Abstract
Exploring efficient and easily implementable prenatal screening strategies aims at birth defect prevention and control. However, there have been limited economic evaluations of non-invasive prenatal screening (NIPS) strategies in China. Furthermore, these studies were predominantly confined to local or geographically proximate provinces and lacked universality and representativeness. This study assesses the health economics of current prenatal screening strategies and NIPS as first-line screening programs, analyzing their efficacy to determine an optimal strategy. From the perspective of health economics, cost-effectiveness, cost-benefit, and single-factor sensitivity were conducted for five different screening strategies using a decision tree model. Among pregnant women aged < 35 years who underwent only one screening for foetal Down syndrome (DS), the detection rate, false positive rate and positive predictive value of NIPS for foetuses with DS were superior to those of the other four serological screening methods. Although applying NIPS as first-line screening method yields the highest efficacy and benefits, it currently lacks cost-effectiveness when compared to serological screening and sequential NIPS screening strategies.
Similar content being viewed by others
Introduction
Down syndrome (DS), also known as trisomy 21 syndrome, is the most common chromosomal aneuploidy in newborns, with an incidence rate of approximately 1/7001. Its incidence rate increases with advanced maternal age; however, it can affect individuals across all age groups and is independent of race or nationality1. This genetic disorder frequently results in miscarriage, stillbirth, and structural abnormalities in the foetus. The clinical manifestations in live births include distinctive facial features, intellectual disabilities, growth retardation, and multiple organ abnormalities, bringing enormous psychological stress and substantial economic burden to families and society. Given the current limitations in medical interventions for such congenital defects and associated conditions, implementing secondary prevention and control through prenatal screening and diagnosis represents an effective strategy to reduce the birth rate of children with chromosomal abnormalities.
Over the past few decades, prenatal screening methods for DS have been developed and updated continuously. The widely implemented clinical strategy for diagnosis is to conduct ultrasound and serum prenatal screening for pregnant women and then obtain foetal samples through invasive prenatal diagnostic procedures such as chorionic villus biopsy, amniocentesis, or umbilical vein puncture for high-risk populations. Due to the proportion of high-risk pregnant women indicated by serum prenatal screening (approximately 5%)2, the number of pregnant women who need to be diagnosed through interventional prenatal procedures remains high, and the existing medical resources for prenatal diagnosis in China are overwhelmed, making the prenatal diagnosis for screening high-risk pregnant women difficult. In addition, only 1–3% of high-risk pregnant women have foetuses with DS3,4,5. In addition, the detection rate of serological screening varies from 60 to 95%, depending on the screening strategy1,4,5,6. However, increasing the detection rate and decreasing the false-negative rate often requires increasing the false-positive rate as a cost.
Therefore, exploring efficient, accurate, and easy-to-perform prenatal screening strategies is a goal and hotspot in birth defect prevention and control. In 2008, Lo and Chiu first published a study on detecting foetal chromosomal aneuploidy abnormalities via cfDNA in maternal plasma7, which is known as non-invasive prenatal testing/screening (NIPT/NIPS). Although NIPS cannot screen for open neural tube defects (ONTDs) compared with traditional serological screening systems, it significantly improves the detection rate of common foetal chromosomal aneuploidy diseases, such as trisomy 21, 18, and 13, while reducing the false-positive rate1,6,8,9, which effectively relieves the pressure of prenatal diagnosis and, to some extent, prevent unnecessary abortion or foetal damage. Currently, NIPS is the subsequent screening after serological screening in China; however, this does not interfere with the right of an ordinary pregnant woman to independently choose NIPS as a first-line test10.
In addition, the current model may have the problems of increased DS screening costs and missed prenatal diagnosis of other chromosomal abnormalities in foetuses. Currently, they are mainly accidentally detected by invasive prenatal diagnosis based on ultrasound abnormalities during pregnancy or for other reasons. NIPS used in clinical practice has not routinely included screening for chromosome microdeletions/microduplications; however, the DS detection and false positive rate of NIPS are superior to serum screening. Whether it will significantly reduce the number of pregnant women undergoing subsequent invasive prenatal diagnosis while reducing the detection of other chromosomal diseases such as sexual chromosome abnormalities and microdeletions/microduplications syndrome remains to be studied.
Few studies have evaluated NIPS screening strategies economically in China. Previous studies have compared the detection efficiency of various screening strategies for DS and conducted health-economic analyses11,12,13,14,15,16. These results suggest that NIPS is a cost-effective and complementary model to traditional serum screening. Therefore, this study aimed to conduct a health economic evaluation of the current prenatal screening program and NIPS as a first-line test through a survey of large prenatal diagnostic institutions with strong radiation capabilities in the Sichuan Province and analyse the effectiveness of these strategies, with a view to obtaining an optimal prenatal screening strategy suitable for our province, providing reasonable suggestions for the clinical application of NIPS and the continuous improvement of our province’s prenatal screening and diagnosis system.
Methods
The survey data in this study comprised the clinical data of single pregnant women aged < 35 years who underwent serum prenatal screening or NIPS at the West China Second University Hospital between 2015 and 2019. All methods were performed in accordance with the Technical standards of prenatal screening and diagnosis for foetal common chromosomal abnormalities and open neural tube defects Part1 Maternal serum prenatal screening in the second trimester (the Health Standards of the People’s Republic of China, 2010), Technical Specification for Prenatal Screening and Diagnosis of NIPS (National Health Commission of the People’s Republic of China, 2016), and other relevant guidelines1,17,18. Every pregnancy had conducted pre-test genetic counselling. Participants provided written informed consent, and the study was authorised by West China Second University Hospital’s Institutional Ethics Committee (No.2024-073). Patients with multiple pregnancies and those whose follow-up sample data were lost were excluded from analysis. Sample data on prenatal screening and diagnosis, including results, abortion rates, and pregnancy outcomes, were collected. The probability of occurrence of each outcome was calculated using the follow-up statistics, and this parameter was incorporated into the subsequent decision-tree model. The cost estimation was derived from on-site research conducted by West China Second University Hospital, encompassing costs associated with prenatal screening and diagnosis, as well as genetic counselling expenses and costs for termination-of-pregnancy. We calculated the standard costs to conduct a preliminary evaluation of the new technology for Down syndrome(DS) screening.
DS prenatal screening process
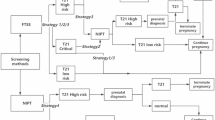
According to the definitions of high risk (≥ 1/270), intermediate risk (1/1000 -1/271), and low risk by serum biochemical screening, the pregnancy outcomes of pregnant women in the clinical pathway of each screening strategy were statistically analysed. The pregnant women independently chose one of the following screening schemes:
Strategy 1–4: Based on serum prenatal screening, we suggested that pregnant women with an intermediate risk of DS receive NIPS, and pregnant women with a high risk of DS receive prenatal diagnosis.
Strategy 1: Serum biochemical screening in the first trimester- NIPS strategy involved serum prenatal screening in the first trimester (PAPP-A, free β-hCG) for pregnant women aged < 35 years at 11–13+6 weeks.
Strategy 2: Combined first-trimester screening - NIPS strategy - NIPS strategy involved combined first-trimester screening (PAPP-A, free β-hCG, and nuchal translucency) for pregnant women aged < 35 years at 11–13+6 weeks.
Strategy 3: Double screening in the second trimester - NIPS strategy involved serum prenatal screening in the second trimester (alpha-fetoprotein [AFP], free β-hCG) for pregnant women aged < 35 years at 15–20+6 weeks.
Strategy 4: Triple screening in the second trimester - NIPS strategy involved serum prenatal screening in the second trimester (AFP, free β- hCG, inhibin-A) for pregnant women aged < 35 years at 15–20+6 weeks.
Strategy 5: NIPS strategy. Pregnant women aged < 35 years underwent NIPS after 12 weeks of pregnancy, and the results suggested that pregnant women at high risk of DS receive a prenatal diagnosis.
Follow-up outcome
The decision tree model in this study included six outcomes: (1) diagnosis of foetus with DS and elective termination of pregnancy; (2) live birth of undiagnosed DS foetus; (3) diagnosis of other chromosomal abnormalities (excluding DS foetus), such as chromosomal aneuploidy, large segment deletion/duplication, microdeletion/microduplication syndrome, etc.; (4) normal foetus aborted due to invasive prenatal diagnosis; (5) spontaneous abortion; (6) live birth of a normal foetus.
Decision tree model and parameter settings
Referring to the “Consolidated Health Economic Evaluation Reporting Standards (CHEERS),” a decision tree model was established using TreeAge Pro 2011 software. The decision tree model incorporated relevant parameters or indicators based on extensive literature review, expert opinions, and local data sources. From the perspective of health economics, cost-effectiveness, cost-benefit, and single-factor sensitivity analyses were conducted to evaluate the five different screening strategies.
To effectively utilise survey parameters for indicator calculation, the decision tree model relied on the following assumptions: (1) Genetic counselling was performed when prenatal screening of pregnant women indicated an intermediate risk of DS, a high risk of DS, or when prenatal diagnosis results indicated that the foetus with DS. (2) When serum prenatal screening indicated an intermediate risk for DS, the acceptance rate for NIPS prenatal screening was 100%. (3) The acceptance rate of invasive prenatal diagnosis was 100% when prenatal screening indicated a high risk of DS. (4) Termination of pregnancy when the foetus was diagnosed with DS through prenatal diagnosis. (5) No overlap between abortion cases resulting from invasive prenatal diagnosis and cases of true positive termination of pregnancy.
Screening efficiency and health-economics evaluation
The screening efficiency indicators included the number of detected cases, the number of missed and surviving children, and positive predictive value (PPV). Health economics evaluation indicators included total cost, cost-effectiveness, cost-benefit, and incremental cost-effectiveness analyses. The chi-square test was used to statistically assess the difference between the detection rates of chromosomal abnormalities and copy number variations (CNV) among the target diseases for each screening strategy. Statistical analyses were performed using the SPSS version 24.0.
Cost refers to the medical expenses required for DS screening. The cost calculation in this study primarily refers to direct medical expenses, including serum screening, nuchal translucency examination, NIPS testing, invasive prenatal diagnosis surgery and testing fees, and termination of pregnancy costs, including foetal termination of pregnancy due to DS or abortion caused by invasive prenatal diagnosis, genetic counselling fees, and childcare costs. Direct nonmedical, indirect, and intangible costs, such as psychological stress caused by invasive prenatal diagnosis for pregnant women were not measured.
The cost calculation formula was as follows.
(1) Prenatal screening cost (yuan) = number of pregnant women undergoing serological screening × acceptance rate (%) × average cost of screening cases + number of pregnant women with NIPS × acceptance rate (%) × average cost of screening cases (yuan).
(2) Prenatal diagnosis cost (yuan) = number of high-risk pregnant women undergoing prenatal screening × acceptance rate of prenatal diagnosis (%) × average cost of prenatal diagnosis (yuan).
(3) Cost of genetic consultation (yuan) = number of pregnant women with positive prenatal screening and diagnosis × acceptance rate (%) × average cost of genetic consultation (yuan).
(4) Cost of terminating pregnancy (yuan) = number of prenatal diagnoses × abortion ratio × average case cost (yuan) + number of detected cases × acceptance rate of pregnancy termination (%) × average cost of terminating pregnancy (yuan).
The cost-benefit analysis (CBA) was performed by calculating the cost-benefit net present value (NPV) and cost-benefit ratio (CBR) of the cost benefits. In this study, the benefit of prenatal screening refers to the economic loss avoided by preventing the birth of a child with DS, which is determined by multiplying the cost of raising a child and the number of detected cases. NPV = B-C (B refers to the potential benefits of screening strategies to avoid the birth of DS children, C represents the cost of screening strategy investment), CBR = B/C.
According to a health economic evaluation of the prenatal screening program for DS in the Hunan Province in 2012, the economic burden for each child with DS after birth was 1.1 million Yuan. The NPV and CBR are used to measure the economy. If the NPV is greater than 0 and the CBR is greater than 1, it indicates that the benefit of the screening strategy outweigh the cost, and the screening strategy is considered economic.
cost effectiveness analysis (CEA) is performed by calculating the cost-effectiveness ratio (CER) and incremental cost-effectiveness ratio (ICER). The evaluation index of effectiveness is the number of cases in which live births of children with DS were prevented. CER refers to the cost invested in different screening strategies to prevent the birth of a child with DS. The smaller the CER, the better the health economics effect of the screening strategy. The ICER is used to compare the relationship between additional inputs and outputs among different screening strategies to determine the value of the increased cost inputs. ICER = (total cost of screening strategy A - total cost of screening strategy B) / (number of DS cases detected by screening strategy A - number of DS cases detected by screening strategy B). Lower incremental costs or costs of raising one child indicate a more advantageous screening strategy. In CEA, CER, and ICER were used to compare the economics of various screening strategies using the prevention of live birth of a child with DS as an outcome indicator. If ICER falls below the willingness to pay (WTP) threshold, it suggests that the scheme is considered economically viable.
WTP is calculated based on per capita Gross Domestic Product (GDP) from the 2020 China Statistical Yearbook and multiplying three with the per capita GDP as the WTP threshold. According to the 2020 China Statistical Yearbook, per capita GDP was 70,892 yuan, and three times per capita GDP was 212,676 yuan.
Results
Screening efficiency evaluation
Table 1 shows the screening results of the four serological screening methods and the NIPS for foetal DS in pregnant women aged < 35 years who underwent only one screening. The detection rate (DR), false positive rate (FPR), and positive predictive value (PPV) of NIPS for foetuses with DS were superior to those of the other four serological screening methods, and the screening efficiency had significant advantages.
Detection of other chromosomal abnormalities
The results in Table 2 show the detection rate of chromosomal abnormalities in foetuses other than the target disease among the five screening strategies. Strategy 5 (NIPS strategy) showed the highest proportion of detecting chromosomal abnormalities other than the target disease (0.22%). They include foetal sex chromosomal abnormality (SCA) (66.7%), other autosomal abnormalities (23.8%), and CNV (9.5%). The chi-square test analysis results revealed a statistically significant difference in the detection rate of chromosomal abnormalities other than the target disease among the different screening strategies (P < 0.001), and the difference in the detection rate of CNV was also statistically significant (P = 0.017).
Health economics analysis
Based on the decision tree model, we simulated and calculated the intervention effects of the five screening strategies for every 10,000 pregnant women under natural conditions using existing parameters.
Costing
The parameters of the decision tree model were sourced from the literature and West China Second University Hospital. Medical expenses were based on the guidance price standards of relevant projects in the Sichuan Province (Table 3).
CBA results
Table 4 shows the CBA results of screening 10,000 pregnant women using the five screening strategies. The effectiveness and benefits of the screening strategies were as follows: Strategy 5 (NIPS strategy) > Strategy 4 (triple screening in the second trimester, NIPS strategy) > Strategy 2 (combined first-trimester screening, NIPS strategy) > Strategy 1 (serum biochemical screening in the first trimester, NIPS strategy) and > Strategy 3 (double screening in the second trimester).
The NPV of all five screening strategies was greater than 0, and the CBR was greater than 1, indicating that the benefits of all five screening strategies were greater than the costs, and all had economic benefits. Among them, strategy 4 (triple screening in the second trimester, NIPS strategy) had the highest cost-benefit NPV (8520318.34 yuan) and the CBR (2.45). The cost of strategy 5 (NIPS strategy) was the highest, at 24857916.29 yuan.
CEA results
Table 5 shows the CEA results of screening 10,000 pregnant women using the five screening strategies. The CER of the screening strategies, from small to large, was as follows: Strategy 4 < Strategy 2 < Strategy 1 < Strategy 3 < Strategy 5. The CER of strategy 4 was the lowest, at 448591.56 yuan per case; the CER of strategy 5 was the highest, at 1052702.21 yuan per case.
Table 6 presents the comparison between the incremental cost-effectiveness analysis results of strategy 5 and the other four screening strategies. Compared to the other four screening strategies, the ICER of Strategy 5 was greater than the WTP value (212676 yuan/case), indicating that Strategy 5 was not cost-effective.
Single factor sensitivity analysis
Figures 1, 2, 3 and 4 show the ICER cyclone plots of strategy 5 compared to the other four screening strategies. The figures show that the primary indicator affecting the ICER is the NIPS detection price. When the price of NIPS testing decreased, the ICER of Strategy 5 gradually decreased compared to the other four screening strategies. Table 7 shows the NIPS prices when the ICER of Strategy 5 is lower than those of the other four screening strategies. From Table 7, it can be seen that when the NIPS testing price drops to around 600 yuan/case, Strategy 5 is more economical compared with Strategy 1, 2, and 3. When the price of NIPS testing further dropped to 478.33 yuan/case, strategy 5 was more cost-effective than strategy 4.
Discussion
CBA and CEA of NIPS for prenatal screening of DS
From the perspective of preventing the birth of a foetus with DS, the NIPS first-line screening strategy prevented 23.61 such births, with superior results compared to other four serological screenings (NIPS sequential screening strategies) and higher than the second-ranked triple screening in the second trimester (13.08 cases). To avoid economic losses by preventing the birth of children with DS, the benefit of prenatal screening was the product of the cost of raising one child and the number of detected cases. Therefore, the first-line screening strategy for NIPS exhibited the highest benefit of 25,971,000 yuan. However, this strategy also had the highest cost at 24857916.29 yuan; therefore, its cost-effectiveness NPV and CER were lower than those of the other four serological screening NIPS sequential screening strategies. Nevertheless, the cost-effectiveness NPV of the NIPS frontline screening strategy was still greater than 0, and the cost-benefit ratio was greater than 1, indicating that the benefits of this screening strategy outweigh the costs.
In addition, from the perspective of CER, the first-line screening strategy of the NIPS had the highest CER, with a cost of 1052702.21 Yuan per case, to prevent the birth of one child with DS. Compared to the other four screening strategies, the ICER of the NIPS frontline screening strategy was greater than the WTP threshold of 212,676 Yuan per case, calculated based on the per capita GDP in the 2020 China Statistical Yearbook. Whether based on the CER or ICER, currently, the cost of the NIPS first-line screening strategy is relatively high and is not cost-effective compared with the cost of the other four screening strategies. Therefore, NIPS is not typically suggested as the initial screening option primarily due to its higher cost relative to other screening strategy, rather than its comparative detection rate. The results of this study were consistent with those of previous studies, and the authors believed that the NIPS, as a conditional screening method after serological screening, was a more economical screening model19,20,21.
Currently, the average cost of NIPS technology in China is approximately 1400–2850 Yuan, and most regions are not covered by medical insurance reimbursement. Pregnant women must bear the testing costs. Some regional health committees provide free NIPS testing for pregnant women with testing indicators in their jurisdiction. According to the Technical Specification for Prenatal Screening and Diagnosis of NIPS (National Health Commission of the People’s Republic of China, 2016), NIPS is applied to pregnant women at intermediate or high risk for serological screening, those with an expected delivery age of ≥ 35 years who are unwilling to undergo invasive prenatal diagnosis, and pregnant women who miss the optimal time for DS serological screening. Previous research has shown that the CER of NIPS as a conditional screening model for high-risk pregnant women in serological screening was superior to that of NIPS as a first-line screening strategy19,20. This study suggests that the cost-effectiveness of providing NIPS as a conditional screening model for the serological screening of intermediate-risk pregnant women was greater than the NIPS first-line screening strategy.
Single-factor sensitivity analysis showed that other parameters remained unchanged. As the average price of NIPS decreased from 2400 Yuan to approximately 600 Yuan, the NIPS first-line screening strategy was more cost-effective than serum biochemical screening in the combined first-trimester screening–NIPS strategy or double screening in the second trimester with NIPS strategy. When the average price of NIPS decreased to 478.33 Yuan, the NIPS first-line screening strategy was more cost-effective than triple screening in the second trimester NIPS strategy. Our study indicates that the cost of NIPS should be significantly reduced. Genevieve’s research results showed that NIPS has a better CER than combined first-trimester screening at specific prices22. Our results are consistent with previously reported findings. At a specific price, considering the life cycle cost of patients with DS after birth, NIPS is expected to become an economically effective alternative to serological screening22,23,24.
Comparative analysis of different prenatal screening strategies
The analysis of the five screening strategies in this study revealed that the cost-benefit NPV obtained by the four serological screening strategies– with NIPS sequential screening strategies and NIPS frontline screening strategies were all greater than 0, and the CBR was greater than 1, indicating that the benefits of the five screening strategies were greater than the costs, and economic feasibility in preventing DS. In our province, the most widely used, which is the triple screening in the second trimester and NIPS strategy, had the highest cost-effectiveness NPV and CEA and lowest CER. From a health economics perspective, this screening strategy was prioritised. Especially for prenatal screening and diagnosing institutions that only perform double screening in the second trimester and NIPS strategy within their jurisdiction, prioritising the selection of triple screening in the second trimester and NIPS strategy is recommended. As the testing cost of triple screening in the second trimester with the NIPS strategy was relatively small compared to double screening in the second trimester with the NIPS strategy, and its acceptance by the women was high, the optimisation of health economic results for screening strategies can be achieved based on small cost growth.
Single-factor sensitivity analysis showed that screening cost was the main factor affecting the economic value of DS prenatal screening strategies. Although the detection rate and PPV of serological screening are relatively low compared with those of NIPS technology, its cost is low and has a positive impact on the CER of screening, ultimately achieving better economic value. Many scholars have conducted CBA of prenatal screening programs for DS; however, their results differ17,25,26,27,28. The reasons for this may be related to the differences in the cost of prenatal screening technology for DS, selection of screening programs, and acceptance of prenatal diagnosis.
Analysis of the efficiency of prenatal screening
This study shows that NIPS outperforms the other four serological screening protocols in terms of detection rate, false positive rate, and PPV for foetuses with DS and has significant advantages in screening efficacy. Among serological screenings, the DR of triple serological screening in the second trimester of pregnancy was the highest. The PPV of the NIPS for foetuses with DS in this study was slightly lower than that reported in the literature1. Because the PPV of the screened target disease was related to the incidence rate of the target disease in the population, the incidence rate of the target disease was low, as was the PPV. Therefore, we considered that the reason for the low PPV was related to the fact that the study participants were pregnant women with an expected delivery age of less than 35 years. According to the Maternal and Child Health Law of China, pregnant women aged < 35 years at delivery are required to undergo serological prenatal screening for common foetal chromosomal abnormalities (DS and trisomy 18) and ONTDs. Therefore, this study selected pregnant women with an expected delivery age of < 35 years as the research participants to analyse the prenatal screening strategy for DS because the prevalence of foetuses with DS increases with an increase in the maternal age. The incidence rate of DS in younger pregnant women was lower than that in older pregnant women, and the corresponding PPV was also lower, but the number of DS was higher in younger pregnant women due to the larger number of pregnancies.
This study analysed the detection status of foetuses with chromosomal abnormalities other than the target disease using five screening strategies. Among them, the NIPS first-line screening strategy showed the highest proportion of detecting chromosomal abnormalities other than the target disease (0.22%). Previously, we anticipated that NIPS, as a first-line screening strategy, may lead to a decrease in detecting chromosomal diseases other than the target disease owing to a decrease in the number of pregnant women undergoing prenatal diagnosis. However, the results of this study were not align with our expectations. The reasons related to this result may be as follows: (1) Technical Specification for Prenatal Screening and Diagnosis of NIPS (National Health Commission of the People’s Republic of China, 2016) clearly recommends that “for abnormal high-risk results other than the target disease, prenatal diagnostic institutions should inform the pregnant woman for further consultation and diagnosis.” Therefore, pregnant women with other abnormal high-risk results through NIPS may have a higher acceptance of invasive prenatal diagnosis after detailed and standardised genetic counselling, and chromosomal abnormalities in the foetus have a greater chance of being detected in a timely manner during pregnancy; (2) Low-risk pregnant women undergoing prenatal screening often opt for further invasive prenatal diagnosis owing to ultrasound indications of foetal abnormalities. If other chromosomal abnormalities do not show significant ultrasound abnormalities during pregnancy, their detection through prenatal diagnosis may be missed. This also suggests that we need to consider whether NIPS can provide additional information on chromosomal disorders compared to prenatal ultrasound examination; (3) foetuses with chromosomal abnormalities other than the target disease may be unexpectedly discovered owing to high-risk screening for invasive prenatal diagnosis, indicating that there may be differences between NIPS high-risk populations and traditional screening high-risk populations; (4) Recently, the advancement in prenatal diagnostic technology, such as the application of molecular genetic testing like chromosome microarray analysis (CMA) and CNV-seq, has significantly enhanced the detection rate of chromosomal abnormalities outside the target disease and expanded the disease spectrum based on the routine application of amniotic fluid chromosome karyotype analysis. Our preliminary findings indicated a necessity for conducting more comprehensive exploration and research on the spill-over value of different screening schemes (abnormal detection beyond the target disease) to obtain more objective and accurate health economics evaluation results.
Conclusion
Our results indicate that the current and widely used sequential screening strategy of NIPS for pregnant women with a risk value of DS between the high-risk cut-off value and 1/1000 based on serology in the second trimester of pregnancy has the highest cost-effective NPV and the best cost-effectiveness and benefit evaluations. This screening strategy should be prioritised from a health economics perspective. Applying NIPS to first-line screening has the highest efficacy and benefit; however, owing to the high cost of NIPS testing, it is not yet cost-effective compared with the serological screening and NIPS sequential screening strategy.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Screening for Fetal Chromosomal Abnormalities. ACOG Practice Bulletin Summary, Number 226. Obstet. Gynecol. 136, 859–867 (2020).
Malone, F. D. et al. First-trimester or second-trimester screening, or both, for Down’s syndrome. N Engl. J. Med. 353, 2001–2011 (2005).
Chen, Y., Wang, X., Li, L., Lu, S. & Zhang, Z. New cut-off values for screening of trisomy 21, 18 and open neural tube defects (ONTD) during the second trimester in pregnant women with advanced maternal age. BMC Pregnancy Childbirth. 20, 776 (2020).
Hsu, J. J., Hsieh, T. T., Soong, Y. K. & Spencer, K. Comparison of Down’s syndrome screening strategies in asians combining serum free beta-hCG and alpha-fetoprotein with maternal age. Prenat Diagn. 17, 707–716 (1997).
Palomaki, G. E., Knight, G. J., McCarthy, J. E., Haddow, J. E. & donhowe, J. M. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am. J. Obstet. Gynecol. 176, 1046–1051 (1997).
Gregg, A. R. et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet. Sci. 18, 1056–1065 (2016).
Lo, Y. M. D. et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 350, 485–487 (1997).
Chiu, R. W. K. et al. Non-invasive prenatal assessment of trisomy 21 by multiplexed maternal plasma DNA sequencing: large scale validity study. Bmj. 342, c7401–c7401 (2011).
Zhang, H. et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146 958 pregnancies. Ultrasound Obstet. Gynecol. 45, 530–538 (2015).
Liu, Y. et al. Clinical performance of non-invasive prenatal served as a first-tier screening test for trisomy 21, 18, 13 and sex chromosome aneuploidy in a pilot city in China. Hum. Genomics. 14, 21 (2020).
Prodan, N. C. et al. Universal Cell Free DNA or Contingent Screening for Trisomy 21: does it make a difference? A comparative study with Real Data. Fetal Diagn. Ther. 49, 85–94 (2022).
Wang, S., Liu, K., Yang, H. & Ma, J. A cost-effectiveness analysis of screening strategies involving non-invasive prenatal testing for Trisomy 21. Front. Public. Health. 10, 870543 (2022).
Cuckle, H., Heinonen, S., Anttonen, A. K. & Stefanovic, V. Cost of providing cell-free DNA screening for Down syndrome in Finland using different strategies. J. Perinat. Med. 50, 233–243 (2022).
Sanchez-Duran, M. A. et al. Clinical application of a contingent screening strategy for trisomies with cell-free DNA: a pilot study. BMC Pregnancy Childbirth. 19, 274 (2019).
Wanapirak, C., Buddhawongsa, P., Himakalasa, W., Sarnwong, A. & Tongsong, T. Fetal Down syndrome screening models for developing countries; part II: cost-benefit analysis. BMC Health Serv. Res. 19, 898 (2019).
Huang, T., Meschino, W. S., Teitelbaum, M., Dougan, S. & Okun, N. Enhanced First Trimester Screening for Trisomy 21 with contingent cell-free fetal DNA: a comparative performance and cost analysis. J. Obstet. Gynaecol. Can. 39, 742–749 (2017).
Dungan, J. S. et al. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 25, 100336 (2023).
Hui, L. et al. Position statement from the International Society for Prenatal Diagnosis on the use of non-invasive prenatal testing for the detection of fetal chromosomal conditions in singleton pregnancies. Prenat. Diagn. 43, 814–828 (2023).
Evans, M. I., Sonek, J. D., Hallahan, T. W. & Krantz, D. A. Cell-free fetal DNA screening in the USA: a cost analysis of screening strategies. Ultrasound Obstet. Gynecol. 45, 74–83 (2015).
Morris, S., Karlsen, S., Chung, N., Hill, M. & Chitty, L. S. Model-based analysis of costs and outcomes of non-invasive prenatal testing for Down’s syndrome using cell free fetal DNA in the UK National Health Service. PLoS ONE; 9. (2014).
Neyt, M., Hulstaert, F. & Gyselaers, W. Introducing the non-invasive prenatal test for trisomy 21 in Belgium: a cost-consequences analysis. BMJ Open.; 4. (2014).
Fairbrother, G., Burigo, J., Sharon, T. & Song, K. Prenatal screening for fetal aneuploidies with cell-free DNA in the general pregnancy population: a cost-effectiveness analysis. J. Maternal-Fetal Neonatal Med. 29, 1160–1164 (2016).
Walker, B. S. et al. A cost-effectiveness analysis of first trimester non-invasive prenatal screening for fetal trisomies in the United States. Plos One; 10. (2015).
Walker, B. S., Jackson, B. R., LaGrave, D., Ashwood, E. R. & Schmidt, R. L. A cost-effectiveness analysis of cell free DNA as a replacement for serum screening for Down syndrome. Prenat. Diagn. 35, 440–446 (2015).
van der Meij, K. R. M., Henneman, L. & Sistermans, E. A. Non-invasive prenatal testing for everybody or contingent screening? Prenat Diagn. 43, 443–447 (2023).
Bowden, B. et al. Implementation of non-invasive prenatal testing within a national UK antenatal screening programme: impact on women’s choices. Prenat Diagn. 42, 549–556 (2022).
Van Den Bogaert, K. et al. Outcome of publicly funded nationwide first-tier noninvasive prenatal screening. Genet. Med. 23, 1137–1142 (2021).
Yang, L. & Tan, W. C. Prenatal screening in the era of non-invasive prenatal testing: a nationwide cross-sectional survey of obstetrician knowledge, attitudes and clinical practice. BMC Pregnancy Childbirth. 20, 579 (2020).
Acknowledgements
We express our gratitude to all participants. We appreciate the efforts made by each team member to ensure the integrity and collection of data.
Funding
This study was funded by grants from the National Key Research and Development Program of China (2023YFC2705605, to Qian Zhu); the National Key Research and Development Program of China (2022YFC2703302); Sichuan Province Science and Technology Support Program, China (2021YFS0078).
Author information
Authors and Affiliations
Contributions
W.L., S.L, Q.Z., and S.L. designed the study, analyzed data, conducted the follow ups and wrote the manuscript. Q.Z. prepared Figs. 1, 2, 3 and 4. B.H., D.H., L.Y., and H.L. analyzed experimental results and issue reports. W.L., B.H., J.L., and C.D. monitored quality control of experiments. F.Z. and Q.F. input basic information. L.P., J.T., K.Z., T.B., X.J., T.X., Y.L., J.C., X.W., L.X., and Y.L. carried out experiments. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Luo, W., Liu, S., He, B. et al. Clinical strategy study on prenatal screening and diagnostic model for Down syndrome. Sci Rep 14, 22269 (2024). https://doi.org/10.1038/s41598-024-73183-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-73183-4