Abstract
Head and neck squamous cell carcinoma (HNSCC) present a significant challenge due to its heterogeneity and limited treatment options, often resulting in severe side effects and poor survival rates with conventional chemoradiotherapy. Here, we investigated the anticancer activity of halogenated benzoate derivatives of cleistanthin A, ECDD-S16 and ECDD-S18, in HNSCC cells. Our findings revealed that ECDD-S18 exhibited remarkable cytotoxicity, surpassing that of cisplatin with minimal impact on normal and cisplatin-sensitive cells. Notably, ECDD-S18 induced apoptosis in a dose-dependent manner and effectively targeted vacuolar ATPase (V-ATPase), impairing lysosomal acidification. Intriguingly, ECDD-S18 inhibited autophagic flux, as evidenced by increased autophagosome but decreased autolysosome formation. Furthermore, proteomic analysis demonstrated downregulation of cathepsin D (CTSD), the lysosomal protease in ECDD-S18-treated HNSCC cells, concurrent with suppressed cell migration. ECDD-S18 also decreased expression of mesenchymal markers, suggesting inhibition of epithelial-mesenchymal transition (EMT). Importantly, cotreatment with ECDD-S18 and cisplatin enhanced the reduction in cell viability. Collectively, our results indicated that the anticancer activity of ECDD-S18 partly stems from its ability to disrupt lysosomal acidification and inhibit autophagy via targeted inhibition of V-ATPase. These findings underscore the therapeutic promise of ECDD-S18 in HNSCC treatment, either alone or in combination with existing drugs, while mitigating toxicity to normal cells.
Similar content being viewed by others
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous malignancy that arises in the epithelium cells lining the upper aerodigestive tracts, including the oral cavity, oropharynx, larynx, and hypopharynx. HNSCC ranks as the sixth most common cancer worldwide, with particularly high prevalence in Australia and Southeast Asia1. The gold standard treatment in clinical practice is chemoradiotherapy (CRT), a combination of radiotherapy and chemotherapeutic drug approaches such as cisplatin, fluorouracil, and paclitaxel. Despite its use to prevent tumor relapse, the success rate of CRT remains low due to patients developing drug resistance, leading to tumor recurrence. Additionally, chemotherapy’s side effects significantly impact the quality of life, including hair loss, kidney damage, decreased immunity, gastrointestinal disorders, hemorrhage, and hearing loss2,3. Despite the implementation of various treatment modalities, the 5-year survival rate for HNSCC remains less than 50%4. Therefore, there is a critical need for effective chemotherapeutic drugs for HNSCC to enhance cytotoxicity and alleviate side effects, ultimately improving survival rates and the quality of life.
The vacuolar ATPases (V-ATPases) are a family of proton pumps expressed in intracellular organelle membranes and the plasma membrane. In mammals, subunits A, B, C, D, E, F, and H form a peripheral V1 domain responsible for hydrolysis adenosine triphosphate (ATP) while subunits a, c, d, and e form membrane-integrated V0 domains involved in proton translocation5. V-ATPases transport protons into extracellular spaces or intracellular compartments like lysosomes, endosomes, and secretory vesicles, playing vital roles in maintaining pH homeostasis necessary for normal cellular processes, including membrane trafficking, protein processing and degradation, and the coupled transport of small molecules6. V-ATPase is upregulated in various types of cancer and plays critical roles on tumor progression including drug resistance, tumor invasion, and metastasis5. Overexpression of V-ATPase has been reported in oral squamous cell carcinoma (OSCC), a common type of HNSCC7, where treatment with V-ATPase inhibitor induced apoptosis8. V-ATPases within lysosomes generate the acidic environment crucial for the degradation of proteins by acid-dependent proteases such as cathepsins. Moreover, the maturation of these cathepsins also required the acidic environment provided by V-ATPases6. Cathepsins are a group of lysosomal proteinases with various subtypes, including cysteine proteases, serine proteases, and aspartic proteases9. They are commonly overexpressed in various human cancers and linked with tumor metastasis by degrading the extracellular matrix (ECM) components, thereby enhancing the loss of cell–cell and cell–matrix adhesion10. Therefore, targeting cathepsins by inhibiting V-ATPase activity could be a promising treatment for cancers. Additionally, V-ATPases also indirectly modulated autophagy in autophagosome-lysosome fusion. Dysregulated autophagy has been linked with genomic damage, metabolic stress, and tumorigenesis, and various studies suggest its association with cancer initiation11,12. In summary, V-ATPases regulate numerous biological pathways associated with tumor initiation and progression, particularly cathepsin activity and autophagy. Defects in V-ATPase activity have been reported in various cancer types13, making the development of effective drugs targeting V-ATPase activity a promising strategy in cancer therapy.
Cleistanthin A (CA), a natural arylnaphthalene lignan glycoside found in Phyllanthus taxodiifolius Beille (Phylanthaceae family), has been recognized for its diverse pharmacological effects, including its anticancer properties14,15. Previous studies conducted on colorectal cancer (CRC) cell lines revealed that CA impaired lysosomal acidification, leading to anticancer effects by inducing apoptosis and restraining cancer cell motility16, although its mechanism through which it targets V-ATPase remains unclear. However, the significant toxicity of CA towards normal cells limits its therapeutic potential, prompting our group to synthesize several CA derivatives with side chain modifications17. Recently, our group synthesized two halogenated benzoate derivatives of CA, ECDD-S16 and ECDD-S18. ECDD-S16, the esterification of CA with 4-fluorobenzoic acid, was found to inhibit pyroptosis in Raw264.7 cells, potentially by impairing endosome acidification through V-ATPase targeting18. However, the anticancer activity as well as their underlying mechanisms of ECDD-S16 and ECDD-S18 remain unexplored, particularly in head and neck squamous cell carcinomas (HNSCCs). Therefore, in this study, we investigated the cytotoxicity of CA and its derivatives compared to cisplatin on HNSCCs. Our results unveiled that ECDD-S18, synthesized through esterification of CA with 4-bromobenzoic acid, exhibited potent cytotoxicity against HNSCC cells while exerting lesser effects on normal cells compared to cisplatin and the parent compound CA. The anticancer activity of ECDD-S18 was attributed to its ability to impair lysosomal acidification by targeting vacuolar ATPase. Thus, ECDD-S18 emerges as a promising therapeutic candidate for treating HNSCC, either alone or in combination with existing drugs, potentially reducing toxic effects on normal cells.
Materials and methods
Cell culture
FaDu (hypopharyngeal carcinoma), Cal-27 (Tongue carcinoma), SH-SY5Y (neuroblastoma), and HK-2 (human kidney) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). HaCaT (human keratinocyte) cell line was from AddexBio (San Diego, CA). Ca9-22 (oral squamous cell carcinoma) cells were obtained from the Japanese Collection of Research Bioresources (JCRB, Osaka, Japan). HaCaT, FaDu, and Cal-27 cells were maintained in high-glucose DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA). Ca9-22 cells were cultured with MEM (Gibco). HK-2 and SH-SY5Y cells were cultured with DMEM/F12 (Gibco). Culture media were supplemented with 10% fetal bovine serum (HyClone, Cytiva, Marlborough, MA), and 1% antibiotic–antimycotic (Gibco). All cells were maintained in a humidified 95% air incubator with 5% CO2 at 37 °C. Cells were regularly tested for mycoplasma contamination before use.
Compounds synthesis and characterization
Cleistanthin A (CA) was acquired as previously described15 with the purity of 94.10% by HPLC [Poroshell 120 EC-C18 col, 50 × 4.6 mm, Agilent, Santa Clara, CA, USA, 2.7 µm, flow rate 0.5 ml/min, detected at 254 nm, 5 µl injection, by the following gradient elution (4 min, 90% H2O, 10% acetonitrile; 9 min, 100% acetonitrile; 15 min, 90% H2O, 10% acetonitrile), with a total run time of 20 min]. For ECDD-S16 synthesis, 4-fluorobenzoic acid (19.3 mg, 0.14 mmol, 1.5 equiv.), N,N´-dicyclohexylcarbodiimide (DCC) (28.3 mg, 0.14 mmol, 1.5 equiv.) and 4-dimethylaminopyridine (DMAP) were dissolved in dry dichloromethane under nitrogen gas with stirring at room temperature for 30 min. A solution of CA (49.5 mg, 0.09 mmol, 1 equiv.) in dry dichloromethane was added and stirred overnight at room temperature. After adding saturated NaHCO3 (aq.), the extraction with CH2Cl2 was performed, and the combined organic layer was washed with saturated NaCl, dried with anhydrous Na2SO4, filtered, and evaporated resulting the white solid (102.5 mg). The compound was purified by preparative TLC, eluting with MeOH-CH2Cl2 (1:5 provided compound ECDD-S16 (49.4 mg, 81%). ECDD-S16 (1); 1H NMR (500 MHz, CDCl3): 8.11 (dd, J = 8.8, 5.4 Hz, 2H), 7.56 (d, J = 3.6 Hz, 1H), 7.14 (br t, J = 8.6 Hz, 2H), 7.01 (s, 1H), 6.94 (dd, J = 7.9, 1.0 Hz, 1H), 6.80 (dd, J = 5.0, 1.4 Hz, 1H), 6.78–6.75 (m, 1H), 6.01 (d, J = 0.9 Hz, 1H), 6.04 (d, J = 1.4 Hz, 1H), 5.58 (t, J = 6.8 Hz, 1H), 5.46 (dd, J = 14.5, 1.6 Hz, 1H), 5.38 (dd, J = 14.6, 4.3 Hz, 1H), 5.23 (dd, J = 6.1, 0.9 Hz, 1H), 4.23 (dd, J = 12.1, 3.4 Hz, 1H), 3.88 (s, 3H), 3.77 (s, 3H), 3.60 (s, 3H), 3.54 (s, 3H), 3.44 (dd, J = 11.8, 7.0 Hz, 1H). 13C NMR (500 MHz, CDCl3): 169.5, 166.1 (C-F, 1JC-F = 253.8 Hz), 164.5, 151.7, 150.2, 147.4, 143.9, 135.6, 132.4 (C-F, 3JC-F = 8.8 Hz), 130.5, 128.3, 126.4 (C-F, 4JC-F = 7.5 Hz), 125.6, 123.50, 119.2, 115.9 (C-F, 2JC-F = 21.3 Hz), 110.6, 108.2, 105.9, 101.2, 100.7, 81.2, 77.9, 72.0, 67.0, 62.7, 60.1, 58.5, 56.0, 55.8. 19F NMR (376 MHz, CDCl3): -104.0 (s). ESI–MS m/z: 663.1879 [M + H]+, (calcd. for C35H32FO12, 663.1878). To synthesize ECDD-S18, 4-bromobenzoic acid (29.5 mg, 0.15 mmol, 1.5 equiv.), N,N´-dicyclohexylcarbodiimide (DCC) (30.3 mg, 0.15 mmol, 1.5 equiv.) and 4-dimethylaminopyridine (DMAP) (catalytic amount) were dissolved in dry dichloromethane (1 mL) under nitrogen gas with stirring at room temperature for 30 min. A solution of Cleistanthin A (52.9 mg, 0.1 mmol, 1 equiv.) in dry dichloromethane (1 mL) was added, then the reaction mixture was left stirring overnight at room temperature. After the addition of saturated NaHCO3 (aq.) with stirring for 10 min, the extraction with CH2Cl2 was performed, and the combined organic layer was respectively washed with sat. NaCl (20 mL), dried with anhydrous Na2SO4, filtered, and evaporated to dryness to give a white solid (87.2 mg). Purification by preparative TLC, eluting with EtOAc-CH2Cl2 (1:19) provided compound ECDD-S18 (61.9 mg, 88%). ECDD-S18 (2); 1H NMR (500 MHz, CDCl3): 7.94 (dd, J = 6.7, 1.8 Hz, 2H), 7.61 (dd, J = 6.7, 1.9 Hz, 2H), 7.55 (d, J = 3.6 Hz, 1H), 7.01 (s, 1H), 6.94 (d, J = 7.7 Hz, 1H), 6.80 (dd, J = 4.9, 1.6 Hz, 1H), 6.78–6.75 (m, 1H), 6.01 (d, J = 1.4 Hz, 1H), 6.04 (d, J = 1.4 Hz, 1H), 5.58 (dd, J = 7.9, 6.2 Hz, 1H), 5.46 (dd, J = 14.5, 1.7 Hz, 1H), 5.37 (dd, J = 14.5, 4.1 Hz, 1H), 5.23 (dd, J = 6.1, 1.2 Hz, 1H), 4.23 (dd, J = 12.0, 3.3 Hz, 1H), 3.89 (s, 3H), 3.77 (s, 3H), 3.60 (s, 3H), 3.53 (s, 3H), 3.46–3.42 (m, 1H). 13C NMR (500 MHz, CDCl3): 169.47, 164.79, 151.68, 150.24, 147.44, 143.92, 135.64, 132.02, 131.20, 130.55, 128.90, 128.28, 126.22, 123.50, 119.17, 110.62, 108.15, 105.94, 101.19, 100.74, 81.19, 77.91, 72.11, 66.98, 62.71, 60.15, 58.55, 55.97, 55.75. ESI–MS m/z: 723.1064 [M + H]+, (calcd. for C35H32BrO12, 723.1077).
Cell viability assay
FaDu, Cal-27, Ca9-22, HaCaT, HK-2 (8 × 103 cells/well), and SH-SY5Y (2 × 104 cells/well) were seeded in 96-well plates and maintained for 24 h at 37 ºC with 5% CO2. Cells were incubated with 0.01, 0.1, 1, 10, and 50 μM of cisplatin and CA; 0.001, 0.01, 0.1, 1, 10, and 20 μM of ECDD-S16 and ECDD-S18 for 24, 48, and 72 h. At indicated time points, cells were incubated with 0.5 mg/ml of MTT for 4 h at 37 °C in humidified 5% CO2 incubator. The MTT solution was removed and DMSO was added to dissolve the formazan crystal. The absorbance value was measured at 570 nm by the Multiskan microplate reader (Thermo Scientific; Wilmington, DE). The half of inhibitory concentration (IC50) was calculated using nonlinear regression analysis by GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA). All experiments were performed in triplicate with at least three independent experiments.
Three-dimensional culture
To establish 3D cultures, Cal-27 (2,000 cells/well), and FaDu (500 cells/well) were seeded in 96-well U-bottom ultralow attachment microplates (Corning, Glendale, AZ) and were maintained at 37 ºC with 5% CO2 for 72 h. After spheroids were formed, each spheroid was incubated with various concentrations of cisplatin and ECDD-S18 for 72 h. Spheroids were stained with Hoechst 33,342 (Invitrogen, Thermo Fisher Scientific, Waltham, MA), and ethidium homodimer-1 (EthD-1) (Invitrogen) for 1 h. Fluorescent signals were captured and analyzed by the Operetta High Content Imaging System (PerkinElmer, Waltham, MA).
Flow cytometry
Cell apoptosis assay was accomplished by a FITC Annexin V/Propidium Iodide (PI) detection kit (BD Biosciences). Cal-27 and FaDu were seeded into 24-well plates at the density of 5 × 104 cells/well and maintained for 24 h in a 37 ºC incubator with 5% CO2. Cells treated with 5% DMSO were used as a positive control. After treatment for 72 h, cells were harvested and stained with FITC Annexin V/PI according to the manufacturer’s instructions. The stained cells were measured using the flow cytometer FACSCanto (BD Bioscience, Becton Drive Franklin Lakes, NJ) and quantified by FlowJo v10 software.
RNA extraction and quantitative RT‑PCR
Cal-27 and FaDu cells were seeded on 60 mm petri dishes at the density of 5 × 105 cells/dish. RNA extraction was carried out using TRIzol™ reagent (Invitrogen). Complementary DNA (cDNA) was synthesized from the total extracted RNA using the iScript™ Reverse Transcription Supermix kit (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction was achieved by an ABI PRISM7500 Sequence Detection System (Applied Biosystems, Waltham, MA) using iTaq™ Universal SYBR Green Supermix (Bio-Rad Laboratories) with specific primers. The expression of target genes was normalized to the expression of GAPDH and analyzed using the comparative 2-(ΔΔCt) method19. The oligonucleotides for the specific primers are as followed: Bax sense strand, 5’-AAGAAGCTGAGCGAGTGT-3’ and antisense strand 5’-GGAGGAAGTCCAATGTC-3’; Bcl-2 sense strand, 5’-CTTCTCCCGCCGCTAC-3’ and antisense strand 5’- CTGGGGCC GTACAGTTC-3′20; CTSD sense strand, 5’-TCTGTGGAGGACCTGATTGC-3’ and antisense strand 5’-GCTGGACTTGTCGCTGTTGTA-3′21; Fibronectin sense strand, 5’-CACCTGTACCCACACGGTC-3’ and antisense strand 5’-TCCAGGAACCCTGAACTGTA AG-3’; Twist sense strand, 5’-GGAGTCCGCAGTCTTACGAG-3’ and antisense strand 5’- TCTGGAGGACCTGGTAGAGG-3’; and GAPDH sense strand, 5’-ATGGGGAAGGT GAAGGTCG-3’ and antisense strand 5’-GGGTCATTGATGGCAACAATAT-3′22.
LysoTracker Red staining
Cal-27 and FaDu cells were plated onto 96-black well plates at the density of 8 × 103 cells/well for 24 h. Cells were treated with ECDD-S18 at 0.1, 1, and 2 µM or 0.1 µM bafilomycin A1 (negative control) for 24 h. Treated cells were then incubated with LysoTracker (Invitrogen) for 2 h. The stained cells were immediately fixed with 4% paraformaldehyde for 20 min and incubated with DAPI (Sigma-Aldrich, St. Louis, MO) for 30 min at room temperature. The images and the fluorescence intensity of stained cells were analyzed by the Operetta High Content Imaging System (PerkinElmer).
Proteomic analysis
Cal-27 cells were seeded into 60 mm petri dishes at the density of 5 × 105 cells/dish for 24 h. Cells were treated with ECDD-S18 at the concentration of 1 µM for 72 h. Cells were harvested in RIPA buffer (50 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% TritonX-100, 1 mM EDTA) supplemented with freshly prepared protease and phosphatase inhibitor cocktail (Roche Diagnostic GmbH, Germany). Protein concentrations were measured using the BCA Protein Assay Kit (Thermo Scientific), following the manufacturer’s instructions. A total protein amount of 30 μg was chemically modified by carbamidomethylation. First, the proteins were reduced using 2 mM tris(2-carboxyethyl)phosphine, followed by alkylation with 10 mM iodoacetamide. The modified proteins were then subjected to digestion with Trypsin/LysC at 37 °C for 6 h. The tryptic peptides were injected to Orbitrap HF mass spectrometry (Thermo Scientific). Raw data obtained from the mass spectrometer was analyzed by Proteome Discoverer software version 2.4 (Thermo Scientific). The peptide ions were matched with The Homo sapiens protein database, which was retrieved from UniProtKB (26,561 sequences; data: 20 April 2022). Proteins that only express in untreated cells were defined as ‘Unique control’ or proteins that only express in treated cells were defined as ‘Unique treatment’. Differentially abundant proteins among untreated and treated cells were identified. Statistical significance of dysregulated proteins was indicated using the criteria of fold change > 2 or < − 2 and p-value by Student t-test < 0.05. For visualizing the differentially abundant proteins between untreated and treated cells, heatmaps were generated with MetaboAnalyst (https://www.metaboanalyst.ca/)23,24, and volcano plot using VolcaNose R (https://huygens.science.uva.nl/VolcaNoseR)25. Pathway analysis in which differentially expressed proteins participated was demonstrated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and Gene Set Enrichment Analysis (GSEA) were performed (https://www.gsea-msigdb.org/). Bubble plot for KEGG analysis was illustrated by SRplot (https://www.bioinformatics.com.cn/) and Gene Ontology (GO) annotation using Metascape (https://metascape.org/)26. The experiments were performed in triplicate with two biologically independent experiments.
Western blot analysis
Cal-27 and FaDu cells were seeded at the density of 5 × 105 cells/dish onto 60 mm petri dishes for 24 h. After treatment with ECDD-S18 or bafilomycin A1 at the indicated concentrations and incubation time, total proteins were extracted using RIPA buffer. Cell lysates were then separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Polyvinylidene Fluoride (PVDF) membrane. Membranes were then blocked with 5% nonfat milk for 1 h at room temperature and incubated with primary antibodies against cathepsin D (Cell Signaling Technology, Danvers, MA) USA; N-cadherin (Abcam, Cambridge, UK), and GAPDH (Thermo Fisher Scientific) at 4 ºC overnight. The membranes were washed with Tris-buffered saline with Tween 20 (TBST) 5 times and incubated with appropriate secondary antibodies conjugated with horseradish peroxide at room temperature for 1 h. The membranes were washed with TBST 5 times, and the protein expressions were detected using an Enhanced Chemiluminescence (ECL) reagent kit (Merck, Darmstadt, Germany) and captured with Amersham Hyperfilm™ (GE Healthcare, Chicago, IL). The band intensities were obtained by the ImageJ 1.53 v software. The levels of protein expression were normalized with GAPDH expression, represented as fold change relative to untreated cells.
Transwell migration assay
Cell migration assay was performed using the 24-well chambers of Transwell Permeable Support (Corning). Cal-27 and FaDu cells were plated in the upper chamber at the density of 1 × 105 cells in 100 µl of the FBS-free medium. The lower chamber was filled with 10% FBS-containing medium, considered as a chemo-attractant. Cells were exposed to various concentrations of ECDD-S18 and allowed to migrate at 37 ºC for 36 h. The migrating cells at the lower surface of the membrane were fixed with cold methanol for 10 min, followed by staining with 0.2% crystal violet for 1 h. The non-migrating cells in the upper chamber were carefully removed by cotton swabs. The migrating cells were captured under a light microscope for 7 image fields in each condition. Migrating cell numbers in each field were counted and averaged using the ImageJ 1.53 v software.
Autophagy assay
Cal-27 and FaDu cells were transfected with 5 μg of cDNAs encoding RFP-GFP-LC3B27 and were seeded onto 96-well black plates at the density of 2 × 104 cells/well. After transfection 24 h, cells were incubated with Earle’s balanced salt solution (EBSS; positive control), ECDD-S18 (0.1, 1, and 2 µM), or 0.1 µM bafilomycin A1 (negative control) for 24 h. Treated cells were immediately fixed with 4% paraformaldehyde for 10 min and incubated with Hoechst 33,342 (Invitrogen) for 15 min at room temperature. Autophagic flux was imaged and analyzed using the Operetta High Content Imaging System (PerkinElmer). All experiments were performed in triplicate with at least three independent experiments.
Computational study of ECDD-S18 binding to vacuolar ATPase
The three-dimensional (3D) structure of V-ATPase from Saccharomyces cerevisiae was downloaded from the RCSB Protein Data Bank (PDB ID: 7TAO28). The protonation states of all ionizable amino acids were predicted at pH 7.4 using the H + + web server29. The chemical structure of ECDD-S18 was sketched using PerkinElmer ChemDraw. Next, the 3D structure of the compound was generated and underwent energy minimization employing the Molecular Mechanics 2 (MM2) force field via Chem3D software. Finally, the protein and ligand were converted to the PDBQT file format using the AutoDockFR software suite30 before performing molecular docking using Autodock Vina 1.231. The resulting 3D binding mode of the best-docked pose of the protein–ligand binding was visualized using the UCSF ChimeraX program32. The 2D interaction diagram of the protein–ligand complex was analyzed using the Discovery Studio Visualizer (BIOVIA, San Diego, CA).
Statistical analysis
Except where otherwise indicated, all experiments were performed at least three independent experiments, and the results were consolidated for identification of the mean ± standard error of the mean (S.E.M.). Statistical analysis was conducted by GraphPad Prism version 8 (GraphPad Software Inc., CA, USA) using one-way ANOVA with Tukey’s multiple comparison test. p values of < 0.05 were considered as statistically significant.
Results
ECDD-S16 and ECDD-S18 potentially reduced HNSCC cell viability
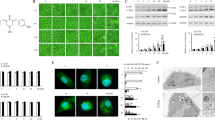
ECDD-S16 and ECDD-S18 were synthesized by the esterification of cleistanthin A (CA) with 4-fluorobenzoic acid (Fig. 1a) and 4-bromobenzoic acid (Fig. 1b), respectively. The structure of these derivatives was confirmed by spectroscopic methods, including NMRs and mass spectrometry (H-NMR, C-NMR, UV, IR, and MS analysis). By HPLC, the purity of ECDD-S16 and ECDD-S18 were 88.8% and 87.92%, respectively. We investigated the cytotoxic activity of CA and CA derivatives in HNSCC cell lines (FaDu, Cal-27, and Ca9-22) by MTT assay. As shown in Table 1 and supplementary Fig. S1, CA, ECDD-S16, and ECDD-S18 exhibited no cytotoxic effect at 24 h, while the cytotoxic effect was observed only for cisplatin. CA reduced the viability of HNSCC cell lines in a dose- and time-dependent manner at 48 and 72 h. Cal-27 and Ca9-22 cells were more sensitive to CA than FaDu cells. The IC50 values of CA in Cal-27, FaDu, and Ca9-22 cells at 48 and 72 h were 0.11 ± 0.07, 18.31 ± 5.53, 0.75 ± 0.02 µM and 0.01 ± 0.003, 0.12 ± 0.03, 0.08 ± 0.03 µM, respectively. At 48 h, ECDD-S18 showed no cytotoxicity, while ECDD-S16 exhibited toxic effects on Cal-27 and Ca9-22 cells with IC50 values of 11.91 ± 2.16 and 8.34 ± 0.87 µM, respectively. At 72 h, both CA derivatives exhibited potent cytotoxicity in HNSCC cell lines. The IC50 values of ECDD-S16 and ECDD-S18 in Cal-27, FaDu, and Ca9-22 at 72 h were 0.30 ± 0.07, 0.03 ± 0.005, 0.32 ± 0.10 µM and 0.81 ± 0.09, 0.15 ± 0.02, 0.86 ± 0.09 µM, respectively. CA and its derivatives exhibited more potent cytotoxicity than cisplatin at 72 h. The therapeutic effect of cisplatin in HNSCC is limited by its adverse effects in normal tissues including nephrotoxicity and neurotoxicity. To examine the potential clinical application of CA derivatives, we investigated their cytotoxic effects in normal keratinocyte cells (HaCaT), human kidney cells (HK-2), and neuroblastoma cells (SH-SY5Y), the neurotoxicity model cell line33. ECDD-S16 and ECDD-S18 had lower toxicity than the parent compound, CA, in HaCaT, HK-2, and SH-SY5Y cells. The cytotoxicity of ECDD-S16 was comparable with cisplatin, while ECDD-S18 revealed higher IC50 values on HaCaT, HK-2, and SH-SY5Y than cisplatin and ECDD-S16. Therefore, ECDD-S18 was selected for further study.
Chemical structure of ECDD-S16 and ECDD-S18 and the cytotoxic effects on HNSCC spheroid. (a) ECDD-S16 and (b) ECDD-S18 are modified from the esterification of cleistanthin A with 4-fluorobenzoic acid and 4-bromobenzoic acid, respectively, in the presence of N,N′-dicyclohexylcarbodiimide (DCC) and a catalytic amount of 4-dimethylaminopyridine (DMAP). (c-f) FaDu cells were seeded in ultralow attachment microplates for 72 h. The formed spheroids were incubated with indicated concentrations of cisplatin (c) and ECDD-S18 (e) for 72 h. Spheroids were stained with Hoechst 33342 (live cells; blue) and ethidium homodimer-1 (EthD-1) (dead cells; orange) for 1 h. Fluorescent signals were captured. Scale bar = 200 µm. The bar graphs represented the mean fluorescence intensity ratio of Hoechst 33342 to EthD-1 of FaDu cells after being treated with cisplatin (d) and ECDD-S18 (f). Data were represented as mean ± S.E.M. (n = 3) and were demonstrated as fold change relative to the DMSO control. *p < 0.05 and ****p < 0.0001, compared with the corresponding control by one-way ANOVA with a Tukey’s multiple comparison test.
ECDD-S18 reduces cell viability on HNSCC spheroids
The cytotoxic effect of ECDD-S18 was further confirmed in the 3D culture model of HNSCC cells. FaDu spheroids were treated with various concentrations of cisplatin and ECDD-S18 for 72 h. Spheroids were stained with Hoechst 33342 for living cells and ethidium homodimer-1 (EthD-1) for dead cells (Fig. 1c,e). The cytotoxic effects were demonstrated as the ratio of the mean fluorescence intensity of Hoechst 33342 to EthD-1 (Fig. 1d,f). Cisplatin treatment caused FaDu spheroid’s death in a dose-dependent manner (Fig. 1c). A higher concentration of cisplatin was required to reduce FaDu spheroid viability. Live/dead cell ratio was significantly reduced at 50 µM (Fig. 1d). ECDD-S18 induced FaDu spheroids death in a dose-dependent manner (Fig. 1e). The effect of ECDD-S18 on FaDu spheroids was more potent than cisplatin. The live/dead cell ratios were significantly decreased after being treated with 0.1 µM ECDD-S18 (Fig. 1f). We failed to establish the Cal-27 spheroid and, therefore, were unable to investigate the cytotoxic effect of cisplatin and ECDD-S18 in Cal-27 3D culture model. Thus, these data support the anticancer activity of CA derivatives in HNSCC.
ECDD-S18 promoted HNSCC cell deaths via apoptosis
We next investigated whether the cytotoxicity of ECDD-S18 in HNSCC cells was mediated by apoptosis using flow cytometric assay after Annexin V/PI staining. As shown in Table 1, Cal-27 and FaDu exhibited different sensitivity to ECDD-S18 at 72 h. Therefore, Cal-27 and FaDu cells were exposed to ECDD-S18 at 0.1, 1, 1.5 µM and 0.01, 0.1, 0.2 µM for 72 h, respectively. Cells treated with 5% DMSO were used as a positive control. ECDD-S18 remarkably induced apoptosis in Cal-27 (Fig. 2a) and FaDu (Fig. 2b) cells in a dose-dependent manner. To further confirm the apoptosis induction by ECDD-S18, the effect of ECDD-S18 treatment on the expressions of pro-apoptotic (Bax) and anti-apoptotic (Bcl-2) genes were determined by quantitative RT-PCR (Fig. 2c,d). The Bax to Bcl-2 expression ratio represents cell death in response to an apoptotic stimulus. Consistent with the flow cytometric assay, treatment with ECDD-S18 significantly elevated the ratio of Bax/Bcl-2 mRNA expression in Cal-27 (Fig. 2c) and FaDu (Fig. 2d). Taken together, our results suggested that the cytotoxic effect of ECDD-S18 in HNSCC cells is mediated via apoptosis induction.
ECDD-S18 induces HNSCC cell death via apoptosis. (a) Cal-27 and (b) FaDu cells were treated with the indicated concentrations of ECDD-S18 and 5% DMSO (positive control) for 72 h. Cells were stained with Annexin V and PI and analyzed by flow cytometry. The bar graphs demonstrated the average percentage of viable, early apoptotic, late apoptotic, and necrotic cells in each group. The expression of Bax and Bcl-2 mRNA in Cal-27 (c) and FaDu (d) cells after treatment with the indicated concentrations of ECDD-S18 for 72 h were assessed by quantitative RT-PCR. The relative expression of target genes was normalized to the expression of GAPDH. Data were illustrated as mean ± S.E.M. (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, compared with the corresponding control by one-way ANOVA with a Tukey’s multiple comparison test.
ECDD-S18 suppresses the activity of vacuolar ATPase in HNSCC cells
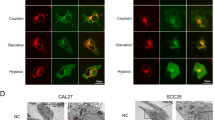
Vacuolar ATPase (V-ATPase) is an ATP-driven-proton pump that transports protons out of the cytosol into either the intracellular compartments such as lysosomes or into the extracellular space. It is crucial in maintaining cellular pH homeostasis and regulating various physiological processes5. Generally, V-ATPase is overexpressed in cancers, enhancing cancer cell proliferation, tumor invasion/metastasis, and drug resistance. Therefore, it is proposed as a promising druggable target for cancer therapy6. Moreover, previous studies discovered that V-ATPase activity is inhibited by CA and its derivatives16,18,27. Hence, we investigated the effect of ECDD-S18 on V-ATPase activity in HNSCC using Lysotracker staining. The red fluorescence of Lysotracker indicated the accumulated lysosomal proton. Cal-27 and FaDu cells were treated with ECDD-S18 (0.1, 1, 2 µM) and a V-ATPase inhibitor, bafilomycin A1 (0.1 µM), for 24 h. High-content analysis was used to capture and quantify the fluorescence intensity (Fig. 3). Bafilomycin A1 inhibited the V-ATPase activity and impaired the lysosome acidification, as demonstrated by the significantly decreased red fluorescence intensity relative to DMSO control. Moreover, ECDD-S18 exhibited dose-dependently inhibition of lysosome acidification in Cal-27 (Fig. 3a), and FaDu (Fig. 3b). These results suggested that ECDD-S18 impairs lysosome acidification may probably be through inhibition of the V-ATPase activity in HNSCC.
ECDD-S18 decreases vacuolar ATPase activity in HNSCC cells. (a) Cal-27 and (b) FaDu cells were seeded onto 96-black well plates. Cells were treated with 0.1, 1, and 2 µM ECDD-S18 or 0.1 µM bafilomycin A1 (positive control) for 24 h. Cells were stained with LysoTracker for 2 h to indicate the lysosomal acidification and then fixed with 4% paraformaldehyde. Fluorescence intensity and images were captured using the Operetta High Content Imaging System. The bar graphs showed the relative fluorescence intensity of the mean LysoTracker (red) intensity normalized with DAPI intensity. Scale bar = 50 µm. (c) Molecular docking result of ECDD-S18 bound at the interface between two adjacent c subunits and subunit a of V-ATPase VO region, along with a close-up view of the binding residues surrounding ECDD-S18. (d) 2D interaction diagrams of the V-ATPase-ECDD-S18 complex. Note that the asterisk in (c) indicates the residues from the second (adjacent) subunit c. Data were illustrated as mean ± S.E.M. (n = 3). *p < 0.05 relative to DMSO control using one-way ANOVA with a Tukey’s multiple comparison test.
Molecular docking was performed to investigate whether ECDD-S18 could target the vacuolar V-ATPase and inhibit its activity. Given that the chemical structure of ECDD-S18 contains a sugar moiety similar to the well-established V-ATPase inhibitor bafilomycin A128,34, it was hypothesized that ECDD-S18 might bind to the V0 region of V-ATPase in the same site as bafilomycin A1. Consequently, ECDD-S18 was docked into the interface between two adjacent c subunits and subunit a of V-ATPase, termed c–c site #1, as previously described28 (Fig. 3c). The docking results revealed that an oxygen atom on the benzodioxole ring of ECDD-S18 formed a hydrogen bond with Tyr142 of subunit c (Fig. 3d), a conserved residue crucial for bafilomycin A1 binding28. The benzene moiety on this ring also interacted with Ala783 from subunit a (Fig. 3c, residue indicated in green) through pi-alkyl interaction. Furthermore, the naphthalene moiety was stabilized by pi-sigma (Ile58 from subunit c) and pi-alkyl (Ile58 from subunit c and Ala783 from subunit a) interactions. Meanwhile, the substituted 4-bromobenzene ring engaged in multiple interactions, including pi-sulfur with Met788 from subunit a, pi-sigma with Val55 from subunit c, pi-alkyl with Phe126 from subunit c, and alkyl with Ile130 from subunit c. Close contact and resulting van der Waals interactions between ECDD-S18 and V-ATPase were observed due to Phe51 and Ile54 from the first c subunit, residues Ile134 and Phe135 from the adjacent c subunit, and residues Ile454, Leu780, Val784, Leu787, and Thr791 from subunit a. Although our docking result may slightly differ from the binding location observed in the previous report of the CA derivative, ECDD-S16, the binding energy obtained from Autodock Vina showed a lower value compared to the previous result (− 8.5 vs − 7.5 kcal/mol)18. This suggests that the defined docking site mentioned above is considered the preferable region for ECDD-S18 binding, leading to the blocking of the rotation of the c ring at the a-c ring interface and inhibition of V-ATPase activity.
ECDD-S18 potently inhibits autophagic flux
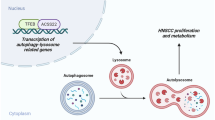
Autophagy is an intracellular degradative process that recycles cellular components and is considered a cell survival mechanism. Numerous previous studies suggest that autophagy can promote the survival and growth of tumors, while suppression of the autophagy process can cause cancer cell death35. The lysosomal acidification by V-ATPase is crucial for lysosomal hydrolase activity and autophagosome-lysosome fusion. Since ECDD-S18 inhibits the V-ATPase activity, we sought to investigate its potential modulatory effects on autophagy. Cal-27 and FaDu cells, transfected with a plasmid encoding mRFP-GFP-LC3B, were exposed to DMSO as a control, varying concentrations of ECDD-S18, and 0.1 µM bafilomycin A1 for 24 h. Quantification of total autophagosomes (RFP+GFP+-LC3B) and autolysosomes (RFP+GFP--LC3B) per cell was conducted using high-content image analysis. Induction of autophagic flux was achieved by incubating cells in Earle’s Balanced Salt Solution (EBSS), the starvation condition, leading to a significant increase in the number of autophagosomes/autolysosomes. Conversely, treatment with ECDD-S18 in Cal-27 cells resulted in an elevation of total LC3B puncta and the number of autophagosomes per cell compared to the DMSO control. This was accompanied by a reduction in the number of autolysosomes per cell relative to the starvation condition (Fig. 4a). Similar results were observed in FaDu cells (Fig. 4b). Collectively, these findings indicate that ECDD-S18 attenuates vacuolar ATPase activity, resulting in compromised lysosomal acidification, inhibition of autophagic flux, and induction apoptosis in HNSCC.
ECDD-S18 inhibits autophagic flux in HNSCC cells. Cal-27 (a) and FaDu (b) cells were transfected with plasmid encoding RFP-GFP-LC3B and then were exposed to EBSS (starvation condition, positive control), 0.1, 1, and 2 µM ECDD-S18 or 0.1 µM bafilomycin A1 (negative control) for 24 h. The number of total LC3B puncta per cell was then analyzed. Scale bar = 20 µm. Bar graphs show the number of RFP+GFP+-LC3B (autophagosomes), RFP+GFP--LC3B (autolysosomes), and total puncta per cell. Data are mean ± S.E.M. (n = 3). *p < 0.05, **p < 0.01, and ****p < 0.0001, relative to DMSO control. #p < 0.05, ##p < 0.01, and ####p < 0.0001, relative to starvation (EBSS; positive control) using one-way ANOVA with a Tukey’s multiple comparison test.
ECDD-S18 reduces cathepsin D expression and suppresses HNSCC cell migration
To examine the effect of ECDD-S18 on protein profiling, proteomic analysis was conducted on Cal-27 cells treated with 0.1 µM ECDD-S18. As illustrated in Fig. 5a, 29 and 22 proteins were exclusively identified in DMSO-treated (unique control) and ECDD-S18-treated cells (unique treatment), respectively. A total of 1527 proteins were identified across both control and treated samples, with 486 proteins were significantly differentially expressed upon ECDD-S18 treatment. Among these, 209 were upregulated and 277 proteins were downregulated (fold change > 2 or < -2, *p < 0.05) (Fig. 5b). Hierarchical clustering analysis of top 50 differentially expressed proteins revealed distinct patterns between treatment and control (Fig. 5c). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment and Gene Set Enrichment Analysis (GSEA) analysis highlighted that these altered proteins are involved in various cancer-related pathways, including ECM-receptor interaction, spliceosome, and focal adhesion (Fig. 5d,e). Additionally, Gene Ontology (GO) annotation analysis was employed to predict the cellular function affected by ECDD-S18 treatment. As shown in Supplementary Fig. S2a, a broad spectrum of biological processes, cellular components, and molecular functions in Cal-27 cells were affected by ECDD-S18. The top ten differentially abundant proteins (DAPs) were ranked based on the least p-value and are shown in Fig. 5f. Notably, cathepsin D (CTSD), an aspartyl lysosomal protease critical for cancer progression, was among the top 10 DAPs as downregulation protein. Consistent with this, The Cancer Genome Atlas Program (TCGA) database revealed a significant upregulation of CTSD (p < 0.01) in HNSCC tissue (n = 520) compared to normal tissue (n = 44) (Fig. 5g). We further validated the impact of ECDD-S18 on CTSD expression at both the protein and mRNA levels using western blot analysis and RT-PCR. The protein levels of both pro-CTSD and mature-CTSD were significantly reduced following ECDD-S18 treatment (Supplementary Fig. S2b). Similar observations were made in FaDu cells (Supplementary Fig. S2c). Furthermore, CTSD mRNA were significantly downregulated in both Cal-27 and FaDu cells after 72 h of ECDD-S18 treatment (Supplementary Fig. S2d). However, the inhibitory effect of ECDD-S18 on CTSD mRNA expression was less pronounced in FaDu cells compared to Cal-27 cells. These results suggest that ECDD-S18 inhibits V-ATPase activity, leading to lysosome dysfunction and subsequent impairment of CTSD expression.
ECDD-S18 alters the protein profiling and downregulates cathepsin D (CTSD) expression in HNSCC cells. Cal-27 cells were treated with 1 µM ECDD-S18 for 72 h before protein extraction for proteomic analysis. (a) Venn diagram illustrated the distribution of the identified proteins. (b) Volcano plots of 486 Differentially Abundant Proteins (DAPs) between log2-fold change of DMSO and ECDD-S18 treated Cal-27 cells with their p-values. The red and blue dots represent the 2 folds of up-regulation and down-regulation proteins, respectively (Student t-test, *p < 0.05). (c) The heatmap of hierarchical clustering analysis of top 50 DAPs. (d) KEGG analysis for the DAPs was plotted in the bulb map. The enrichment score (ES) represented the enrichment degree of DAPs. (e) Enrichment plots for cancer-related pathways data sets enriched in GSEA KEGG analysis showed the ES score and the rank-ordered list. NES, normalized enrichment score. (f) The least p-value top ten DAPs. Proteomic analysis was performed in two independent experiments in triplicate. (g) Comparison of CTSD gene expression obtained from The Cancer Genome Atlas (TCGA) database between normal (n = 44) and HNSCC (n = 520) tissues. Data were mean ± S.E.M. (n = 3) and transcripts per kilobase million (TPM). **p < 0.01, relative to normal using Student t-test.
CTSD functions as a proteolytic enzyme that degrades extracellular matrix (ECM) components, contributing to cancer cell metastasis10. We, therefore, investigated the effect of ECDD-S18 on HNSCC migration using transwell assay. Cal-27 and FaDu cells were treated with ECDD-S18 at 0.1 and 1 µM for 36 h. ECDD-S18 significantly reduced the number of migrating cells in both Cal-27 and FaDu cells compared to the DMSO control group in a dose-dependent manner (Fig. 6a,b). CTSD activation requires an acidic pH in the lysosome to cleave its pro-CTSD form and convert it into the mature CTSD form. Therefore, dysfunction in lysosomal acidification could lead to a decrease in mature CTSD levels. Indeed, the protein expression of mature CTSD was downregulated after treatment with ECDD-S18 or bafilomycin A1 for 36 h compared to the DMSO control in both Cal-27 (Fig. 6c) and FaDu (Fig. 6d) cells. Furthermore, the mRNA expression of CTSD was also significantly decreased following treatment with ECDD-S18 or bafilomycin A1 (a V-ATPase inhibitor) compared to the DMSO control in both Cal-27 and FaDu cells (Fig. 6e). These findings suggest that ECDD-S18 inhibits V-ATPase activity, leading to impaired lysosomal acidification and reduced mature CTSD production.
ECDD-S18 inhibits HNSCC migration via diminished CTSD expression. (a, b) ECDD-S18 inhibits HNSCC migration. Cal-27 (a) and FaDu (b) cells were plated in the upper chamber of 8 µm pore-size transwell insert and treated with ECDD-S18 (0.1 and 1 µM) and DMSO for 36 h. Cells at the lower surface of the transwell membranes were fixed and stained with crystal violet and then captured by light microscopy for 7 fields in each group. Bar graphs are the average number of migrating cells and are presented as percent of DMSO control. (c, d) The expression of CTSD protein in HNSCC. Cal-27 (c) and FaDu (d) cells were treated with ECDD-S18 (0.1, 1, and 2 µM), bafilomycin A1 (0.1 µM) or DMSO for 36 h. Western blotting analyzed the protein lysates for pro-CTSD, mature-CTSD, and N-cadherin expression. GAPDH was used as a loading control. The expression was normalized to GAPDH and represented as fold change. (e) The mRNA expression of CTSD in Cal-27 and FaDu. Cells were treated with ECDD-S18 (0.1, 1, and 2 µM), bafilomycin A1 (0.1 µM) or DMSO for 36 h. The total RNA was extracted and analyzed for CTSD mRNA expression by quantitative RT-PCR. The expression was normalized to GAPDH and represented as fold change relative to the DMSO control. (f) Proposed anticancer mechanism of ECDD-S18 in HNSCC cells. Data are mean ± S.E.M. (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, relative to DMSO control using one-way ANOVA with a Tukey’s multiple comparison test.
Cathepsin D promotes cancer cell metastasis by facilitating epithelial-mesenchymal transition (EMT). Therefore, we investigated whether the reduction of CTSD expression by ECDD-S18 would inhibit EMT by examining the expression of EMT markers. We found that treatment with ECDD-S18 significantly downregulated N-cadherin protein expression in a dose-dependent manner compared to DMSO control in Cal-27 and FaDu cells (Fig. 6c,d). We also examined the expression of Twist and Fibronectin gene after 36 h of ECDD-S18 exposure using quantitative RT-PCR. Consistently, the expression of Twist and Fibronectin transcripts were significantly downregulated in Cal-27 cells (Supplementary Fig. S3a). However, no significant change was observed in FaDu cells (Supplementary Fig. S3b). Moreover, treatment with ECDD-S18 and the V-ATPase inhibitor bafilomycin A had no effect on the expression of E-cadherin mRNA and protein (Supplementary Fig. S4), indicating that the effect of ECDD-S18 is context-dependent manner. These results implied that ECDD-S18 suppresses EMT-mediated HNSCC cell migration through impairment of lysosomal acidification, mediated by V-ATPase activity inhibition concomitant with the reduction of the mature CTSD production.
ECDD-S18 enhances the anticancer activity of cisplatin in HNSCC cells
Cisplatin is an effective chemotherapeutic agent for head and neck cancers. Therefore, we investigated whether ECDD-S18 could enhance the anticancer activity of cisplatin. Cal-27 and FaDu cells were treated with 1 µM of ECDD-S18, cisplatin, or a combination of both for 48 and 72 h. Consistently, ECDD-S18 treatment reduced HNSCC cell viability, while cisplatin showed only a slight suppression of cell viability at 48 and 72 h (Fig. 7). In Cal-27 cells, co-treatment with 1 µM of ECDD-S18 and cisplatin further reduced cell viability compared to treatment with ECDD-S18 or cisplatin alone at 48 h. However, this combination effect was not observed at 72 h (Fig. 7a). In contrast, co-treatment with ECDD-S18 and cisplatin markedly reduced the viability of FaDu cells at both 48 and 72 h compared to ECDD-S18 or cisplatin alone (Fig. 7b). These data may suggest a synergistic effect between ECDD-S18 and cisplatin, enhancing their anticancer activity against HNSCC cells.
Combined treatment of cisplatin and ECDD-S18 reduces HNSCC cell viability. Cal-27 (a) and FaDu (b) cells were exposed to 1 µM of ECDD-S18, cisplatin, or a combination of both for 48 and 72 h. Cell viability was measured by using MTT assay. The results are means ± S.E.M. (n = 3). *p < 0.05, **p < 0.01, and ****p < 0.0001, relative to DMSO control using one-way ANOVA with a Tukey’s multiple comparison test.
Discussion
Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous malignancy, and its incidence is projected to increase by 30% to 1.08 million annually by 2030, as reported by GLOBOCAN36. Cisplatin, approved by the FDA, is widely used as a chemotherapeutic agent for HNSCC patients in combination with radiation. However, drug resistance and undesirable side effects such as kidney problems, allergic reactions, decreased immunity, gastrointestinal disorders, hemorrhage, and hearing loss have been reported in patients receiving cisplatin37. Moreover, chemotherapy is not preferred for elder patients with coexisting conditions, which is the major group in HNSCC patients. Furthermore, the 5-year survival rate of HNSCC patients remains poor, at less than 50%4. Therefore, effective chemotherapeutic drugs for HNSCC are required to enhance cytotoxicity and alleviate side effects, resulting in improved survival rates and quality of life. Here, we investigated the anticancer activity of halogenated benzoate derivatives of cleistanthin A (CA), ECDD-S16 and ECDD-S18, compared to cisplatin on HNSCCs and normal cells. Our results unveiled that ECDD-S18, synthesized through the esterification of CA with 4-bromobenzoic acid, exhibited potent cytotoxicity against HNSCC cells while demonstrating lesser effects on normal cells compared to cisplatin and CA. Treatment with ECDD-S18 significantly reduced HNSCC cell viability through the induction of apoptosis and inhibition of migration. Mechanistically, the anticancer activity of ECDD-S18 was partly due to its ability to inhibit V-ATPase, which disrupts lysosomal acidification, impairs CTSD maturation, and consequently arrests autophagic degradation (Fig. 7). This finding sheds light on a novel pathway for therapeutic intervention in HNSCC.
Concurrent chemoradiotherapy (CRT) utilizing platinum-based chemotherapy and/or surgery is considered a standard treatment for locally advanced (LA) HNSCC patients. Cisplatin emerges as the primary chemotherapeutic agent in CRT for LA-HNSCC patients38. Despite yielding promising outcomes when combined with radiation, the therapeutic utility of cisplatin in HNSCC is hampered by its adverse effects on normal tissues, notably nephrotoxicity and neurotoxicity39. Thus, there is a need for new anticancer compounds with high efficacy toward HNSCC but fewer undesirable effects on normal cells. Cleistanthin A (CA) is a phytoconstituent of Phyllanthus taxodiifolius Beille, exhibiting diverse pharmacological effects, including diuretic effect40, and notable anticancer potential against various cancer types such as melanoma cells41 and breast cancer cells42. Our recent investigation has elucidated the anticancer efficacy of CA in colon cancer cells16. Despite its promising therapeutic effects, the cytotoxicity of CA towards normal cells presents a significant challenge. Consequently, we synthesized two CA derivatives, ECDD-S16 and ECDD-S18, by introducing 4-fluorobenzoic acid and 4-bromobenzoic acid onto the side chain of CA at the para-position, respectively. Herein, we explore the anticancer activity of these novel derivatives in the context of head and neck squamous cell carcinoma (HNSCC). Interesting, the cytotoxic impact of CA and its derivatives proved significantly greater than that of cisplatin in HNSCC. The enhancement of therapeutic efficacy through halogenated-modified natural compounds has gradually gained attention43. We have previously demonstrated that the addition of a halogenated benzoate group to altholactone increased its anticancer activity in cholangiocarcinoma cells44. However, the halogenated modification did not improve the anticancer efficacy of CA, as the effect of CA derivatives on HNSCC viability did not differ from that of their parent compound, cleistanthin A. Nevertheless, compared to CA, the cytotoxicity of the derivatives, especially ECDD-S18, was less pronounced in normal cells. It remains unclear whether cellular selectivity results from halogenated modification. Nephrotoxicity and neurotoxicity are known side effects of cisplatin treatment. We assessed the effect of ECDD-S18 on HK-2 cells (human kidney proximal tubule epithelial cells) and SH-SY5Y neuroblastoma cells, a well-established model for studying cisplatin-induced neurotoxicity. As shown in Table 1, the IC50 of ECDD-S18 for HK-2 and SH-SY5Y cells was slightly higher than that of cisplatin. Furthermore, the IC50 of cisplatin is similar between HNSCC and HK-2 cells, suggesting that cisplatin is toxic to both HNSCC cells and normal cells at its effective concentration. In contrast, ECDD-S18 exhibits significantly lower IC50 values for HNSCC cell lines compared to HK-2 cells. This indicates that ECDD-S18 can effectively target HNSCC cells at concentrations that are less toxic to normal cells. Collectively, these findings suggest that ECDD-S18 may offer a superior safety profile compared to cisplatin, with reduced toxicity to both normal kidney cells and neurons.
While there are currently no studies on the anticancer activity and toxicity of cleistanthin A derivatives in animal models, previous research has demonstrated the anticancer potential and toxicity of parent compound cleistanthin A. For instance, cleistanthin A significantly reduced tumor volume in mice bearing Dalton’s lymphoma and S-180 sarcoma and prolonged their lifespan. In healthy mice, treatment with cleistanthin A did not affect body weight or lymphocyte count, whereas mice receiving cisplatin experienced more than 30–40% body weight loss and a marked decrease in WBC and RBC levels45. Additionally, several studies have demonstrated the lower cytotoxicity of cleistanthin A derivatives in normal cells relative to cancer cells. For example, Zhao et al. reported that twelve new glycosides and alkane derivatives of cleistanthin A exhibited significant antiproliferative activity against HCT-116, HepG2, A549, and HeLa cancer cell lines, with minimal impact on HEK293 cells46. Furthermore, the diphyllin glycoside derivative ECDD-S27 decreased the viability of cervical adenocarcinoma cells with an IC50 of 0.04 µM, while the IC50 on HK-2 cells was greater than 50 µM27. These findings, along with our study, suggest that ECDD-S18 has the potential to be a promising therapeutic agent with a better safety profile than cisplatin. However, to fully confirm these observations, further research, including direct in vivo comparative studies, is essential.
Vacuolar ATPase (V-ATPase) is ATP-driven proton pump responsible for transporting protons into the intracellular compartment, where they play pivotal roles in various processes including membrane protein trafficking, protein degradation, and autophagy. At the plasma membrane of specialized cells, V-ATPases transport protons out of the cytosol into the extracellular space, contributing to functions such as bone resorption, sperm maturation, and urinary acidification47. Additionally, V-ATPases are found to be overexpressed in various cancers, promoting drug resistance, tumor migration and invasion, and inhibiting the apoptosis process5. Thus, they represent promising druggable targets in cancer therapy. The V-ATPase has been implicated in autophagy; a process critical for cell survival6. The final stage of autophagy involves the fusion of autophagosomes with lysosomes to form autolysosomes, wherein the acidic environment activates enzymes responsible for degrading biological material. The V-ATPase plays a key role in maintaining a low intra-lysosome pH essential for lysosomal hydrolase activity. Inhibition of V-ATPase activity by its inhibitor, bafilomycin A1 results in a blockade of autophagic flux11. Consequently, inhibition of autophagy results in the suppression of cancer cell proliferation and tumor progression48. Previous studies have identified upregulated expression of V-ATPase in oral squamous cell carcinoma (OSCC), a common type of HNSCC7,49. Moreover, the previous evidence also suggests the involvement of autophagy in therapeutic resistance in HNSCC50. Indeed, elevated levels of autophagy have been observed in HNSCC in response to adverse conditions from cisplatin treatment51.This information suggests the critical roles of V-ATPase and autophagy in HNSCC progression. Previously, we found that treatment with CA reduced CRC migration and invasion, although the mechanism through which it targets target V-ATPase remains unclear16. Recently, halogenated benzoate derivatives of CA such as ECDD-S16 was shown to target V-ATPase and inhibit pyroptosis in Raw264.7 cells18. However, the effects on cancer cells remain unknown. Additionally, another derivative resulting from the esterification of CA with 3,5-dimethoxybenzoic acid, ECDD-S27, was identified as an autophagy inhibitor via V-ATPase targeting27. Expanding on these insights, our study revealed that ECDD-S18, a bromobenzoate derivative, suppressed HNSCC viability through apoptosis induction, mediated by inhibition of autophagy via V-ATPase targeting. Furthermore, through comparison of molecular docking data, we found that the binding energy of ECDD-S18 (-8.50 kcal/mol) was lower than that of CA (-6.96 kcal/mol), indicating a stronger binding affinity of ECDD-S18 to V-ATPase. Moreover, in contrast with CA, both ECDD-S16 and ECDD-S18 displayed a remarkable preference for cancer cells over normal cells. While the mechanism underlying this selectivity remains unclear, the incorporation of halobenzene moiety could potentially enhance ECDD-S18’s binding affinity against V-ATPase, primarily through hydrophobic contact and hydrogen bonding52 and specificity towards malignant cells53. Our finding suggests the potential of ECDD-S18 as a more effective therapeutic agent.
Lysosomal acidification is crucial for the maturation of acid-dependent proteases, particularly the cathepsin protein family6. Cathepsin D (CTSD) is an aspartyl lysosomal protease distributed in lysosomes to degrade proteins in an acidic environment, working synergistically with other cathepsins54. CTSD plays pivotal roles in several tumor progression steps, including cell proliferation, angiogenesis, apoptosis, and autophagy55. Its overexpression correlates with therapeutic resistance in hepatocellular carcinoma 56, pancreatic cancer57, and ovarian cancer58. Consistent with our TCGA database analysis, which showed significant CTSD overexpression in HNSCC compared to normal tissue. However, the effects of the CA derivative, ECDD-S18, on CTSD expression in HNSCC remain unknown. CTSD is synthesized as an inactive pro-form and is activated through processing that is facilitated by the acidic environment maintained by V-ATPases in the lysosome59. Thus, inhibition of V-ATPases can impair the maturation of CTSD. Consistent with this, our data show that treatment with ECDD-S18 significantly downregulated the protein expression of mature CTSD in HNSCC cells. The effect of CA derivative ECDD-S16 on the reduction of mature form of cathepsin D was also observed in Raw264.7 cells18. CTSD is also critical for maintaining the lysosomal environment necessary for autophagy. During autophagy, damaged organelles and proteins are sequestered into autophagosomes, which then fuse with lysosomes where CTSD assists in breaking down these cargoes, thereby facilitating autophagic flux60. Therefore, disruption of lysosomal acidification by ECDD-S18 through V-ATPase inhibition could impair CTSD maturation and consequently arrest autophagic degradation.
CTSD functions as a proteolytic enzyme that degrades extracellular matrix (ECM) components, thereby promoting cancer cell migration and invasion, ultimately contributing to tumor metastasis10. Loss of function study revealed that CTSD acts as positive regulator of epithelial-mesenchymal transition (EMT)61. Elevated CTSD expression levels have been consistently observed in oral carcinomas (OSCC) with regional lymph node metastasis compared to node-negative tumors62. Furthermore, several independent clinical studies have suggested a correlation between high CTSD expression and an increased risk of HNSCC metastasis63,64. Consistent with roles of CTSD on cancer cell metastasis, our study found that treatment with ECDD-S18 reduced mature CTSD levels, concomitant with markedly suppressed HNSCC cell migration. Additionally, ECDD-S18 treatment suppressed the expression of mesenchymal markers, including N-cadherin, Twist, and fibronectin, indicating its potential inhibitory effects on the epithelial-mesenchymal transition (EMT) process. However, treatment with ECDD-S18 and the V-ATPase inhibitor bafilomycin A had no effect on the expression of E-cadherin mRNA and protein. Consistent with our findings, previous studies have shown that bafilomycin A1 suppressed EMT in NSCLC cells by reducing mesenchymal markers such as N-cadherin, while E-cadherin levels remained unchanged65. Although the exact mechanism remains unclear, these results indicate that ECDD-S18 may suppress EMT through pathways independent of E-cadherin expression. Moreover, a previous study in breast cancer cells found that N-cadherin plays a direct role in promoting cell motility regardless of E-cadherin expression66. In our study, ECDD-S18 suppressed the expression of N-cadherin, suggesting that the reduction in cell migration by ECDD-S18 may be primarily due to the suppression of N-cadherin. Notably, ECDD-S18 treatment also suppressed the expression of CTSD mRNA. The expression of the CTSD gene has been shown to be regulated by transcription factor EB (TFEB), a member of the MiT/TFE transcription factor family67. However, the effect of ECDD-S18 on CTSD mRNA expression remains unknown and warrants further investigation.
In conclusion, our study delved into the anticancer effects and underlying mechanism of halogenated benzoate derivative of CA ECDD-S18 on HNSCC. Notably, ECDD-S18 demonstrated superior cytotoxic effects compared to cisplatin, the standard drug for treating HNSCC. Furthermore, these derivatives exhibited a remarkable selectivity for cancer cells over normal cells, surpassing their parent compound CA. Mechanistically, the anticancer activity of ECDD-S18 partly stems from its ability to disrupt lysosomal acidification and impede autophagy via targeted inhibition of vacuolar ATPase. As a result, HNSCC cell viability and migration were significantly suppressed. Our findings illuminate the therapeutic potential of CA derivatives in HNSCC, suggesting their promising role in combination with current therapeutic modalities.
Data availability
The data generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Leemans, C. R., Snijders, P. J. F. & Brakenhoff, R. H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer18, 269–282. https://doi.org/10.1038/nrc.2018.11 (2018).
Fraval, H. N., Rawlings, C. J. & Roberts, J. J. Increased sensitivity of UV-repair-deficient human cells to DNA bound platinum products which unlike thymine dimers are not recognized by an endonuclease extracted from Micrococcus luteus. Mutat. Res.51, 121–132. https://doi.org/10.1016/0027-5107(78)90014-3 (1978).
Gupta, T., Kannan, S., Ghosh-Laskar, S. & Agarwal, J. P. Systematic review and meta-analyses of intensity-modulated radiation therapy versus conventional two-dimensional and/or or three-dimensional radiotherapy in curative-intent management of head and neck squamous cell carcinoma. PLoS One13, e0200137. https://doi.org/10.1371/journal.pone.0200137 (2018).
Colevas, A. D. et al. NCCN guidelines insights: head and neck cancers, version 1.2018. J. Natl. Compr. Cancer Netw.16, 479–490. https://doi.org/10.6004/jnccn.2018.0026 (2018).
Chen, F., Kang, R., Liu, J. & Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Therapy29, 1529–1541. https://doi.org/10.1038/s41417-022-00477-y (2022).
Stransky, L., Cotter, K. & Forgac, M. The Function of V-ATPases in Cancer. Physiol. Rev.96, 1071–1091. https://doi.org/10.1152/physrev.00035.2015 (2016).
Garcia-Garcia, A. et al. Immunohistochemical localization of C1 subunit of V-ATPase (ATPase C1) in oral squamous cell cancer and normal oral mucosa. Biotech. Histochem.87, 133–139. https://doi.org/10.3109/10520295.2011.574647 (2012).
Kiyoshima, T. et al. Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression. PLoS One8, e80998. https://doi.org/10.1371/journal.pone.0080998 (2013).
Olson, O. C. & Joyce, J. A. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer15, 712–729. https://doi.org/10.1038/nrc4027 (2015).
Tan, G. J., Peng, Z. K., Lu, J. P. & Tang, F. Q. Cathepsins mediate tumor metastasis. World J. Biol. Chem.4, 91–101. https://doi.org/10.4331/wjbc.v4.i4.91 (2013).
Mauvezin, C., Nagy, P., Juhász, G. & Neufeld, T. P. Autophagosome–lysosome fusion is independent of V-ATPase-mediated acidification. Nat. Commun.6, 7007. https://doi.org/10.1038/ncomms8007 (2015).
Yun, C. W. & Lee, S. H. The roles of autophagy in cancer. Int. J. Mol. Sci.https://doi.org/10.3390/ijms19113466 (2018).
Whitton, B., Okamoto, H., Packham, G. & Crabb, S. J. Vacuolar ATPase as a potential therapeutic target and mediator of treatment resistance in cancer. Cancer Med.7, 3800–3811. https://doi.org/10.1002/cam4.1594 (2018).
Pornpongrungrueng, P., Chantaranothai, P., Parnell, J. A. N. & Hodkinson, T. R. Two new species of Phyllanthus (Phyllanthaceae) from Thailand. PhytoKeys136, 35–44 (2019).
Tuchinda, P. et al. Cytotoxic Arylnaphthalide Lignan Glycosides from the Aerial Parts of Phyllanthus taxodiifolius. Planta Med.72, 60–62. https://doi.org/10.1055/s-2005-873141 (2006).
Jearawuttanakul, K. et al. Cleistanthin A induces apoptosis and suppresses motility of colorectal cancer cells. Eur. J. Pharmacol.889, 173604. https://doi.org/10.1016/j.ejphar.2020.173604 (2020).
Neumann, C. S., Fujimori, D. G. & Walsh, C. T. Halogenation strategies in natural product biosynthesis. Chem. Biol.15, 99–109. https://doi.org/10.1016/j.chembiol.2008.01.006 (2008).
Ekchariyawat, P. et al. ECDD-S16 targets vacuolar ATPase: A potential inhibitor compound for pyroptosis-induced inflammation. PLoS One18, e0292340. https://doi.org/10.1371/journal.pone.0292340 (2023).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Ray, S. K. et al. Molecular evidence of apoptotic death in malignant brain tumors including glioblastoma multiforme: Upregulation of calpain and caspase-3. J. Neurosci. Res.69, 197–206. https://doi.org/10.1002/jnr.10265 (2002).
Paquette, M. et al. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy17, 3957–3975. https://doi.org/10.1080/15548627.2021.1898748 (2021).
Raimondo, S. et al. Citrus limon -derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotargethttps://doi.org/10.18632/oncotarget.4004 (2015).
Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res.49, W388–W396. https://doi.org/10.1093/nar/gkab382 (2021).
Pang, Z. et al. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protocols17, 1735–1761. https://doi.org/10.1038/s41596-022-00710-w (2022).
Goedhart, J. & Luijsterburg, M. S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep.10, 20560. https://doi.org/10.1038/s41598-020-76603-3 (2020).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun.10, 1523. https://doi.org/10.1038/s41467-019-09234-6 (2019).
Paha, J. et al. A novel potent autophagy inhibitor ECDD-S27 targets vacuolar ATPase and inhibits cancer cell survival. Sci. Rep.9, 9177. https://doi.org/10.1038/s41598-019-45641-x (2019).
Keon, K. A., Benlekbir, S., Kirsch, S. H., Muller, R. & Rubinstein, J. L. Cryo-EM of the yeast V(O) Complex reveals distinct binding sites for macrolide V-ATPase inhibitors. ACS Chem. Biol.17, 619–628. https://doi.org/10.1021/acschembio.1c00894 (2022).
Anandakrishnan, R., Aguilar, B. & Onufriev, A. V. H++ 3.0: automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res40, W537–W541. https://doi.org/10.1093/nar/gks375 (2012).
Ravindranath, P. A., Forli, S., Goodsell, D. S., Olson, A. J. & Sanner, M. F. AutoDockFR: advances in protein-ligand docking with explicitly specified binding site flexibility. PLoS Comput. Biol.11, e1004586. https://doi.org/10.1371/journal.pcbi.1004586 (2015).
Eberhardt, J., Santos-Martins, D., Tillack, A. F. & Forli, S. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings. J. Chem. Inf. Model61, 3891–3898. https://doi.org/10.1021/acs.jcim.1c00203 (2021).
Pettersen, E. F. et al. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci.30, 70–82. https://doi.org/10.1002/pro.3943 (2021).
Cheung, Y.-T. et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. NeuroToxicology30, 127–135. https://doi.org/10.1016/j.neuro.2008.11.001 (2009).
Wang, R. et al. Molecular basis of V-ATPase inhibition by bafilomycin A1. Nat. Commun.12, 1782. https://doi.org/10.1038/s41467-021-22111-5 (2021).
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer12, 401–410. https://doi.org/10.1038/nrc3262 (2012).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin.68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Polat, O. A. et al. Evaluation of histologic, antiapoptotic and antioxidant effects of melatonin against the acute ocular toxicity of Cisplatin. Tissue Cell85, 102226. https://doi.org/10.1016/j.tice.2023.102226 (2023).
Demirci, N. S. et al. Modified docetaxel, cisplatin and fluorouracil therapy as the first-line treatment for patients with recurrent/metastatic squamous cell carcinoma of the head and neck cancer: a retrospective study. Curr. Med. Res. Opin.33, 401–407. https://doi.org/10.1080/03007995.2016.1257984 (2017).
Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem.88, 102925. https://doi.org/10.1016/j.bioorg.2019.102925 (2019).
Parasuraman, S. & Raveendran, R. Diuretic effects of Cleistanthin A and Cleistanthin B from the leaves of Cleistanthus Collinus in Wistar Rats. J. Young Pharmacists4, 73–77. https://doi.org/10.4103/0975-1483.96616 (2012).
Pan, S., Cai, H., Gu, L. & Cao, S. Cleistanthin A inhibits the invasion and metastasis of human melanoma cells by inhibiting the expression of matrix metallopeptidase-2 and -9. Oncol. Lett.14, 6217–6223. https://doi.org/10.3892/ol.2017.6917 (2017).
Liu, S. et al. Cleistanthin A inhibits the invasion of MDA-MB-231 human breast cancer cells: involvement of the β-catenin pathway. Pharmacol. Rep.72, 188–198. https://doi.org/10.1007/s43440-019-00012-1 (2020).
Hernandes, M. Z., Cavalcanti, S. M., Moreira, D. R., de Azevedo Junior, W. F. & Leite, A. C. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets11, 303–314. https://doi.org/10.2174/138945010790711996 (2010).
Kitdumrongthum, S. et al. Inhibition of topoisomerase IIalpha and induction of DNA damage in cholangiocarcinoma cells by altholactone and its halogenated benzoate derivatives. Biomed. Pharmacother.127, 110149. https://doi.org/10.1016/j.biopha.2020.110149 (2020).
Pradheepkumar, C. P. & Shanmugam, G. Anticancer potential of cleistanthin A isolated from the tropical plant Cleistanthus collinus. Oncol. Res.11, 225–232 (1999).
Zhao, Y., Lu, Y., Ma, J. & Zhu, L. Synthesis and Evaluation of Cleistanthin A Derivatives as Potent Vacuolar H(+) -ATPase Inhibitors. Chem. Biol. Drug Des.86, 691–696. https://doi.org/10.1111/cbdd.12538 (2015).
Breton, S. & Brown, D. Regulation of luminal acidification by the V-ATPase. Physiology28, 318–329. https://doi.org/10.1152/physiol.00007.2013 (2013).
Yang, A. et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov.4, 905–913. https://doi.org/10.1158/2159-8290.Cd-14-0362 (2014).
Pérez-Sayáns, M. et al. Measurement of ATP6V1C1 expression in brush cytology samples as a diagnostic and prognostic marker in oral squamous cell carcinoma. Cancer Biol. Therapy9, 1057–1064. https://doi.org/10.4161/cbt.9.12.11880 (2010).
Sannigrahi, M. K., Singh, V., Sharma, R., Panda, N. K. & Khullar, M. Role of autophagy in head and neck cancer and therapeutic resistance. Oral Dis21, 283–291. https://doi.org/10.1111/odi.12254 (2015).
Chen, Y. et al. Autophagy regulates the cancer stem cell phenotype of head and neck squamous cell carcinoma through the noncanonical FOXO3/SOX2 axis. Oncogene41, 634–646. https://doi.org/10.1038/s41388-021-02115-7 (2022).
Xu, Z. et al. Halogen bond: its role beyond drug-target binding affinity for drug discovery and development. J. Chem. Inf. Model54, 69–78. https://doi.org/10.1021/ci400539q (2014).
Huwaimel, B. I. et al. Discovery of halogenated benzothiadiazine derivatives with anticancer activity*. ChemMedChem16, 1143–1162. https://doi.org/10.1002/cmdc.202000729 (2021).
Redecker, B., Heckendorf, B., Grosch, H.-W., Mersmann, G. & Hasilik, A. Molecular organization of the human cathepsin D gene. DNA Cell Biol.10, 423–431. https://doi.org/10.1089/dna.1991.10.423 (1991).
Berchem, G. et al. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene21, 5951–5955. https://doi.org/10.1038/sj.onc.1205745 (2002).
Shen, S. et al. Molecular mechanism of C-reaction protein in promoting migration and invasion of hepatocellular carcinoma cells in vitro. Int. J. Oncol.50, 1289–1298. https://doi.org/10.3892/ijo.2017.3911 (2017).
Dumartin, L. et al. AGR2 is a novel surface antigen that promotes the dissemination of pancreatic cancer cells through regulation of cathepsins B and D. Cancer Res71, 7091–7102. https://doi.org/10.1158/0008-5472.Can-11-1367 (2011).
Zhao, X. et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy11, 1849–1863. https://doi.org/10.1080/15548627.2015.1017185 (2015).
Kitazawa, S. et al. Cancer with low cathepsin D levels is susceptible to vacuolar (H(+) )-ATPase inhibition. Cancer Sci108, 1185–1193. https://doi.org/10.1111/cas.13240 (2017).
Di, Y. Q. et al. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy17, 1170–1192. https://doi.org/10.1080/15548627.2020.1752497 (2021).
Lee, S. G. et al. Cathepsin D promotes polarization of tumor-associated macrophages and metastasis through TGFBI-CCL20 signaling. Exp Mol Med56, 383–394. https://doi.org/10.1038/s12276-024-01163-9 (2024).
Vigneswaran, N. et al. Variable expression of cathepsin B and D correlates with highly invasive and metastatic phenotype of oral cancer. Hum Pathol31, 931–937. https://doi.org/10.1053/hupa.2000.9035 (2000).
Gandour-Edwards, R., Trock, B. & Donald, P. J. Predictive value of cathepsin-D for cervical lymph node metastasis in head and neck squamous cell carcinoma. Head Neck21, 718–722. https://doi.org/10.1002/(sici)1097-0347(199912)21:8%3c718::aid-hed6%3e3.0.co;2-w (1999).
Maurizi, M. et al. Cathepsin D concentration in primary laryngeal cancer: correlation with clinico-pathological parameters, EGFR status and prognosis. Int. J. Cancer69, 105–109. https://doi.org/10.1002/(sici)1097-0215(19960422)69:2%3c105::Aid-ijc6%3e3.0.Co;2-4 (1996).
Alizadeh, J. et al. Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochim. Biophys. Acta Mol. Cell Res.1865, 749–768. https://doi.org/10.1016/j.bbamcr.2018.02.007 (2018).
Nieman, M. T., Prudoff, R. S., Johnson, K. R. & Wheelock, M. J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol147, 631–644. https://doi.org/10.1083/jcb.147.3.631 (1999).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science325, 473–477. https://doi.org/10.1126/science.1174447 (2009).
Acknowledgements
This research project is supported by Mahidol University (Basic Research Fund: fiscal years 2022 to AC) and National Research Council of Thailand (NRCT) and Mahidol University (N42A660523 to AC) and the Science Achievement Scholarship of Thailand (SAST to AW).
Funding
The Science Achievement Scholarship of Thailand; National Research Council of Thailand (NRCT) and Mahidol University (Grant nos. N42A660523); This research project is supported by Mahidol University (Basic Research Fund: fiscal years 2022)
Author information
Authors and Affiliations
Contributions
A.W, W.P., A.C. Conceptualization; A.W., W.P., S.K., B.N., P.K., K.J. T.K., and S.P. Conducted the experiments and analyzed the data; N.C., B.M., and P.T. compound synthesis and characterization; A.C., M.P., and S.B. Supervised all research; A.W. and A.C. Wrote the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wongpan, A., Panvongsa, W., Krobthong, S. et al. Cleistanthin A derivative disrupts autophagy and suppresses head and neck squamous cell carcinoma progression via targeted vacuolar ATPase. Sci Rep 14, 22582 (2024). https://doi.org/10.1038/s41598-024-73186-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73186-1