. & Kovalchuk, O. Cannabinoids alleviate the LPS-induced cytokine storm via attenuating NLRP3 inflammasome signaling and TYK2-mediated STAT3 signaling pathways in vitro. Cells 11, 9 (2022).
Li, H. et al. Glycyrrhetinic acid: A potential drug for the treatment of COVID-19 cytokine storm. Phytomedicine 2022, 102 (2022).
You, J. et al. Inspiration for COVID-19 treatment: Network analysis and experimental validation of baicalin for cytokine storm. Front. Pharmacol. 2022, 13 (2022).
Lin. X, Zhao. Q, Fu. B, Xiong. Y, Zhang. S, Xu. S, Wu. H. ISOC1 Modulates Inflammatory Responses in Macrophages through the AKT1/PEX11B/Peroxisome Pathway. Molecules 27(18), 5896 (2022).
Wang. R, Wang. Y, Wu. J, Guo. Y, Xiao. H, Zhang. Y, & Ma. K. Resveratrol Targets AKT1 to Inhibit Inflammasome Activation in Cardiomyocytes Under Acute Sympathetic Stress. Frontiers in Pharmacology 13, https://doi.org/10.3389/fphar.2022.818127 (2022).
Yang. J, Cheng. M, Gu. B, & Wang. J. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-κBaxis, Cell Death & Disease 11(10), https://doi.org/10.1038/s41419-020-03038-z (2020).
Arranz, A. et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. 109(24), 9517–9522 (2012).
Martin, M. et al. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6, 777–784 (2005).
Seiler, F. et al. FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J. Immunol. 190(4), 1603–1613 (2013).
Sun, K. et al. Schisandrin attenuates lipopolysaccharide-induced lung injury by regulating TLR-4 and Akt/FoxO1 signaling pathways. Front. Physiol. 2018, 9 (2018).
Lee, J., Jang, J., Park, S.-M. & Yang, S.-R. An update on the role of Nrf2 in respiratory disease: Molecular mechanisms and therapeutic approaches. Int. J. Mol. Sci. 22, 16 (2021).
Lin, X. et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PloS one 14, e0216711 (2019).
Liu, X. et al. Mollugin prevents CLP-induced sepsis in mice by inhibiting TAK1-NF-κB/MAPKs pathways and activating Keap1-Nrf2 pathway in macrophages. Int. Immunopharmacol. 125, 111079 (2023).
Luo, L. et al. Astaxanthin attenuates ferroptosis via Keap1-Nrf2/HO-1 signaling pathways in LPS-induced acute lung injury. Life Sci. 311, 121091 (2022).
Acknowledgements
The authors thank Ping Lin, Jie Zhang and Qin Lin from the lab of experimental oncology for their great help in this study. The authors gratefully appreciate BioRender's modifications to the figures. The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for their linguistic assistance during the preparation of this manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (NO.82260490), Sichuan Provincial Nature Science Foundation (2022NSFSC1379); Sichuan Science and Technology Programme (2022YFSY0054) and Technology Innovation Project of Chengdu Science and Technology (2020-YF05-00059-SN); Natural Science Foundation of Hainan Province (NO.821QN394); Science and technology research project on novel corona-virus pneumonia outbreak, West China Hospital, Sichuan University (HX-2019-nCoV-069).
Author information
Authors and Affiliations
Contributions
Xu, Li, Yang, Li, Xiao, You, Li, Zheng, Li, Yi, and Huang contributed to this study. Xu, Li,ang contributed equally to this study. Yi, Li andHuang directed the design of this study, supervised its implementation and revised draft. Xu, Li, Yang, Li, Xiao participated in the specific experimental process, data analysis and paper writing. You, Li, Zheng were involved in the charting of the paper. All authors have read and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article Li Zhaojun was incorrectly affiliated with ‘Department of Radiation Oncology, Hainan Affiliated Hospital of Hainan Medical University (Hainan General Hospital), No.31, Longhua Road, Haikou, 570100, China’. The correct affiliation is given here: ‘Department of Radiation Oncology, Hainan Affiliated Hospital of Hainan Medical University (Hainan General Hospital), No.17, Xiuhua Road, Haikou 570100, China.’ The original Article has been corrected.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, J., Li, Y., Yang, X. et al. Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages. Sci Rep 14, 20913 (2024). https://doi.org/10.1038/s41598-024-71569-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71569-y
Keywords
This article is cited by
-
Quercetin alleviates radiation-induced erectile dysfunction by modulating oxidative stress and apoptosis through the Nrf2/HO-1 pathway
European Journal of Medical Research (2026)
-
Effect of Quercetin on inflammatory markers in diabetes mellitus: a systematic review and meta-analysis of animal studies
Inflammopharmacology (2026)
-
Inflammation: a matter of immune cell life and death
npj Biomedical Innovations (2025)