Abstract
Improved solubility and anti-inflammatory (AI) properties are imperative for enhancing the effectiveness of poorly water-soluble drugs, particularly non-steroidal anti-inflammatory drugs (NSAIDs). To address these critical issues, our focus is on obtaining NSAID materials in the form of inclusion complexes (IC) with methyl-beta-cyclodextrin (MCD). Ketoprofen (KTP) is selected as the NSAID for this study due to its potency in treating various types of pain, inflammation, and arthritis. Our objective is to tackle the solubility challenge followed by enhancing the AI activity. Confirmation of complexation is achieved through observing changes in the absorbance and fluorescence intensities of KTP upon the addition of MCD, indicating a 1:1 stoichiometric ratio. Phase solubility studies demonstrated improved dissolution rates after the formation of ICs. Further analysis of the optimized IC is conducted using FT-IR, NMR, FE-SEM, and TG/DTA techniques. Notable shifts in chemical shift values and morphological alterations on the surface of the ICs are observed compared to their free form. Most significantly, the IC exhibited superior AI and anti-arthritic (AA) activity compared to KTP alone. These findings highlight the potential of ICs in expanding the application of KTP, particularly in pharmaceuticals, where enhanced stability and efficacy of natural AIs and AAs are paramount.
Similar content being viewed by others
Introduction
Ketoprofen (KTP) is a nonsteroidal anti-inflammatory drug (NSAID) known for its analgesic and antipyretic properties. It is commonly prescribed for rheumatic disorders like rheumatoid arthritis, osteoarthritis, and various nonrheumatoid pains1,2,3. Despite its therapeutic benefits, KTP faces challenges due to its poor water solubility, low dissolution rate, and consequently, reduced bioavailability. These limitations hinder its effectiveness in therapy4,5. Improving bioavailability often involves enhancing the aqueous solubility and dissolution rate of KTP6,7. Various strategies have been explored to address this, including solid dispersion8, KTP-dextran ester prodrugs9, microencapsulation of KTP elixir10, and inclusion complexation with cyclodextrin11. Cyclodextrin [CD], known for its interaction with NSAIDs, forms inclusion complexes (IC) with KTP, thereby improving its solubility and bioavailability6.
CDs serve as versatile additives utilized across industries including agriculture, medicine, chemistry, environmental engineering, and cosmetics. Primarily, CDs function as carriers of active substances to enhance the solubility of water-insoluble ingredients12,13,14. Structurally, CDs consist of six (α-CD), seven (β-CD), or eight (γ-CD) glycopyranose units linked by α-(1–4) bonds, forming a truncated conical shape with a relatively hydrophobic inner cavity and a hydrophilic outer surface. The exterior of cyclodextrin displays hydrophilic characteristics due to primary hydroxyl groups at the C-6 position and secondary hydroxyl groups at the C2 and C3 positions15,16. Conversely, the inner surface of cyclodextrin is lined with hydrophobic C3-H and C5-H hydrogen atoms along with ether-like anomeric oxygen atoms16. CDs are widely favored as host molecules for molecular encapsulation of low molecular weight hydrophobic molecules (guest molecules) to form inclusion complexes. This involves placing the hydrophobic guest molecule within the hydrophobic cavity of cyclodextrin, facilitated by various non-covalent interactions such as electrostatic interactions, hydrogen bonds, hydrophobic effects, and van der Waals forces17,18. These interactions alter the physical, chemical, and biochemical properties of encapsulated molecules, which can be assessed through methods examining changes in parameters like absorbance, fluorescence, NMR chemical shifts, and chemical stability19,20.
In general, small drug molecules exhibit significant complexing activity with CDs, particularly those compounds with the lowest water solubility, which display a notable increase in solubility proportional to CD concentration. As a result, CDs have been widely employed in pharmaceutical formulations to improve the solubility of poorly water-soluble drugs. Natural CDs have been extensively utilized for this purpose; however, their relatively low solubility in water restricts their application. Consequently, chemically modified CDs have garnered considerable attention for enhancing the physicochemical properties of CDs21,22,23. For instance, methyl substitution of CDs offers advantages such as increased solubility, reduced hemolytic activity, and diminished nephrotoxicity24.
The analysis of KTP with native BCD has been previously conducted, demonstrating enhanced anti-inflammatory activity at certain oral dosages in rats11. However, the complex materials synthesized via the solvent evaporation method present certain drawbacks25. Additionally, native BCD faces solubility issues in water26, leading to the recent exploration of BCD derivatives in research27,28. Previous studies have analyzed the interactions of KTP with BCD in both solution and solid states29, revealing significantly improved solubility upon the formation of IC between KTP and BCD29,30,31,32. However, anti-inflammatory studies for the complexes of KTP with derivatives of BCD have not been conducted. A synthesis of ICs of KTP with BCD using the supercritical anti-solvent process has been reported33,34,35, which is deemed superior to other methods by the Paulo research group, despite requiring an expensive experimental setup. Recognizing the limitations and advantages of different synthetic methods for KTP-CD complexes, ultrasonication emerges as a cost-effective and less complex alternative36,37,38, avoiding the use of toxic solvents. With the considerations from existing literature, we designed the current study to synthesize complex materials of KTP with MCD using ultrasonication followed by a freeze-dried process. The obtained complex material undergoes thorough characterization using IR and NMR for chemical identification, while SEM and TGA analysis is employed for surface analysis. Furthermore, thorough investigations have been conducted into optimized structures for the IC, particularly focusing on energy parameters and hydrogen bonding interactions. Furthermore, in-vitro anti-inflammatory is also conducted for the ICs, comparing their efficacy with KTP alone.
Materials and methods
Materials
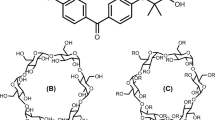
The necessary chemicals, KTP (molecular formula: C16H14O3, molecular weight: 254.28, purity: 99%), and MCD (molecular formula: C54H94O35, molecular weight: ~ 1303.311, purity: 98%), were obtained from Sigma-Aldrich Chemical Co (Seoul, South Korea). Distilled water was utilized as the solvent for all experimental procedures. Figure 1 depicts the structural representations of KTP and MCD. Human keratinocytes immortalized cells were used in the in-vitro experiments. These cells were purchased from AddexBio.
Phase solubility (PS) study
The phase solubility study is a widely employed method utilized to ascertain the stoichiometric binding or affinity between MCD and the guest molecule, KTP. This method provides insights into how the solubility of KTP changes upon the formation of ICs within the MCD cavity39. Phase solubility diagrams40 for the complexes were generated by introducing an excess of the drug (18 mg KTP/ml) into CD (21 mg of MCD) solutions with gradually increasing concentrations. The pH of the KTP solution was adjusted to 3.0 using a buffer solution, ensuring it remained below the pKa of the drug to maintain its unionized state and consequently, its low solubility. Subsequently, the prepared solutions were stirred at room temperature for 24 h to attain equilibrium, followed by filtration through 0.45 μm filters. The filtrates were then analyzed using a UV–Vis spectrophotometer, and measurements were conducted in triplicate.
Preparation of KTP:MCD ICs
The formation of ICs involving KTP with MCD commenced with the dissolving of KTP (0.47 mM) in ethanol, along with MCD (0.47 mM) in 20 ml of distilled water. The resulting solution underwent sonication in an ultrasonic bath (30 kHz, 100 W, Hielsher UP200St, Iran) for 5 min to ensure thorough blending. To prepare the ICs, the KTP solution was slowly added dropwise into the MCD solution with an identical concentration, resulting in a 1:1 molar ratio. The mixture was consistently heated to 60.0 °C with magnetic stirring. Notably, the resulting ICs exhibited water solubility, as evidenced by the clear appearance of the entire solution. Subsequently, a water-based removal technique was employed. The resulting solutions were filtered using a 0.45 μm syringe filter and then frozen at -81.0 °C for 48 h, followed by lyophilization at -80.50 °C under 1.09 Pa for 72 h in a freeze dryer41. This process yielded white-colored powder representative of the ICs, designated as IC (KTP:MCD IC).
Results and discussion
Effect of KTP on the addition of MCD
The absorption spectra of KTP in aqueous solution (approximately pH 7) at various concentrations of MCD are recorded and are illustrated in Fig. 2A. A clear absorption peak with significant absorbance (0.38 a.u) is observed at 260 nm for KTP alone32,42. As depicted in Fig. 2, the absorbance of KTP increases with rising concentrations of MCD. Notably, there are no alterations in the absorption maximum of KTP during the addition of MCD, indicating the absence of any structural changes in the KTP. This phenomenon is attributed to the encapsulation of KTP into the cavities of MCD without inducing any structural changes and to the absorption of KTP onto the surface of the container walls. This occurs as the environment of KTP transitions from a more protic state (bulk aqueous phase) to a less protic state (CD's cavity). All measurements are repeated approximately 12 and 48 h after solution preparation, revealing no significant change in absorbance values. The encapsulation remained intact, indicating the stability of the IC, which did not decompose even after prolonged storage.
Moreover, Fig. 2B illustrates the impact of adding MCD on the fluorescence spectra of an aqueous solution of KTP at a constant concentration. In the absence of MCD, fluorescence spectra are obtained with very low-intensity values, and no specific spectral maximum (λemi is at 335 nm) is found43. As depicted in the figures, the rise in MCD concentration leads to a distinct enhancement in fluorescence intensity, reaching up to 12 × 10–3 M. This enhancement likely arises from the complexation between KTP and MCD. At short intermolecular distances, there is radiation-less energy transfer from the excited state of the guest to the ground state of the CD, thereby amplifying the fluorescence intensity44,45.
The binding constant (K) and stoichiometric ratios for the ICs are found using the Benesi–Hildebrand (BH) relation46,47,48. This relation is applied to the changes observed in the absorbance and fluorescence intensities of KTP with varying concentrations of MCD, confirming the formation of a 1:1 complex. The K values for the 1:1 complex are calculated using the following equations.
Using Eqs. (1) and (2) mentioned above, linear plots are constructed to calculate the binding constant (K). BH plot exhibits a robust linear regression, offering compelling evidence for the formation of a 1:1 complex with MCD, as shown in Fig. 3. The values of the binding constants are presented in Table S1.
To further investigate the spontaneity of IC formation, one can utilize thermodynamic parameters to analyze the displacement of KTP within a cavity of MCD and the subsequent exchange of water molecules during IC formation. The Gibbs free energy change (ΔG) is a crucial thermodynamic parameter that can be employed to assess the spontaneity of complexation and elucidate the influence of temperature on the ICs45,49. In an aqueous environment at neutral pH and room temperature, negative ΔG values are typically observed for the formation of ICs (refer to Table S1). This indicates that the process of IC occurs spontaneously, providing valuable insights into the binding affinity between MCD and KTP. Furthermore, the negative ΔG value suggests that the reaction is exergonic, meaning it releases energy, and thus proceeds spontaneously at room temperature. This offers significant insights into the underlying thermodynamics governing the formation of IC.
PS diagram
A PS diagram has been constructed for the IC, illustrating the solubility of KTP relative to the concentrations of MCD50. The PS analysis unveils a trend characterized by a linear increase in KTP concentration with increasing concentrations of MCD, denoted by AL-type linearity (as depicted in Fig. 4A), indicating the formation of a 1:1 stoichiometric ratio51,52. In the absence of MCD, KTP exhibits a water solubility of approximately ~ 0.001 mM. However, as the concentrations of MCD increase, the water solubility of KTP rises by approximately ~ 0.021 at the highest concentrations of MCD. Minimal alterations are observed in the saturation concentration of KTP, suggesting that the dissolution behavior of KTP:MCD primarily arises from the weak interaction between KTP and MCD in the buffer solution50.
Dissolution rate
The dissolution profiles of KTP and its IC with MCD are shown in Fig. 4B. Initially, KTP dissolves very slowly, with only about 13% of the drug dissolving in 70 min. In contrast, the IC shows a significantly higher dissolution rate, achieving almost complete solubility (up to 96%) within the same period (Table S2). By forming an IC with MCD, the dissolution behavior of KTP improves to approximately 83%. NMR and FT-IR analyses confirm the successful formation of the IC. This complexation enhances the drug's wettability, thereby increasing its dissolution rates53.
Analysis of KTP by 1H NMR spectrum
Figure S4 displays the NMR spectrum of KTP. Aromatic protons are detected in the downfield region, ranging from 6.40 to 7.68 ppm. Assigning individual proton chemical shifts involved consideration of integral values, multiplicity, coupling constants, and the electronegativity effect of substituents. The doublets of H2'/H6' merged at 7.73 ppm, with a coupling constant attributed to H2'/H6' (inset Fig. 5). Similarly, signals from H2''/H6'' merged at 7.68 ppm with a coupling constant of 7.8 Hz. Two triplets appeared at 7.52 and 7.58 ppm, with coupling constants of 7.5 and 7.8 Hz, respectively. One proton integral each is assigned to H3'/H5' and H4’. A singlet and a doublet, each representing one proton, merged into a multiplet at 7.60 ppm with a coupling constant of 7.8 Hz, corresponding to H2'' and H6'' respectively. The remaining signal at 7.61 ppm is assigned to H3''/H4'' protons, observed as doublets with a coupling constant of 7.8 Hz. A proton signal at 12.46 ppm, with a single proton integral, is attributed to the acid proton of ketoprofen. Additionally, a multiplet and a doublet in the upfield region, around 3.83 and 1.39 ppm, are conveniently assigned to the methine and methyl protons of the propionic acid moiety. Proton chemical shifts of KTP are summarized in Table S2.
Analysis of IC by 1H NMR spectrum
Figure 5 displays the 1H NMR spectrum of the IC. In this spectrum, both the aromatic protons of KTP and MCD are detected. The protons of the phenyl ring attached to the carbonyl group experienced a shielding effect of 0.01–0.02 ppm. Conversely, the carboxylic acid proton is deshielded by 0.03 ppm, and the methyl and methane protons of the propanoic acid moiety of KTP are shifted to the upfield region by 0.02 ppm. The rest of the protons of the guest molecule (KPT) remained unaffected. Similarly, for MCD protons, except H2 and H4 protons, all others are shielded by 0.16–1.32 ppm. H2 remained unaffected, while H4 experienced deshielding by 0.16 ppm. Additionally, the methyl protons of the methoxy groups are shielded by 0.37 ppm. These observations confirm the formation of an IC between KTP and MCD. Further confirmation is obtained by studying the ROESY of the aforementioned complex. Proton chemical shifts of MCD, KTP, and the IC are collectively presented in Table S2.
Analysis of ROESY of IC
Figure 6A displays the ROESY of the IC, from which cross-peaks labeled a, b, c, d, e, and f (Labeled ROESY is provided in Figures S5 and S6) are obtained at 3.82/1.37, 3.82/3.24, 3.82/3.46–3.72, 3.82/4.16, 3.82/4.34, and 3.82/7.69 ppm, respectively. These cross-peaks indicate correlations between the methine proton of the guest KTP and other protons of both the aromatic protons of the guest and the MCD moiety. The singlet observed at 3.24 ppm is attributed to the methyl proton of the methoxy group attached to the methylene carbon at C-5. The proton chemical shift at 7.69 ppm is assigned to aromatic protons (H2”/H6”), while the signal at 1.37 ppm is already assigned to the methyl protons of the phenyl propanoic acid part. Proton signals at 3.46–3.72, 4.16, and 4.34 ppm are conveniently assigned to H3, H5, and H2 respectively. Cross-peaks a and f are correlations attributed to the KTP, while cross-peaks b, c, d, and e indicate correlations between KTP and the MCD. All protons experience a shift to the upfield region, suggesting that the propanoic acid part resides outside the bucket and interacts with the methoxy methyl proton at C-5 and other CD protons, confirming the formation of the inclusion complex of MCD and KTP. In addition to the above cross-peaks, two more significant cross-peaks labeled g and h are observed at 7.56/7.53 and 7.56/7.73 ppm. These cross-peaks indicate correlations between the aromatic protons H2’/H6’, H3’/H5’, and H4’ of another phenyl group of KTP (attached to the carbonyl group). This aromatic proton experiences slight shielding of about 0.01 to 0.02 ppm, confirming that the phenyl ring with the propanoic acid part is outside the cavity, while the unsubstituted phenyl is inside the cavity. Based on the obtained NMR results and the correlations from ROESY, it strongly suggests that the structure of the IC involves the acid part of the guest outside the cavity and the counterpart inside the bucket, as illustrated in Fig. 6B.
FT-IR spectral analysis
Figure 7A illustrates the FT-IR spectra of KTP and its IC, demonstrating the interaction between KTP and MCD cavities. The spectrum of KTP exhibits absorption bands corresponding to the C=O stretching vibration of the acidic group, the C=O stretching vibration of the ketone, the O–H band, and the C=C stretching vibration of the aromatic ring at 1697, 1655, 3380, and 3100 cm−1, respectively. Furthermore, a strong absorption peak at 697 cm−1 is observed, attributed to the phenyl group of KTP54. The FTIR spectra of MCD display characteristic bands, including one at approximately 3426 cm−1 associated with the stretching vibration of hydrogen-bonded hydroxyl groups in MCD (Fig. S7A). Another band at 1646 cm−1 arises from the bending vibration of H–O–H in water molecules anchored within the CD cavities. Additionally, a strong absorption peak at 1155 cm−1 is attributed to the C–O stretching vibration, primarily assigned to the ether groups present in MCD52,55. Comparatively, no evidence of new bond formation is observed in the ICs, and there are no significant shifts in the spectral peaks of stretching and bending vibrations when compared to MCD alone. The stretching frequencies of the analyzed peaks are nearly obscured by MCD, highlighting the predominant role of CDs in the inclusion process of KTP56,57.
Raman spectral analysis
FT-Raman spectra provide rich information, where peak positions, intensities, widths, and shapes all offer valuable insights. Moreover, this technique is relatively quick and straightforward. Figure 7B illustrates the Raman spectra of KTP and its IC, demonstrating the interaction between KTP and CD cavities. In the spectra of KTP, three main peaks are identified as follows: The C=C stretching of the phenyl group in KTP observed at 1589 cm−1 and 1649 cm−1, the C–H stretching region in the range of 2950–3150 cm−1, and the ring deformation peak appearing at 998 cm−132,54,58. MCD exhibits a broad peak at 3400 cm−1, indicating the presence of –OH groups within the cavity (Fig. S7B). Additionally, a distinct peak at 2942 cm−1, attributed to C–H aliphatic bonds in the MCD cavity, is observed55. In the case of the IC, a reduced broad peak at 3400 cm−1 suggests the involvement of OH groups in interactions with KTP through hydrogen bonding. Notably, while there is no ring deformation peak in MCD, it becomes evident in the ICs, indicating the presence of both KTP and MCD in the complex products. The formation of ICs with MCD results in a significant shift in the peaks corresponding to the main groups of KTP, highlighting the influence of MCD on the KTP.
FE-SEM analysis
FE-SEM analysis is conducted to investigate the morphological properties of the IC and its raw sample of KTP. SEM images of both free KTP and the IC are presented in Fig. 8. As anticipated, bare KTP displayed rock-like particles with a colloidal nature, characterized by irregularly shaped crystals and larger particle sizes59,60. The complexation of KTP with MCD resulted in morphological changes, consistent with findings from FT-IR analysis. The particles observed in the SEM images of the IC exhibited smaller and finer dimensions, forming a layered structure61,62,63.
Thermal stability analysis
Thermogravimetry (TG), characterized by a continuous temperature rise, stands as one of the most straightforward and commonly utilized techniques for examining the thermal behavior of any formulation. TGA is employed to elucidate the thermal behavior of the IC. The TG curves of both KTP and the IC are depicted in Fig. 9. KTP, being a stable organic drug, displays resistance to mass loss with a gradual temperature increase up to 210 °C. Beyond this temperature, there is a gradual weight loss in KTP, indicating decomposition occurring between 210 and 298 °C, with more than 98% weight loss observed during this temperature range64,65. DTA curves for KTP reveal a sharp peak at 290 °C, signifying weight loss and indicating thermal stability without losses before 250 °C. In the case of the IC, the TGA curve remains stable up to 218 °C. A slight and insignificant reduction in mass is observed up to 100 °C, attributed to the release of moisture content from the IC. Beyond 218 °C, there is a notable but modest weight decrease (approximately 20%) up to 290 °C, followed by a gradual decline starting at 290 °C. The presence of MCD in the IC impedes the gradual decrease in the weight percentage of KTP within this temperature range, where decomposition of KTP occurs. Between 290 and 360 °C, there is a nearly 60% weight loss attributed to the major decomposition of both KTP and MCD in the IC. The DTA peaks at 288 °C and 356 °C correspondingly support the observed weight losses. Consequently, the experiment highlights that the thermal stability of KTP in the IC significantly surpasses that of bare KTP66,67. This suggests a stronger binding interaction of KTP with MCD, leading to enhanced thermal stability and improved ability to withstand temperature variations68,69.
DFT calculations
To get further elucidate the experimental results, we used quantum chemical calculations to explore the potential mode of interaction within the inclusion complexes. The most stable configuration, reflecting the lowest energy among the optimized conformations, is determined using the DFT/B3LYP/6-36-1G(d) level of theory70,71. In terms of configurations, in orientation 1, the phenyl group of KTP is positioned inside the cavity near the wider rim of MCD, as depicted in Fig. 10a, b. In orientation 2, the propanoic acid group of KTP stayed within the cavity adjacent to the wider rim of MCD, as illustrated in Fig. 10c, d. The binding energy results are summarized in Table 1, indicating significant negative binding energies for both orientations of complexes, suggesting favorability in the complexation process. The optimized geometrical parameters of orientation 1, and orientation 2 are summarized in Tables S3 and S4. The complexation energy calculations revealed that the KTP/MCD configuration in Orientation 1 (ΔE = −39.2821 kcal/mol) is more favorable than in orientation 2 (ΔE = −29.6184 kcal/mol). Consistently, DFT analysis suggested orientation 1 as the most favored configuration, a finding corroborated by 1H NMR and ROESY results.
Additionally, DFT calculation predicted non-covalent interaction specifically intermolecular hydrogen bonding (H-bond) between KTP and MCD (Table 2), and this kind of H-bond plays an important role in stabilizing the guest molecule inside the cavity of the host72. In orientation 1, the acid proton and phenyl C-H proton of KTP participate with the hydroxyl ‘O’ atom on the wider rim and the ether ‘O’ atom of MCD respectively. Notably, in Fig. 11a, b, the proton (H222) in the –COOH group of KTP form H-bonding with the -CH-OH ‘O’ atom (O16) of MCD, while another H-bonding interaction occurs when the aromatic –CH (H209) group of KTP acts as a hydrogen bond donor with the HC–O-CH ether ‘O’ atom (O24) of MCD. Conversely, in orientation 2 (Fig. 11c, d), the protons of the COOH (H222) and phenyl C–H (H216) groups of KTP engage in hydrogen bonding interactions with the ether (HC–O–CH) ‘O’ (O25 and O28) atoms of MCD, respectively.
The electronic quantum parameters, encompassing the energies associated with the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbital (LUMO), as well as the HOMO–LUMO gap (ΔEgap), and the global dipole moment of the analyzed IC, are computationally determined using B3LYP/6-31G(d) levels of theory. These findings are detailed in Table 2.
The HOMO–LUMO gap observed in both inclusion complexes signifies the presence of charge transfer interaction within host–guest molecules. The comparatively larger gap in both orientations indicates the formation of a more stable IC. Specifically, the frontier molecular orbital (FMO) gaps for orientation 1 and orientation 2 are determined to be 4.72 and 4.66 eV, respectively, with orientation 1 exhibiting a higher energy gap than orientation 2. These findings are consistent with complexation energy results. Illustrative FMO diagrams of the KTP/MCD orientations, depicted in Fig. 12, reveal the localization of the HOMO orbital on the MCD and the LUMO orbitals on KTP, indicating established mutual interaction between KTP and MCD. Through DFT analysis, we also observed an increase in the dipole moment of both uncomplexed MCD and KTP when the hydrophobic guest enters the MCD cavity and forms the complex, indicating an augmentation of polarity with the system.
In-vitro cytotoxicity by MTT assay
The cytotoxic effects of KTP and its ICs with MCD are evaluated in vitro by assessing their impact on cell proliferation using a normal cell line (HaCaT cells) treated with various concentrations of the aforementioned samples for 6 and 24 h. Our findings indicate that there is no significant alteration in cell viability among the treated cells (Fig. 13A). Additionally, no significant change in the incidence of cell death is observed in cultures exposed to KTP and MCD compared to controls (Fig. S8). Upon closer examination of the cell assay, some notable instances of cell death are observed for KTP samples at both 6 and 24-h time points. The elongation observed had minimal impact on cell function, suggesting it did not significantly contribute to cell death. However, after 24 h of treatment with 100% KTP in the form of IC, certain HaCat cells exhibited signs of cell death at concentrations of 200 µg/ml. Comparison between the cell death induced by KTP and its ICs revealed a significant improvement in cell death with the formation of ICs, resulting in substantially greater inhibition of cell growth compared to KTP alone while maintaining safety for the cell lines.
LDH assay results
In addition to MTT assay, LDH is the other method to check the cytotoxicity of the samples. The LDH release assay serves as an essential technique for assessing the integrity of cellular membranes. Elevated LDH release signifies compromised membrane integrity, indicative of high toxicity, whereas lower LDH release suggests enhanced membrane integrity and reduced toxicity. Our findings reveal that there is no significant alteration in LDH release from HaCat cells following treatment with KTP and IC for both 6 and 24 h (Fig. 13B). These results suggest that our compound exhibits minimal toxicity in cellular models, as evidenced by the preserved membrane integrity observed in the LDH release assay.
Detection of apoptotic cells with flow cytometry using annexin-V
Apoptosis represents a methodically regulated process marked by distinct morphological alterations, such as chromatin condensation, fragmented nuclei, cell shrinkage, and nuclear fragmentation73,74. To assess the effectiveness of KTP and KTP:MCD IC, we examined apoptosis levels using Annexin-V staining and flow cytometry at two different concentrations (0, 50, and 100 µg/ml). Figure 14 showcases representative images of Annexin V/7AAD-stained HaCaT cells subjected to KTP treatment and its IC. Interestingly, no apoptotic cells are detected in the treatment groups at 50 and 100 µg/ml concentrations. Thus, both KTP and its IC exhibited no discernible impact on normal cell behavior (Figs. S9, and S10). These results suggest that neither KTP nor KTP:MCD IC prompted apoptosis under the tested conditions. The absence of apoptotic cells in both treated and control groups implies a lack of programmed cell death induction or cytotoxic effects in fibroblast cells, indicating a promising safety profile for these treatments about programmed cell death induction.
In-vitro anti-inflammation (AI) assay
The experimental model of inflammation, capable of measuring inflammation, has proven to be of paramount importance in testing and assessing the analgesic and anti-inflammatory (AI) effects of bare KTP or the IC with MCD. Here, the preliminary assessment of AI activity is conducted in vitro using the albumin denaturation assay, as protein denaturation is implicated in inflammation and rheumatoid arthritis75,76. Protein denaturation is a process by which a protein loses its secondary and tertiary structure, potentially leading to the loss of its biological function. This denaturation of protein is recognized as a marker for inflammation77, as denatured proteins can form antigens that contribute to AI and related diseases like rheumatoid arthritis. The BSA assay is widely accepted and utilized in-vitro protein denaturation assays to evaluate the AI properties of substances78,79. In this assay, denatured proteins are generated using BSA at a pH of 7.4, and the AI of materials can be assessed by adding the substance to the BSA-induced inflammation (through the generation of denatured proteins). Inhibition of protein denaturation by substances indicates AI property, with a higher degree of inhibition suggesting greater AI potential80,81. Given KTP's notable AI properties, the albumin denaturation assay is employed to explore the anti-inflammatory activity of the studied IC, and the results are presented in Fig. 15, indicating enhanced AI activity for both the samples (KTP and IC) up to 400 μg/ml. The release of KTP from the IC is higher than that from bare KTP. The IC notably protects BSA from denaturation, with inhibition exceeding 85%. This protection is particularly pronounced with KTP, reaching 80%. This observation aligns well with the KTP release rate (IC > KTP). The significant role of KTP in AI activity in the form of IC is reflected in its binding constant.
Conclusions
In this study, we have successfully synthesized and characterized the IC of KTP with MCD, further evaluating its in-vitro AI and AA activities. The BH plots and PS study revealed that KTP forms a 1:1 stoichiometric ratio with MCD. The PS study indicated a significant increase in the solubility of KTP in the IC. FT-IR, 1H NMR, ROESY, FE-SEM, and TG/DTA results confirmed the formation of the IC. The complexation energy of orientation 1 (ΔE = −39.2821 kcal/mol) is demonstrated to be more favorable compared to orientation 2 (ΔE = −29.6184 kcal/mol). This finding is further supported by 1H NMR and ROESY results. MTT and LDH assays explored the KTP, and ICs with MCD have not been produced any toxic to the normal cell (HaCaT cell lines). Preliminary in-vitro studies demonstrated that the IC enhances the AI and AA activities of KTP by protecting it from protein denaturation, facilitated by the enhanced release of KTP from the IC. This strategy of preparing ICs in optimized ratios holds promise for facilitating the exploration of other poorly water-soluble NSAIDs. Such an approach can be followed to enhance the anti-inflammatory (AI) activity without inducing toxicity in normal cells.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yu, Y. et al. The anti-inflammation and anti-nociception effect of ketoprofen in rats could be strengthened through co-delivery of a H2S donor, S-propargyl-cystein. J. Inflamm. Res. 14, 5863–5875 (2021).
Sakeena, M. H. F., Yam, M. F., Elrashid, S. M., Munavvar, A. S. & Azmin, M. N. Anti-infl ammatory and analgesic effects of ketoprofen in palm oil esters nanoemulsion. J. Oleo. Sci. 59, 667–671 (2010).
Carla Aragoni, G. P. M. et al. Fluorescent sensing of non-steroidal anti-inflammatory drugs naproxen and ketoprofen by dansylated squaramide-based receptors. Org. Biomol. Chem. 21, 2968–2975 (2023).
Van Hees, T. et al. Application of supercritical carbon dioxide for the preparation of a piroxicam beta-cyclodextrin inclusion compound. Pharm. Res. 16, 1864–1870 (1999).
Van Hees, T., Piel, G., Henry de Hassoville, S., Evrard, B. & Delattre, L. Determination of the free/included piroxicam ratio in cyclodextrin complexes: Comparison between UV spectrophotometry and differential scanning calorimetry. Eur. J. Pharm. Sci. 15, 347–353 (2002).
Avouac, B. & Teule, M. Ketoprofen: The European experience. J. Clin. Pharm. 28, S2–S7 (1988).
Soliman, O.E.-A., Mohamed, E. A. M., El-Dahan, M. S. & Ali Khatera, N. A. Potential use of cyclodextrin complexes for enhanced stability, anti-inflammatory efficacy, and ocular bioavailability of loteprednol etabonate. AAPS PharmSciTech. 18, 1228–1241 (2017).
Chan, S.-Y., Chung, Y.-Y., Cheah, X.-Z., Tan, E.Y.-L. & Quah, J. The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers. Asian J. Pharm. Sci. 10, 372–385 (2015).
Lee, Y. et al. Evaluation of dextran-flufenamic acid ester as a polymeric colon-specific prodrug of flufenamic acid, an anti-inflammatory drug, for chronotherapy. J. Drug Target. 19, 336–343 (2011).
Yamada, T., Onishi, H. & Machida, Y. Sustained release ketoprofen microparticles with ethylcellulose and carboxymethylethylcellulose. J. Control Rel. 75, 271–282 (2001).
Grecu, M., Nastasa, V., Ilie, C., Miron, L. & Mares, M. Comparative assessment of effectiveness of ketoprofen and ketoprofen/beta-cyclodextrin complex in two experimental models of inflammation in rats. Lab. Anim. 48, 20–26 (2014).
Liu, L. et al. Inclusion complexes of laccaic acid A with β-cyclodextrin or its derivatives: Phase solubility, solubilization, inclusion mode, and characterization. Dyes. Pig. 139, 737–746 (2017).
Wadhwa, R. et al. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ-cyclodextrin. J. Cancer 7, 1755–1771 (2016).
Betc Kalepu, S. & Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharmaceut. Sin. B 5, 442–453 (2015).
Saha, S., Roy, A., Roy, K. & Roy, M. N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 6, 35764 (2016).
Sun, H.-L., Zhang, Y.-M., Chen, Y. & Liu, Y. Polyanionic cyclodextrin induced supramolecular nanoparticle. Sci. Rep. 6, 27 (2016).
He, D. et al. Molecular encapsulation of rifampicin as an inclusion complex of hydroxypropyl-beta-cyclodextrin: Design; characterization and in vitro dissolution. Coll. Surf. B. 103, 580–585 (2013).
Choi, J. M. et al. Solubility and bioavailability enhancement of ciprofloxacin by induced ovalshapedmono-6-deoxy-6-aminoethylamino-betacyclodextrin. Carb. Pol. 163, 118–128 (2017).
Sambasevam, K. P., Mohamad, S., Sarih, N. M. & Ismail, N. A. Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int. J. Mol. Sci. 14, 3671–3682 (2013).
Jambhekar, S. S. & Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug. Discov. Today. 21, 356–362 (2016).
Fenyvesi, É., Szemán, J., Csabai, K., Malanga, M. & Szente, L. Methyl-beta-cyclodextrins: The role of number and types of substituents in solubilizing power. J. Pharm. Sci. 103, 1443–1452 (2014).
Béni, S., Szakács, Z., Csernák, O., Barcza, L. & Noszál, B. Cyclodextrin/imatinib complexation: Binding mode and charge dependent stabilities. Eur. J. Pharm. Sci. 30, 167–174 (2007).
Mannila, J., Järvinen, T., Järvinen, K., Tarvainen, M. & Jarho, P. Effects of RM-beta-CD on sublingual bioavailability of Delta9-tetrahydrocannabinol in rabbits. Eur. J. Pharm. Sci. 26, 71–77 (2005).
Varga, E. et al. Comparative analysis of the full set of methylated β-cyclodextrins as chiral selectors in capillary electrophoresis. Electrophoresis. 40, 2789–2798 (2019).
Zhou, J., Jia, J., He, J., Li, J. & Cai, J. Cyclodextrin inclusion complexes and their aplication in food safety analysis: Recent developments and future prospects. Foods. 11, 3871 (2022).
Liu, J. Y., Zhang, X. & Tian, B. R. Selective modifications at the different positions of cyclodextrins: a review of strategies. Turk. J. Chem. 44, 261–278 (2020).
Sá Couto, A. R., Ryzhakov, A., Larsen, K. L. & Loftsson, T. Interaction of native cyclodextrins and their hydroxypropylated derivatives with carbamazepine in aqueous solution. Evaluation of inclusion complexes and aggregates formation. ACS Omega 4, 1460–1469 (2019).
Ann, H. J., Kim, K. M., Choi, J.-S. & Kim, C.-K. Effects of cyclodextrin derivatives on bioavailability of ketoprofen. Drug. Dev. Indus. Pharm. 23, 397–401 (1997).
Mura, P. et al. Interactions of ketoprofen and ibuprofen with b-cyclodextrins in solution and in the solid state. Int. J. Pharm. 166, 189–203 (1998).
Nagarsenker, M. S., Tantry, J. S. & Shenai, H. Influence of hydroxypropyl-B-cyclodextrin on the dissolution of ketoprofen and irritation to gastric mucosa after oral administration in rats. Pharm. Sci. 3, 44345 (1997).
Guidi, G. D., Condorelli, G., Giuffrida, S., Puglisi, G. & Giammon, G. Effect of/3-cyclodextrin complexation on the photohaemolitic activity induced by ketoprofen and naproxen sensitization. J. Incl. Phenom. Mol. Recogn. Chem. 15, 43–58 (1993).
Obaid, A., Jamil, A. K. M., Prabu, S., Saharin, S. M. & Mohamad, S. Spectroscopic studies for the inclusion complexation of ketoprofen enantiomers with β-cyclodextrin. Spectrochim. Acta Part A. 241, 118674 (2020).
Franco, P. & Marco, I. D. Preparation of non-steroidal anti-inflammatory drug/β-cyclodextrin inclusion complexes by supercritical antisolvent process. J. CO2 Utiliz. 44, 101397 (2021).
Bounaceur, A., Rodier, É. & Fages, J. Maturation of a ketoprofen/beta-cyclodextrin mixture with supercritical carbon dioxide. J. Supercrit. Flu. 41, 429–439 (2007).
Novarita, P. & Sumarno, T. The effect of water addition in inclusion formation of ketoprofen/β-cyclodextrin using supercritical CO2. AIP Conf. Proc. 2085, 020052 (2019).
Su, J. et al. Formation of β-cyclodextrin inclusion enhances the stability and aqueous solubility of natural borneol. J. Food. Sci. 77, C658–C664 (2012).
Grgac, S. F. et al. The Effect of ultrasonic treatment on the binding of the inclusion complex β-cyclodextrin-peppermint oil with cellulose material. Materials. 15, 470 (2022).
Yao, Y. et al. Preparation of inclusion complex of perfluorocarbon compound with β-cyclodextrin for ultrasound contrast agent. RSC Adv. 5, 6305–6310 (2015).
Das, S. K. et al. Cyclodextrins-the molecular container. Res. J. Pharm. Biol. Chem. Sci. 4, 1694–1720 (2013).
Higuchi, T. & Connors, K. A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 4, 117–212 (1965).
Kfoury, M. et al. Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem. 196, 518–525 (2016).
Guo, H., Cai, C., Gong, H. & Chen, X. Multi-spectroscopic method study the interaction of anti-inflammatory drug ketoprofen and calf thymus DNA and its analytical application. Spectrochim. Acta Part A 79, 92–96 (2011).
Bi, S., Yan, L., Sun, Y. & Zhang, H. Investigation of ketoprofen binding to human serum albumin by spectral methods. Spectrochim. Acta Part A 78, 410–414 (2011).
Chandrabose, L. et al. Characterization and molecular docking analysis for the supramolecular interaction of lidocaine with β-cyclodextrin. Polycyc. Arom. Comp. 43, 1202–1218 (2023).
Murugan, M., Anitha, A., Sivakumar, K. & Rajamohan, R. Effect of pH and structural orientation on supramolecular complexation of chloroquine in β-cyclodextrin medium. J. Mol. Liq. 325, 115157 (2021).
Benesi, A. & Hildebrand, J. H. The Benesi–Hildebrand method for determination of Kf for DA association and ε values for DA CT absorption. J. Am. Chem. Soc. 71, 2703–2707 (1949).
Wang, R. & Yu, Z. Validity and reliability of Benesi–Hildebrand method. Acta Phys. Chim. Sin. 23, 1353–1359 (2007).
Kim, D., Scranton, A. B. & Stansbury, J. W. Analysis of association constant for ground-state dye-electron acceptor complex of photoinitiator systems and the association constant effect on the kinetics of visible-light-induced polymerizations. J. Pol. Sci. Part A. 47, 1429–1439 (2009).
Iglesias, E. Inclusion complexation of novocaine by beta-cyclodextrin in aqueous solutions. J. Org. Chem. 71, 4383–4392 (2006).
Shota, S. et al. Relationship between phase solubility diagrams and crystalline structures during dissolution of cimetidine/cyclodextrin complex crystals. J. Pharm. Sci. 109, 2206–2212 (2020).
Rajaram, R. et al. Formulation of ensulizole with beta-cyclodextrins for improved sunscreen activity and biocompatibility. J. Pharm. Sci. https://doi.org/10.1016/j.xphs.2023.12.019 (2023).
Rajaram, R. et al. Enhanced photostability and biocompatibility of sunscreen formulation of 2-phenylbenzimidazole-5-sulfonic acid with methyl-beta-cyclodextrin. J. Mol. Liq. 390, 123013 (2023).
Lo, Y.-K., Hsu, C.-P., Tsai, T.-R. & Cham, T.-M. Comparison of the solubility and dissolution rate of gliclazide-β-cyclodextrin inclusion complexes prepared by liquid/liquid extraction and neutralization. J. Food. Drug. Anal. 14, 230–235 (2006).
Choi, S.-H., Kim, S.-Y., Ryoo, J. J., Park, J. Y. & Lee, K.-P. FT-Raman and FT-IR spectra of the non-steroidal anti-inflammatory drug ketoprofen included in cyclodextrins. Anal. Sci. 17, i786–i788 (2001).
Rajamohan, R. et al. A novel and water-soluble material for coronavirus inactivation from oseltamivir in the cavity of methyl and sulfated-β-cyclodextrins through inclusion complexation. J. Pharm. Biomed. Anal. 221, 115057 (2022).
Cheng, J.-H. et al. Preparation and properties of octenyl succinate β-cyclodextrin and its application as an emulsion stabilizer. Food Chem. 218, 116–121 (2017).
Dongmei, K. et al. Preparation and characterization of octenyl succinate β-cyclodextrin and vitamin E inclusion complex and its application in emulsion. Molecules 25, 654 (2020).
Vueba, M. L., Pina, M. E., Veiga, F., Sousa, J. J. & Batista de Carvalho, L. A. E. Conformational study of ketoprofen by combined DFT calculations and Raman spectroscopy. Int. J. Pharm. 307, 56–65 (2006).
Yadav, P. S., Kumar, V., Singh, U. P., Bhat, H. R. & Mazumder, B. Physicochemical characterization and in vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and d-mannitol. Saudi Pharm. J. 21, 77–84 (2013).
Mohammadian, M., Kashi, T. S. J., Erfan, M. & Soorbaghi, F. P. In-vitro study of ketoprofen release from synthesized silica aerogels (as drug carriers) and evaluation of mathematical kinetic release models. Iran. J. Pharm. Res. 17, 818–829 (2018).
Kou, X., Zhang, X., Ke, Q. & Meng, Q. Pickering emulsions stabilized by β-CD microcrystals: Construction and interfacial assembly mechanism. Front. Nut. 10, 1161232 (2023).
Acar, T. et al. Host–guest interactions of caffeic acid phenethyl ester with β-cyclodextrins: Preparation, characterization, and in vitro antioxidant and antibacterial activity. ACS Omega. 9, 3625–3634 (2024).
Alshehri, S. et al. Host-guest complex of β-cyclodextrin and pluronic F127 with Luteolin: Physicochemical characterization, anti-oxidant activity and molecular modeling studies. J. Drug Del. Sci. Tech. 55, 101356 (2020).
Junior, H. O. et al. A new crystalline ketoprofen sodium salt: Solid-state characterization, solubility, and stability. J. Pharm. Sci. 111, 1674–1681 (2022).
Tiţa, B., Fuliaş, A., Bandur, G., Marian, E. & Tiţa, D. Compatibility study between ketoprofen and pharmaceutical excipients used in solid dosage forms. J. Pharm. Biomed. Anal. 56, 221–227 (2011).
Shivani, U. et al. Encompassment of benzyl isothiocyanate in cyclodextrin using ultrasonication methodology to enhance its stability for biological applications. Ultrason. Sonochem. 39, 25–33 (2017).
Praveena, A., Prabu, S. & Rajamohan, R. Encapsulation of quercetin in β-cyclodextrin and (2-hydroxypropyl)-β-cyclodextrin cavity: In-vitro cytotoxic evaluation. J. Macromol. Sci. Part A. 54, 894–901 (2017).
Li, W. et al. Preparation and characterization of inclusion complex of benzyl isothiocyanate extracted from papaya seed with β-cyclodextrin. Food Chem. 184, 99–104 (2015).
Periasamy, R., Kothainayaki, S., Rajamohan, R. & Sivakumar, K. Spectral investigation and characterization of host–guest inclusion complex of 4, 4′-methylene-bis (2-chloroaniline) with beta-cyclodextrin. Carb. Pol. 114, 558–566 (2014).
Snor, W., Liedl, E., Weiss-Greiler, P., Viernstein, H. & Wolschann, P. Density functional calculations on meloxicam-beta-cyclodextrin inclusion complexes. Int. J. Pharm. 381, 146–152 (2009).
Ignaczak, A. & Orszanski, Ł. In search of the most stable molecular configuration of heptakis(2,6-O-dimethyl)-β-cyclodextrin and its complex with mianserin: A comparison of the B3LYP-GD2 and M062X-GD3 results. J. Phys. Chem. B. 125, 13077–13087 (2021).
Aree, T. Advancing insights on β-cyclodextrin inclusion complexes with SSRIs through lens of X-ray diffraction and DFT calculation. Int. J. Pharm. 609, 121113 (2021).
Subramaniyan, S. et al. Fabrication of nanofiber coated with l-arginine via electrospinning technique: a novel nanomatrix to counter oxidative stress under crosstalk of co-cultured fibroblasts and satellite cells. Cell Commun. Adhes. 24, 19–32 (2018).
Klempaiová, M. et al. Cytotoxicity testing of a polyurethane nanofiber membrane modified with chitosan/β-cyclodextrin/berberine suitable for wound dressing application: evaluation of biocompatibility. Cell Tissue Bank. 17, 665–675 (2016).
Vipin, D. B. et al. Characterisation and anti-inflammatory evaluation of the inclusion complex of ellagic acid with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocyc. Chem. 82, 361–372 (2015).
Kumari, K. D. K. P., Weerakoon, T. C. S., Handunnetti, S. M., Samarasinghe, K. & Suresh, T. S. Anti-inflammatory activity of dried flower extracts of Aegle marmelos in Wistar rats. J. Ethnopharmacol. 151, 1202–1208 (2014).
Ruiz-Ruiz, J. C., Matus-Basto, A. J., Acereto-Escoffié, P. & Segura-Campos, M. R. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from melipona beecheii honey. Food Agric. Immun. 28, 1424–1437 (2017).
Heendeniya, S. N., Ratnasooriya, W. D. & Pathirana, R. N. In vitro investigation of anti-inflammatory activity and evaluation of phytochemical profile of syzygium caryophyllatum. J Pharm. Phytochem. 7, 1759–1763 (2018).
Ashwini, T. et al. Sinapis arvensis-wild mustard as an anti-inflammatory agent: An in-vitro study. J. Clin. Diag. Res. 16, FC06–FC08 (2022).
Luana, H. et al. Pharmacological effects of a complex α-bisabolol/β-cyclodextrin in a mice arthritis model with involvement of IL-1β, IL-6 and MAPK. Biomed. Pharmacother. 151, 113142 (2022).
Eloísa, P. B. S. S. S. et al. Nerolidol-beta-cyclodextrin inclusion complex enhances anti-inflammatory activity in arthritis model and improves gastric protection. Life Sci. 265, 118742 (2021).
Acknowledgements
This project is supported by Researchers Supporting Project number (RSPD2024R712), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
R. R : Conceptualization, methodology, resources, formal analysis, writing—original draft. E. K : Software, formal analysis. P. M : Formal analysis. K. M : Formal analysis. C. G : Formal analysis. D.S. P: Methodology, resources. T. M: Formal Analysis, Writing—review & editing. Y. R. L: Writing—review & editing. Supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rajamohan, R., Kamaraj, E., Muthuraja, P. et al. Enhancing ketoprofen's solubility and anti-inflammatory efficacy with safe methyl-β-cyclodextrin complexation. Sci Rep 14, 21516 (2024). https://doi.org/10.1038/s41598-024-71615-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71615-9