Abstract
Prematurity is associated with lower exercise capacity, which relies on the integrity of the cardiovascular, pulmonary, and skeletal muscle systems. Our animal model mimicking prematurity-associated conditions showed altered muscle composition and atrophy in adulthood. This study aimed to compare muscle composition and strength in adults born preterm versus full-term controls. This observational cohort study recruited 55 adults born preterm, ≤ 29 weeks’ of gestation and 53 full-term controls who underwent musculoskeletal ultrasound imaging to assess morphology of the rectus femoris at rest and during a maximal voluntary contraction. Maximal voluntary contraction of the hands and legs were measured by manual dynamometry. In adults born preterm, there was lower muscle strength (handgrip: − 4.8 kg, 95% CI − 9.1, − 0.6; knee extensor: − 44.6 N/m, 95% CI − 63.4, − 25.8) and smaller muscle area (− 130 mm2, 95% CI − 207, − 53), which was more pronounced with a history of bronchopulmonary dysplasia. Muscle stiffness was increased in the preterm versus term group (0.4 m/s, 95% CI 0.04, 0.7). Prematurity is associated with alterations in skeletal muscle composition, area, and function in adulthood. These findings highlight the necessity to implement preventive and/or curative approaches to improve muscle development and function following preterm birth to enhance overall health in this population.
Similar content being viewed by others
Introduction
Improved perinatal care has allowed the survival of infants born extremely preterm (< 28 weeks of gestational age (GA). As individuals born preterm reach adulthood, studies have shown their increased vulnerability for chronic health diseases1. Indeed, preterm birth and its associated conditions can alter the normal sequence of organ development with lasting effects on future cardiovascular, pulmonary, or renal health1. Moreover, adults born preterm who had complications such as bronchopulmonary dysplasia (BPD), the chronic lung disease of prematurity, are more susceptible to display organ system dysfunction in adulthood2.
Several studies in children and adults born preterm reported limited exercise capacity, mostly explained by cardiopulmonary impairment3. Exercise capacity is also determined by the integrity of the skeletal muscle system, which is important to maintain posture, voluntary movements, and to support involuntary actions, such as breathing and protective reflexes4. Skeletal muscles are further involved in organ and tissue regulation through the release of signaling molecules, the myokines5,6. Alteration of skeletal muscle tissue can disrupt homeostasis of several systems and contribute to heighten the risk for chronic diseases7. The third trimester of pregnancy is an important period for skeletal muscle ontogenesis, characterized by maturation of muscle fibers and excitation–contraction coupling, and fiber type determination8.
We previously conducted a systematic review and meta-analysis to summarize the impact of preterm birth on skeletal muscles and found a reduction of muscle thickness and power in individuals born preterm versus term. However, studies remain scarce in adults born preterm with limited data on other characteristics of skeletal muscles, such as muscle area composition, and strength, highlighting the knowledge gap in understanding the effect of preterm birth on skeletal muscle development9.
In a preclinical model mimicking deleterious conditions associated with preterm birth, preterm birth-related conditions cause an oxidative stress and inflammatory response associated with activation of protein degradation pathways, muscle atrophy, lower mitochondrial oxidative capacity, and muscle fatigability at juvenile and adult stages10,11.
We therefore postulated that preterm birth is associated with altered composition and function of the skeletal muscle in adults born very preterm which could be worse with a history of bronchopulmonary dysplasia. To tackle this hypothesis, we assessed skeletal muscle composition by musculoskeletal ultrasound of the rectus femoris and muscle strength using dynamometry in adults born preterm and compared their measures to full-term controls.
Materials and methods
Study population
The Health of Adults born Preterm Investigation (HAPI) cohort recruited individuals born at ≤ 29 weeks’ GA and full-term (≥ 37- to 42 weeks 0 day of GA) controls. Participants were identified from a list of patients born at one of the three neonatal intensive care units in Montreal, Canada, between 1987 and 199712. Full-term controls were born with birth weight ≥ 2500 g and group matched for sex and age (± 2 years). When available, we recruited them among friends and siblings of individuals born preterm, to take into consideration the shared environment. Exclusion criteria for all participants were the presence of severe neurosensory deficit preventing test completion or being pregnant (Supplementary Fig. 1). Of the 247 individuals reached, 109 were included. One participant was excluded due to gestational age (32 weeks). All participants gave written consent to participate in the study. They were assessed at Centre Hospitalier Universitaire Sainte-Justine from June 2021 to October 2023.
Muscle composition
Image acquisition by ultrasound
B-mode ultrasound images of the rectus femoris (quadricep muscle) were captured with a Canon Aplio i800 device (Canon Medical Systems, Otawara-Shi, Japan) using a 14L5 linear probe set at 14 MHz to assess muscle area, thickness, echogenicity, and elasticity/stiffness. Images were acquired by two operators trained for high intra- and inter-observer reliability (ICC 0.94–0.99). Throughout the study, the gain was set at 75 dB (dynamic range of 75 gradations) and remained constant whereas the depth ranged between 5 and 7 cm to allow full visualization of the muscle and the focal zone was positioned at the middle of this muscle. All participants were in a sitting position with the knee in a 90-degree flexion. Images were taken from the non dominant leg (defined as the opposite leg use to kick a ball or climb stairs) halfway along the line from the anterior superior iliac spine to the superior border of the patella. Three B-mode images were taken for each plane: transverse and longitudinal at rest and under maximal voluntary contraction. The elasticity/stiffness of the rectus femoris was also evaluated in triplicates using single shot shear wave elastography (SWE) at rest and under maximal voluntary contraction in a longitudinal plane.
Image analysis
Muscle area (cross sectional area, mm2) was assessed using images captured in the transverse plane. Sub-cutaneous thickness (cm) (distance between the skin and the superior fascia of the muscle), echogenicity (average pixel value in a region of interest, expressed in arbitrary unit—AU), and shear wave elastography (m/s) were assessed using images captured in the longitudinal plane. Muscle area and echogenicity analyses were performed by a single operator using a custom program (USLBP_GUI; version R2018b) developed with MATLAB's Image Processing Toolbox (The MathWorks Inc., Natick, MA, USA), as used in previous studies (Supplementary Fig. 2)13 Muscle area and thickness were normalized to the body mass index (BMI)14.
Handgrip and quadriceps strength
Grip strength was assessed according to a standardized protocol15,16 with participants seated, both feet flat on the ground. Arm was unsupported with elbow flexion at 90°. Participants were encouraged to squeeze the handle of the dynamometer (Lafayette Hand Dynamometer, model 78010) to generate a maximal voluntary effort held during at least 3 s, for three measures for both hands. Maximum values obtained on the dominant and non-dominant hand were averaged to calculate the mean maximal grip strength. The absolute maximal grip strength out of all six trials was also reported17,18. Values are reported as kilograms (kg) and normalized to height (m)19. For the leg extensor strength, we adapted an existing standardized protocol20,21,22 to allow concomitant ultrasound imaging integration during the assessment. An instrumented dynamometer (EasyForce, Meloq AB, Sweden) was fixed from the table to the ankle of the dominant and non-dominant legs with the knee and hip flexed at 90° with participants seated. After three submaximal trials to warm up, participants performed three maximal isometric voluntary contractions on the dominant and non-dominant legs with a one-minute rest period between each measurement. Maximum values from each leg were averaged to calculate leg extensor strength. Values are reported in Newton meters (Nm) and normalized to body weight (kg)23. For normalization of the strength to muscle surface area (cm2) (evaluated by ultrasound), value from the non-dominant leg was used.
Additional assessments
Height, weight, thigh circumference, body mass index, daily count step, physical activity, estimated VO2 max (based on Huet questionnaire)24 and neonatal characteristics are described in the online supplementary material.
Statistical analysis
Descriptive statistics were calculated as mean with standard deviation (SD) for continuous variables and counts with proportions for categorical variables. We checked for normality of outcome variables of muscle composition and strength using visual inspection and the Shapiro–Wilk test. All between-group comparisons were performed using linear regression analyses and adjusted for sex. Given the ultrasound absorbance properties of adipose tissue, we further adjusted for sub-cutaneous thickness when comparing muscle echogenicity. To examine the relationship between skeletal muscle strength, muscle composition and aerobic capacity, linear regression analyses were conducted. We also created an interaction term between the independent variable and prematurity status to assess whether relationships differed between adults born preterm and term. All analyses were performed using SPSS software version 27 and the graphical representations of correlations were done with R software version 4.3.2 and package version ggplot2 version 3.4.4.
Results
Study population
We included 108 participants: 55 very preterm (24 men, 31 women; mean GA: 26.9 ± 1.3 weeks) and 53 full-term controls (23 males, 30 women; mean GA 39.4 ± 1.3 weeks) (Table 1). Among adults born preterm, participants who were recruited were of lower GA (non-participants: 27.2 ± 1.3 weeks, P = 0.383) and birthweight (non-participants: 1033 ± 274 g, P = 0.045), but there was no sex difference (non-participants: 55% females, P = 0.940). Mean age at assessment was 29.9 years. In the preterm group, 16 (29%) had a history of moderate to severe BPD. In addition, 5 had some level of neuromotor deficits (i.e., cerebral palsy), but were still able to complete the study protocol (Table 1). Individuals born preterm had smaller anthropometric measures compared to full-term controls. Estimated VO2max [ml/(kg/min)] was lower in the preterm group. There were no observed between-group differences in the number of steps, sedentary time, moderate-to-vigorous and vigorous physical activity time per day.
Muscle structure
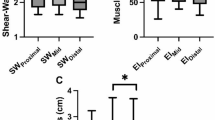
Individuals born preterm, in comparison to term-born peers, had a reduction of 13.5% of the muscle area at rest (mean difference, MD: of – 130 mm2; 95% CI − 206 to − 54, P = 0.001) and 12.5% during maximal contraction (MD – 119 mm2; 95% CI − 202 to − 35, P = 0.006) (Table 2 and Supplementary Fig. 3) even after adjusting for sex. However, when normalizing to BMI, differences in muscle area and thickness between individuals born preterm and full-term were no longer observed. Muscle stiffness (shear wave elastography, SWE) was higher in adults born preterm during maximal contraction adjusting for sex (MD: + 0.4 m/s; 95% CI 0.0–0.7, P = 0.026). A similar trend was observed at rest, although it did not reach statistical significance. No between-group differences in muscle echogenicity were found at rest or at maximal contraction (Table 2).
Muscle function
Individuals born preterm, compared to full-term controls, had lower maximal knee extensor strength (MD − 44.7 Nm; 95% CI − 61.4, − 28.0, P < 0.001), even after normalizing for body weight and accounting for sex (Table 3). Combined and absolute maximal handgrip were lower for the preterm group in comparison to their full-term counterparts (Table 4) (MD − 4.8 kg; 95% CI − 9.1, − 0.6, P = 0.025 and − 4.3 kg; 95% CI − 8.8, 0.1, P = 0.058, respectively). When stratified by sex, the preterm-term differences were larger among men than among women.
Bronchopulmonary dysplasia and skeletal muscle
To explore factors that could contribute to skeletal muscle alterations, we compared skeletal muscle composition and function between adults born preterm with moderate to severe BPD and those without BPD. Muscle area at rest was lower in those with BPD versus without BPD (MD – 119 mm2, 95% CI − 235, − 3, P = 0.044) even after adjusting for birth weight. We did not identify any statistically significant differences for the other parameters (Table 5).
Relationship between skeletal muscle strength, muscle composition and aerobic capacity
Individuals with greater skeletal muscle mass at the quadriceps typically displayed greater knee extensor strength. For each cm2 increase in cross-sectional area of the quadriceps, knee extensor strength increased by 9.4 Nm (95% CI 3.7, 15.1) in the preterm group and by 10.4 Nm (95% CI 3.8, 16.9) in the full-term group (Fig. 1A) There was no difference in this association when comparing the preterm versus term group (p-value = 0.752 for the interaction between prematurity status and cross-sectional area). Conversely, higher skeletal muscle stiffness was associated with lower strength. Each unit increase in SWE resulted in a decrease in knee extensor strength of − 18.2 Nm (− 27.8, − 8.7) and − 25.6 Nm (− 39.9, − 11.4) for the preterm and full-term born groups, respectively (Fig. 1B) (P-value for the interaction between prematurity status and SWE = 0.838).
Association between cross sectional area, shear wave elastrography and knee extension strength. (A) Increased cross sectional area correlates with higher strength. (B) Increased shear wave elastography is associated with reduced strength. A linear regression line is displayed for the association between the two variables with its computed 95% CI. Correlation coefficients (R) and P-values were calculated using the Pearson’s method.
Finally, predicted VO2 max was associated with muscle strength in both groups (Supplementary Table 1). However, we did not find any association between level of physical activity and muscle area and strength (Supplementary Table 1).
Discussion
This study shows that adults born very preterm have altered skeletal muscle health. Compared to full-term controls, they have lower muscle mass, even more so with a history of BPD, lower strength, and higher muscle stiffness. However, there is no significant difference in muscle echogenicity and level of physical activity among the participants. Increased muscle mass and lower stiffness are associated with greater strength, the latter also correlating with predicted VO2max. To our knowledge, very few studies have concomitantly investigated muscle composition and strength in adults born very preterm. None has evaluated muscle stiffness nor the effect of BPD on skeletal muscle health.
Skeletal muscle health is an important determinant of functioning and quality of life. In older individuals, sarcopenia (i.e., the loss of skeletal muscle mass and strength) is associated with a reduced capacity to perform activities of daily living25. The loss of function can result in lower mobility and frailty, but also metabolic problems, which, in turn, can predispose to adverse health conditions and premature death26. Preterm birth is associated with limited exercise capacity, glucose intolerance, and increased risk of cardiovascular diseases1,3,27,28. The extent to which the skeletal muscle could contribute to these dysfunctions is unknown. The first step was therefore to examine whether skeletal muscle tissue was altered in adults born preterm, which could potentially point towards a target for intervention to improve health.
Preterm birth can disrupt several maturational processes important to healthy organ development, including the skeletal muscle tissue29. Mechanisms are still under investigations, but could involve early life exposure to oxidative stress30,31 and systemic inflammation32. Complications like BPD or sepsis, and treatments, including steroids or anti-inflammatory agents, could also influence skeletal muscle tissue development33,34. In a preclinical rodent model mimicking preterm birth-related conditions, markers of inflammation and oxidative stress were found within the skeletal muscle tissue of juvenile pups exposed to transient neonatal hyperoxia. These exposed tissues displayed smaller fiber size, increased proportion of fast fatigable fibers, and increased deposition of collagen over time and decreased muscle strength, which was more pronounced in males than females10,11. This regulatory axis between inflammation and muscle has also been observed in other conditions. For instance, low-grade systemic inflammation has been associated with muscle atrophy in chronic obstructive pulmonary disease (COPD) and aging35,36. Muscle biopsies in patients with COPD have further shown upregulation of pro-inflammatory cytokines37,38,39 along with muscle weakness. In addition, birth size in itself could also play a role as reported in a study of adults born at term (37–42 weeks’ GA) which showed that birth size predicted handgrip strength in adulthood40.
In infants born preterm, muscle thickness was diminished compared to full-term controls41, and even more so with BPD42. We found similar findings, suggesting that smaller muscle size may track from infancy to adulthood. In addition, we observed that decreased muscle area was associated with lower muscle strength of the quadriceps. The smaller height and weight, which characterize the body phenotype of individuals born preterm, could explain why muscle area and thickness are inferior. Indeed, when we adjusted for body size, the observed differences were no longer statistically significant. However, leg strength remained lower in the preterm group, in agreement with other studies43,44,45, suggesting that there may be other contributors beside anthropometric differences. Lower leg strength could indicate altered myofibers function due to inadequate excitation–contraction coupling and/or ATP supply secondary to mitochondrial deficiency. In humans, autopsy studies found lesser mitochondria energy metabolism in the muscles of newborns born very preterm versus full-term46,47. In a rodent model mimicking preterm birth-related conditions, lower muscle mitochondrial biogenesis and oxidative activity, and compensatory higher glycolytic enzyme expression were documented when compared to control rats; these differences were observed in males but not in female rats10. We also found lower strength in the upper extremities for the preterm group with a handgrip estimated to be equivalent to that of a 65-year-old male and a 60-year-old female48. This corroborates results from Morrison and colleagues on 95 adults in their thirties with birth weight < 1000 g, who displayed an absolute maximal grip strength comparable to individuals aged 65 years for males and 55–60 years for females17, suggesting premature muscle wasting. Adult males born preterm appeared more affected than females. Male infants born preterm are more vulnerable than females with higher mortality and morbidities49,50,51. Some of these morbidities involve injury associated with oxidative stress and inflammation52,53. Female neonates versus males have greater antioxidant capacity54,55, which may confer better protection.
We assessed muscle stiffness/elasticity using elastography imaging. Measurement of muscle elasticity at rest provides relevant baseline physiological characteristics. However, measuring stiffness during muscle contraction is superior at discriminating a normal from a pathological state56,57. In our study, we observed at rest a slight increase of SWE, and significant differences with full-term controls during maximal contraction. Interestingly, higher stiffness was associated with lower muscular strength. Considering that muscle stiffness increases with age58,59, longitudinal follow-up will allow to determine whether muscle aging occurs more prematurely and/or is accelerated in preterm population.
Physical activity is a critical determinant of skeletal muscle health. Cohort studies have shown that adults born very preterm self-report decrease leisure time physical activities compared to full-term peers60. We objectively quantified physical activity and sedentary time using accelerometers and found no between-group differences, like others61,62,63, suggesting that the observed skeletal muscle dysfunction is not solely the result of deconditioning. Moreover, these findings indicate that the reduction observed in the estimated aerobic capacity and the muscular strength are not a limit to physical activity in early adulthood. Possibly performing the same physical activity will require more efforts in preterm born individuals. The estimated aerobic capacity was surprisingly high, especially when considering both groups’ modest levels of physical activity. In previous studies, estimated VO2max using the Huet questionnaire was higher in our participants when compared to measured peak VO2 by cardiopulmonary exercise testing64,65. Nevertheless, estimated and measured VO2max are highly correlated24, and the magnitude of the between-group differences using estimated values was comparable to what was previously reported with measured peak VO212,65.
Study limitations must be acknowledged. First, selection bias may have resulted in recruiting adults born preterm of higher socio-economic status and in better health. This would lead to an underestimate of the effect of preterm birth on skeletal muscle health. However, participants born preterm compared to non-participants were of smaller gestational age and lower birthweight, which could result in an overestimation of the difference. In addition, our cohort was predominantly white and representative of the general population in the province of Québec, Canada, born during that time period. Replication in other populations is required for generalizability. A portable electronic instrumented dynamometer was used to maximize the feasibility and generalizability; however, it comes inherently with limits if compared with a high-tech motorized instrumented dynamometer that would have provided added stability and reduced risk of movement compensation. This being said, a standardized protocol was followed in the present study as described above throughout the study using the same table and foam cushions for adjustment. Participants were familiarized to the testing procedure prior to performing the measures. Finally, the use of a questionnaire to estimate VO2max is not as objective as a direct measure. Future studies would gain in precision measuring VO2max.
Conclusions
Our findings indicate that very preterm birth leads to alteration in skeletal muscle composition and function in adulthood. These alterations could contribute to the higher risk of developing cardiopulmonary disorders observed in preterm born individuals and hinder maintenance of an active lifestyle with aging. Interventions to improve skeletal muscle health may represent a novel avenue to prevent chronic health diseases following preterm birth. Physical activity can reverse aging in sarcopenic patient66; an adapted exercise program targeting muscle mass and function from the neonatal intensive care unit to adulthood could bring benefits.
Data availability
Data is available for sharing upon reasonable request to the corresponding authors.
References
Luu, T. M., Katz, S. L., Leeson, P., Thébaud, B. & Nuyt, A. M. Preterm birth: Risk factor for early-onset chronic diseases. CMAJ 188(10), 736–746 (2016).
Dartora, D. R. et al. Association of bronchopulmonary dysplasia and right ventricular systolic function in young adults born preterm. Chest 160(1), 287–296 (2021).
Edwards, M. O. et al. Effect of preterm birth on exercise capacity: A systematic review and meta-analysis. Pediatr. Pulmonol. 50(3), 293–301 (2015).
Frontera, W. R. & Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 96(3), 183–195 (2015).
Severinsen, M. C. K. & Pedersen, B. K. Muscle-organ crosstalk: The emerging roles of myokines. Endocr. Rev. 41(4), 594–609 (2020).
Gomarasca, M., Banfi, G. & Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 94, 155–218 (2020).
Wolfe, R. R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 84(3), 475–482 (2006).
Draeger, A., Weeds, A. G. & Fitzsimons, R. B. Primary, secondary and tertiary myotubes in developing skeletal muscle: A new approach to the analysis of human myogenesis. J. Neurol. Sci. 81(1), 19–43 (1987).
Deprez, A. et al. Impact of preterm birth on muscle mass and function: A systematic review and meta-analysis. Eur. J. Pediatr. 183, 2989–2002 (2024) (in press).
Tetri, L. H. et al. Sex-specific skeletal muscle fatigability and decreased mitochondrial oxidative capacity in adult rats exposed to postnatal hyperoxia. Front. Physiol. 9, 326 (2018).
Deprez, A. et al. Transient neonatal exposure to hyperoxia, an experimental model of preterm birth, leads to skeletal muscle atrophy and fiber type switching. Clin. Sci. (Lond.) 135(22), 2589–2605 (2021).
Flahault, A. et al. Increased incidence but lack of association between cardiovascular risk factors in adults born preterm. Hypertension 75(3), 796–805 (2020).
Lalumiere, M. et al. Proposing a minimal data set of musculoskeletal ultrasound imaging biomarkers to inform clinical practice: An analysis founded on the achilles tendon. Ultrasound Med. Biol. 46(9), 2222–2235 (2020).
Kara, M. et al. Star-sonographic thigh adjustment ratio: A golden formula for the diagnosis of sarcopenia. Am. J. Phys. Med. Rehabil. 99(10), 902–908 (2020).
Toolbox TN. Grip strength test. NIH Toolbox. An assessment of upper extremity strength. Participants are asked to squeeze a hand dynamometer as hard as they can, one hand at a time (2024).
Physiology CSFE. The Canadian Physical Activity, Fitness and Lifestyle Approach Supplement to the Third Edition 28 (2010).
Morrison, K. M. et al. Grip strength is lower in adults born with extremely low birth weight compared to term-born controls. Pediatr. Res. 89(4), 996–1003 (2021).
Roberts, H. C. et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 40(4), 423–429 (2011).
Kasović, M. et al. Allometric normalization of handgrip strength in older adults: Which body size parameter is the most appropriate?. BMC Sports Sci. Med. Rehabil. 15(1), 18 (2023).
Petitclerc, É., Hébert, L. J., Mathieu, J., Desrosiers, J. & Gagnon, C. Lower limb muscle strength impairment in late-onset and adult myotonic dystrophy type 1 phenotypes. Muscle Nerve 56(1), 57–63 (2017).
Palmieri-Smith, R. M., Brown, S. R., Wojtys, E. M. & Krishnan, C. Functional resistance training improves thigh muscle strength after acl reconstruction: A randomized clinical trial. Med. Sci. Sports Exerc. 54(10), 1729–1737 (2022).
Abdalla, P. P. et al. One-repetition submaximal protocol to measure knee extensor muscle strength among older adults with and without sarcopenia: A validation study. BMC Sports Sci. Med. Rehabil. 12, 29 (2020).
Šarabon, N., Kozinc, Ž & Perman, M. Establishing reference values for isometric knee extension and flexion strength. Front. Physiol. 12, 767941 (2021).
Trivel, D. et al. Validity and reliability of the huet questionnaire to assess maximal oxygen uptake. Can. J. Appl. Physiol. 29(5), 623–638 (2004).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48(1), 16–31 (2019).
Zhou, H. H. et al. Association of muscle wasting with mortality risk among adults: A systematic review and meta-analysis of prospective studies. J. Cachexia Sarcopenia Muscle 14(4), 1596–1612 (2023).
Lovering, A. T. et al. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann. Am. Thorac. Soc. 11(10), 1528–1537 (2014).
Rogers, M., Fay, T. B., Whitfield, M. F., Tomlinson, J. & Grunau, R. E. Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<or=800 g) survivors at 17 years of age compared with term-born control subjects. Pediatrics 116(1), e58-65 (2005).
Romero, N. B., Mezmezian, M. & Fidziańska, A. Main steps of skeletal muscle development in the human: Morphological analysis and ultrastructural characteristics of developing human muscle. Handb. Clin. Neurol. 113, 1299–1310 (2013).
Cannavò, L., Perrone, S., Viola, V., Marseglia, L., Di Rosa, G., Gitto, E. Oxidative stress and respiratory diseases in preterm newborns. Int. J. Mol. Sci. 22(22) (2021).
Torres-Cuevas, I. et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 12, 674–681 (2017).
Humberg, A. et al. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 42(4), 451–468 (2020).
Schakman, O., Kalista, S., Barbe, C., Loumaye, A. & Thissen, J. P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 45(10), 2163–2172 (2013).
Duchesne, E., Dufresne, S. S. & Dumont, N. A. Impact of inflammation and anti-inflammatory modalities on skeletal muscle healing: From fundamental research to the clinic. Phys. Ther. 97(8), 807–817 (2017).
Remels, A. H., Gosker, H. R., Langen, R. C. & Schols, A. M. The mechanisms of cachexia underlying muscle dysfunction in copd. J. Appl. Physiol. 114(9), 1253–1262 (2013).
Antuña, E. et al. Inflammaging: Implications in sarcopenia. Int. J. Mol. Sci. 23(23), 15039 (2022).
Montesde Oca, M. et al. Skeletal muscle inflammation and nitric oxide in patients with copd. Eur. Respir. J. 26(3), 390–397 (2005).
Spruit, M. A. et al. Muscle force during an acute exacerbation in hospitalised patients with copd and its relationship with cxcl8 and igf-i. Thorax 58(9), 752–756 (2003).
Maltais, F. et al. An official american thoracic society/european respiratory society statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 189(9), e15-62 (2014).
Garay, J. L., Barreira, T. V., Wang, Q. & Brutsaert, T. D. Intra-uterine effects on adult muscle strength. Early Hum. Dev. 163, 105490 (2021).
Bertini, G., Elia, S. & Dani, C. Using ultrasound to examine muscle mass in preterm infants at term-equivalent age. Eur. J. Pediatr. 180(2), 461–468 (2021).
Dassios, T., Kaltsogianni, O., Krokidis, M., Hickey, A. & Greenough, A. Deltoid muscle morphometry as an index of impaired skeletal muscularity in neonatal intensive care. Eur. J. Pediatr. 177(4), 507–512 (2018).
Mata Zubillaga, D. et al. Evaluation of isometric force in lower limbs and body composition in preterm infants. An. Pediatr. (Barc) 83(4), 229–235 (2015).
Takken, T., van Haastert, I., Scholman, W. L., Block, A. J., Eijsermans, M. J. C., Vries, L., Helders, P. J. M. Aerobic exercise capacity and its relationship with pulmonary function, muscle strength, physical activity and motor competence in preterm born children: The premafit-pilot study. In Aerobic Exercise and Athletic Performance: Types, Duration and Health Benefits 293–306 (2010).
Smuall, E. W., Baror, O., Mil, E. V. & Saigal, S. Muscle function of 11- to 17-year-old children of extremely low birthweight. Pediatr. Exerc. Sci. 10, 327–336 (1998).
Honzik, T. et al. Activities of respiratory chain complexes and pyruvate dehydrogenase in isolated muscle mitochondria in premature neonates. Early Hum. Dev. 84(4), 269–276 (2008).
Wenchich, L. et al. Mitochondrial energy metabolism in very premature neonates. Biol. Neonate 81(4), 229–235 (2002).
Dodds, R. M. et al. Grip strength across the life course: Normative data from twelve british studies. PLoS One 9(12), e113637 (2014).
Stevenson, D. K. et al. Sex differences in outcomes of very low birthweight infants: The newborn male disadvantage. Arch. Dis. Child Fetal Neonatal Ed. 83(3), F182-185 (2000).
Boghossian, N. S., Geraci, M., Edwards, E. M., Horbar, J. D. Sex differences in mortality and morbidity of infants born at less than 30 weeks' gestation. Pediatrics 142(6) (2018).
Binet, M. E., Bujold, E., Lefebvre, F., Tremblay, Y. & Piedboeuf, B. Role of gender in morbidity and mortality of extremely premature neonates. Am. J. Perinatol. 29(3), 159–166 (2012).
Thébaud, B. & Abman, S. H. Bronchopulmonary dysplasia: Where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am. J. Respir. Crit. Care Med. 175(10), 978–985 (2007).
Zhang, L. et al. Nesfatin-1 alleviates hyperoxia-induced bronchopulmonary dysplasia (bpd) via the nuclear factor-κb (nf-κb) p65 signaling pathway. J. Biochem. Mol. Toxicol. 38(4), e23680 (2024).
Lavoie, J. C. & Chessex, P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic. Biol. Med. 23(4), 648–657 (1997).
Harrison, A., Khashu, M., Friel, J., Lavoie, J. C. & Chessex, P. Variations in metabolic response to tpn are influenced more by sex than by light exposure. J. Pediatr. Gastroenterol. Nutr. 45(5), 577–581 (2007).
Botanlioglu, H. et al. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral pain syndrome. Skelet. Radiol. 42(5), 659–666 (2013).
Bedewi, M. A. et al. Shearwave elastography of the sartorius muscle. Medicine (Baltimore) 100(11), e25196 (2021).
Alfuraih, A. M., Tan, A. L., O’Connor, P., Emery, P. & Wakefield, R. J. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin. Exp. Res. 31(12), 1755–1763 (2019).
Ikezoe, T., Asakawa, Y., Fukumoto, Y., Tsukagoshi, R. & Ichihashi, N. Associations of muscle stiffness and thickness with muscle strength and muscle power in elderly women. Geriatr. Gerontol. Int. 12(1), 86–92 (2012).
Tikanmäki, M. et al. Leisure time physical activity in young adults born preterm. J. Pediatr. 189, 135-142.e132 (2017).
Tikanmäki, M. et al. Objectively measured physical activity and sedentary time in young adults born preterm-the ester study. Pediatr. Res. 81(4), 550–555 (2017).
Lowe, J., Watkins, W. J., Kotecha, S. J. & Kotecha, S. Physical activity and sedentary behavior in preterm-born 7-year old children. PLoS One 11(5), e0155229 (2016).
Tardif, C. B. et al. Hapi fit: An exercise intervention to improve peak aerobic capacity in young adults born very preterm. Med. Sci. Sports Exerc. 56(1), 44–52 (2024).
Delfrate, J., Girard-Bock, C., Curnier, D., Perie, D., Cloutier, A., Gascon, G., Landry, J. S., Masse, B., Stickland, M. K., Nuyt, A. M. et al. Cardiopulmonary response to exercise in adults born very preterm. Eur. Respir. J. 2023.
Xie, L. F. et al. The long-term impact of very preterm birth on adult bone mineral density. Bone Rep. 10, 100189 (2019).
Shen, Y. et al. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J. Cachexia Sarcopenia Muscle 14(3), 1199–1211 (2023).
Acknowledgements
We thank Hakim Mecheri for contributing to ultrasound images analyses, Dr Alexis Vivoli for the graphical generation on R software, and Mi-Suk Kang-Dufour for her helpful review of the statistical analyses. We thank all the participants for their contribution to this study. We thank the Meloq AB Company for kindly providing us the dynamometer used in this study.
Funding
Alyson Deprez was supported by a scholarship from the FRQNT (Fonds de recherche du Québec—Nature et Technologies, 275929). Dany H Gagnon holds a Senior Research Career Award from the FRSQ and holds the Initiative for the Development of New Technologies and Practices in Rehabilitation (INSPIRE) research chair. Marie Eve Mathieu holds a Canada Research Chair—Tier 2 on Physical Activity and Juvenile Obesity. Nicolas Alexandre Dumont was supported by a FRQS Junior-2 award, and by a research grant from the CIHR (PJT-174993). Anne Monique Nuyt was supported by the Cercle de Sainte-Justine DOHaD Research Chair and a Tier 1 Canada Research Chair in Prematurity and Developmental Origins of Cardiovascular Health and Diseases. Thuy Mai Luu was supported by a CIHR (PJT-173404) and FRQS (Fonds de Recherche du Québec—Santé) senior award. The other authors received no additional funding.
Author information
Authors and Affiliations
Contributions
A.D., R.E.J., D.H.G., N.A.D., A.M.N. and T.M.L. conceptualized and designed the study. A.D., R.E.J., A.G.H., A.C., M.E.M. and T.A.K. designed the data collection instruments, collected data, carried out the initial analyses. A.D. drafted the initial manuscript, prepared the figures and tables and revised the manuscript. T.M.L., A.M.N. and N.A.D. coordinated the project and supervised data collection. R.E.J., D.H.G., M.E.M., N.A.D., A.M.N. and T.M.L. critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical guidelines
The study was approved by the appropriate clinical ethics committee of CHU Sainte-Justine and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants gave their informed consent prior to their inclusion in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Deprez, A., El-Jalbout, R., Cloutier, A. et al. Adults born preterm have lower peripheral skeletal muscle area and strength. Sci Rep 14, 21457 (2024). https://doi.org/10.1038/s41598-024-72533-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72533-6
Keywords
This article is cited by
-
Cardiometabolic characteristics of school-aged children born preterm with very low birth weight
Pediatric Research (2025)
-
Skeletal muscle alterations in Marfan syndrome: a systematic review
Journal of Muscle Research and Cell Motility (2025)