Abstract
Rodents are the synanthropic mammals that are existing in close proximity to humans and their belongings and have the potential to act as the reservoir for a variety of parasites having zoonotic potential. Present study was designed to report the molecular prevalence and phylogenetic evaluation of Toxoplasma gondii in the blood samples of four wild rodent species [Rattus rattus (N = 122), Mus musculus (N = 64), Rattus norvegicus (N = 57) and Dryomys nitedula (N = 1)] that were trapped during May 2022 till July 2023 from three districts in Punjab (Jampur, Dera Ghazi Khan and Multan) and three districts (Upper Dir, Mardan and Bunar) in Pakistan. Results revealed that 44/244 (18%) rodents amplified ITS-1 gene of Toxoplasma gondii through PCR. Parasite prevalence varied between the rodent species. Highest rate of infection was found in Rattus norvegicus followed by Rattus rattus and Mus musculus. For both rat species, Toxoplasma gondii infection significantly varies between the sampling districts. DNA sequencing and BLAST analysis confirmed the presence of Toxoplasma gondii in rodent blood samples. Phylogenetic analysis showed that Pakistani isolates were genetically diverse and clustered with the isolates that were reported from worldwide countries. Complete blood count analysis revealed that parasite infected rodents had disturbed lymphocyte, mean platelet volume, mean corpuscular volume (and mean corpuscular hemoglobin concentration. Markers of oxidative stress analysis revealed that infected rodent had elevated malondialdehyde levels in liver and kidney while disturb catalase concentrations in kidney and heart as compared to uninfected animals. In conclusion, we are reporting a relatively high prevalence of Toxoplasma gondii in Pakistani rodents. Infection leads to disturbed complete blood count and markers of oxidative stress in the vital organs. We recommend large scale studies in various geo-climatic regions of Pakistan to report the incidence and prevalence of this pathogen among the rodents in order to prevent their infections in local people as well as in livestock.
Similar content being viewed by others
Introduction
Rodents are successfully combating with different environments (aquatic, semiaquatic or dry conditions) and among them synanthropic rodents are especially important as they share the common environment with human and are considered as serious reservoirs of the pathogens1. Among the mammals, the largest order is Rodentia that included a number of rats and mice species that not only acts as pest but they are also involved in the transmission of a number of parasitic infections including toxoplasmosis2.
Toxoplasma gondii is an intracellular protozoan that infects almost all warm-blooded animals and several cold-blooded organisms across the globe3. Rodents are known to have different susceptibility to various strains of this parasite. Rodents that are sensitive to a particular strain usually die soon while the resistant rodents develop lifelong chronic infection that plays a major role in the transmission of parasite4. The prevalence of Toxoplasma gondii in wildlife is correlated with the presence of final hosts (felids) as the oocysts of this parasite is excreted in the fecal material and ingested by new host5,6. Since the rat and mice are omnivorous and consumes seeds and insects and materials from the environment that can be contamination by Toxoplasma gondii’s oocysts, hence they have high susceptibility to get this parasitic infection7. These infected rodents are hunted and consumed by cats, dogs and other animals that carry this infection to new hosts and destinations5. The most common symptoms of toxoplasmosis in animals include fever, loss of appetite and lethargy but symptoms vary with the nature of infection: acute or chronic8.
Literature review revealed just a couple of studies regarding the Toxoplasma gondii mediated infection in wild rodents from Pakistan. Both studies were from Punjab province: Rizwan et al.9 used PCR while Ahmad et al.10 used ELISA to report the prevalence of this protozoan in rodent species. Hence the present study was designed to report the prevalence of Toxoplasma gondii in the blood samples of four wild rodent species (Mus musculus, Rattus norvegicus, Rattus rattus and Dryomys nitedula) that were trapped from six districts in two provinces (Punjab and Khyber Pakhtunkhwa) in Pakistan. We are also reporting the risk factors and effect of parasite on the complete blood count and on the markers of oxidative stress from the vital organs of these rodents.
Materials and methods
Study area and subjects
In the present work, all methods and experiments were approved by the ethical review committee of Bahauddin Zakariya University Multan, Pakistan (BZU/Ethics/Zool-29-2022). Importantly, all methods were performed in accordance with the relevant guidelines and regulations.
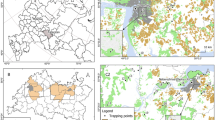
An active epidemiological survey was conducted to determine the prevalence of Toxoplasma gondii in wild rat and mice that were trapped from three regions in Punjab (Jampur, Dera Ghazi Khan and Multan) and three regions in Khyber Pakhtunkhwa (KPK) (Sheringal, Mardan and Buner) in Pakistan (Fig. 1). A total of 244 wild rodents were trapped from two provinces during May 2022 till July 2023. Standard rodent traps with bate or glue traps were set in houses, storage sites, fields and in shops the sampling areas in Punjab. Along with the urban regions, traps were also set in forest and mountain regions in Khyber Pakhtunkhwa along with the usual sites as in Punjab province. The traps were checked at regular intervals so that live rodents can be handled for blood collection under isoflurane inhalation. Standard taxonomic keys were used to identify the species of the trapped rodents11.
Data collection
A questionnaire was filled for each animal on sampling site to gather basic information about each trapped rodent including species, sex, sampling site, body weight and length and presence of ectoparasites on animal’s body.
Blood collection, complete blood count and DNA extraction
Blood samples were collected from each animal from direct cardiac puncture. Blood samples were collected into the screw-capped tubes containing 0.5 M Ethylene Diamine Tetra Acetic acid (EDTA) as an anticoagulant and frozen at − 20 °C till the further molecular analysis. Hematological parameters, red, white blood cell and platelet counting and associated parameters were analyzed in all collected rodent blood samples by using an automated hematological analyzer (Mythic™ 18 Vet, Orphee, Switzerland). Genomic DNA was extracted from the rodent blood by using Wizard® Genomic DNA Purification Kit (Promega, Madison, WI, USA) following the manufacturer’s instructions.
Molecular detection by PCR
The extracted DNA samples were analyzed for the presence of Toxoplasma gondii (target was ITS-1 gene) by using the species-specific primers (Forward 5′-AGTTTAGGAAGCAATCTGAAAGCACATC-3′ and Reverse 5′-GATTTGCATTCAAGAAGCGTGATAGTAT-3′) following Halova et al.12. A reaction mixture of 25 µl was prepared that contained 13 mM Tris–HCl (pH 8.3), 65 mM KCl, 2.5 mM MgCl2, 300 µM of each dNTP,1U of DNA Polymerase, 0.5µM of each primer and 5 µl of template DNA. Reaction conditions comprised an initial denaturation step of 94 °C for 3 min followed by 30 cycles of denaturation 94 °C for 30 s, primer annealing 55 °C for 45 s and extension 72 °C for 30 s. A final extension at 72 °C for 5 min was performed. During each reaction, distilled water was used as negative control while DNA of Toxoplasma gondii (that was available at our lab from previous studies) was used as positive control.
DNA sequencing
Amplified PCR products were sequenced by a commercial company (First Base, Malaysia). All sequences were submitted to NCBI’s GenBank and were assigned accession numbers: OR797081, OR797082, OR797083 and OR797084 for Toxoplasma gondii.
Phylogenetic analysis of ITS-1 gene of Toxoplasma gondii
The DNA sequences generated in this study underwent initial trimming by using FinchTV (version 1.4.0) to eliminate the primer-contaminated regions and any misread nucleotides at the start and end of the sequence. Additional similar sequences were retrieved using the Basic Local Alignment Search Tool (BLAST) algorithm on NCBI’s platform. Subsequently, all sequences were aligned using the ClustalW multiple sequence alignment algorithm in BioEdit (version 7.2.5). The aligned sequences were imported into MEGA X (version 10.2.6). A model selection test was conducted for all sequences, using MEGA’s integrated model selection tool. The best-fit model was chosen based on Bayesian Information Criteria (BIC) and Akaike Information Criteria (AIC) values, with the model exhibiting the lowest BIC and AIC considered as the “best-fit” substitution model. Phylogenetic trees were constructed using the Maximum Likelihood algorithm in MEGA X with 1000 bootstraps. The final version of the inferred tree was generated using the iTOL server (https://itol.embl.de/, accessed on November 1st, 2023).
Oxidative stress marker analysis
Animals were sacrifices under Isoflurane anesthesia and vital organs (heart, kidney, lungs and liver) were surgically removed and markers of oxidative stress: Superoxide dismutase, Malondialdehyde and Catalase levels were determined in them following Hussain et al.13.
Statistical analysis
Statistical package Minitab (Minitab, Pennsylvania, USA) was used for the statistical analysis of data. Data was expressed as mean values ± standard error of mean (SEM) where applicable. Probability levels of P < 0.05 were considered significant. PCR based pathogen prevalence between various rodents’ species was calculated by Chi-square (χ2) test. One way ANOVA was applied to analyze the prevalence of a parasite between sampling sites, Association between the presence of pathogen and studied epidemiological factor was assessed by contingency table analysis using the Fisher’s exact test (for 2 × 2 tables). Two sample t-test was calculated to compare hematological between parasite positive and negative rodents.
Results
Subject details
From Punjab, 40, 48 and 36 rodents were trapped from Jampur, Dera Ghazi Khan and Multan districts respectively belonging to three species: Mus musculus, Rattus rattus and Rattus norvegicus. While 58, 34 and 27 rodents were captured from Upper Dir, Mardan and Buner districts in Khyber Pakhtunkhwa respectively belonging to four species: Mus musculus, Rattus rattus, Rattus norvegicus and Dryomys nitedula. The rodents included in this study were 57% male (139/244) and 43% females (105/244).
Prevalence of Toxoplasma gondii in rodents blood samples
Polymerase chain reaction had amplified a 300 base pairs amplicon specific for ITS-1 gene of Toxoplasma gondii in 44 out of 244 (18%) blood samples that were collected from four wild rodent species during present study (Table 1).
Genetic diversity of Toxoplasma gondii in rodents
The phylogenetic tree was inferred based on these four partial ITS-1 gene sequences by Maximum-Likelihood method. The label in the tree includes the accession number, name of the parasite and the country from where this haplotype was deposited. Host of Toxoplasma gondii is also shown as graphic image. The haplotypes generated in this study displayed genetic diversity as three of them clustered together while one: ‘Toxoplasma gondii OR797083.1 (isolated from Rattus rattus) Pakistan’, clustered with Toxoplasma gondii ITS-1 sequences reported from Brazil (FJ176233, JF810943, OL323108), China (JX456457, MH553292), Sweden (U16161), Japan (LC722483), Australia (L49390), Germany (EU025025), Italy (HG793394), Tunisia (ON072255, ON514614), Iraq (OR672854), Mongolia (MH423902), and Thailand (KP895872). Whereas, the other three haplotypes: Toxoplasma gondii OR797081.1 (isolated from Mus musculus), OR797082.1 (isolated from Rattus rattus) and OR797084.1 (isolated from Rattus norvegicus)’ generated during the present study clustered together with Toxoplasma gondii sequences from Pakistan (OR727859.1, MW885251.1), Malaysia (OP490603.1) and Brazil (MH793503.1). The partial ITS-1 sequence of Neospora caninum’s (GQ899204) isolated from aborted fetus of Western Black Rhinoceros of Australia was used as an outgroup in this phylogenetic analysis (Fig. 2).
Phylogenetic tree of Toxoplasma gondii based on the partial ITS-1gene sequences. The four new sequences of Toxoplasma gondii obtained in this study are highlighted in red (OR797081-OR797084). Scale bar represents 0.02 substitutions per nucleotide position. Bootstrap value is shown as number on the node. The evolutionary history was inferred by using the Maximum Likelihood method and Kimura 2-parameter model52. The tree with the highest log likelihood (− 511.25) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The proportion of sites where at least 1 unambiguous base is present in at least 1 sequence for each descendent clade is shown next to each internal node in the tree. This analysis involved 26 nucleotide sequences. There was a total of 242 positions in the final dataset. Evolutionary analyses were conducted in MEGA X 53.
Risk factor analysis
Four rodent species were identified during present investigation. Chi square test results indicated that the prevalence of Toxoplasma gondii significantly varied among the enrolled rodent species (P < 0.001). Dryomys nitedula was most highly susceptible to this haemoparasite infection (100%) followed by Rattus norvegicus (36.8%), Rattus rattus (12.3%) and Mus musculus (10.9%) (Table 1).
Rodent blood samples were collected from six different districts in two provinces during present study. When the prevalence of Toxoplasma gondii was compared among the sampling site, it was observed that prevalence of Toxoplasma gondii varied non significantly between the sampling sites (P = 0.2). A significant variation in Toxoplasma gondii prevalence with sampling sites was observed for Rattus rattus (P < 0.001). The highest parasite prevalence was observed in rats trapped from Jampur (100%) followed by Buner (31%), Upper Dir (19%), Multan (4%) and Dera Ghazi Khan District (3%). None of the Rattus rattus trapped from Mardan district was Toxoplasma gondii infected. Prevalence of Toxoplasma gondii in Rattus norvegicus also varied significantly between the sampling sites (P < 0.001). The highest parasite prevalence was observed in Rattus norvegicus captured from Mardan (70%) followed by Buner (67%), Jampur (50%), Dera Ghazi Khan (50%) and Multan (8%) district. Toxoplasma gondii infection was not detected in Rattus norvegicus captured from Upper Dir District (Table 2).
Association of rodent sex was also determined with the prevalence of Toxoplasma gondii. Fisher’s exact test results indicated that Toxoplasma gondii infection was not associated with the sex or either Mus musculus, Rattus rattus or Rattus norvegicus. This analysis was not possible for Dryomys nitedula as just a single male animal of this species was trapped during this study (Table 3).
Complete blood count analysis
Analysis of complete blood cell count indicated that Toxoplasma gondii infected Mus musculus had elevated lymphocyte count (P = 0.05) and mean platelet volume (P = 0.03) while decreased mean corpuscular volume (P = 0.05) and mean corpuscular hemoglobin concentration (P < 0.001) as compared to mice where Toxoplasma gondii infection was not detected (Table 4).
Analysis of complete blood cell count indicated that mean corpuscular volume (P = 0.001) was the only parameter that was significantly elevated in Toxoplasma gondii infected Rattus norvegicus. While all the studied parameters varied non significantly (P > 0.05) when compared between Toxoplasma gondii infected and uninfected Rattus rattus enrolled during present investigation (Table 4).
Markers of oxidative stress analysis
Our results indicated that Superoxide Dismutase levels varied non significantly (P > 0.05) when compared in kidney, liver, heat and lungs of Toxoplasma gondii infected and uninfected mice (Table 5). A decrease in catalase concentration in kidney (P = 0.02) while elevation in heart (P = 0.03) was documented for Toxoplasma gondii infected mice as compared to uninfected mice. While catalase levels remained unaffected when compared in liver and lungs of Toxoplasma gondii positive and negative mice enrolled during present study (Table 5). A significant decrease in Malondialdehyde levels was observed in kidney and liver of Toxoplasma gondii infected mice while Malondialdehyde levels in heart and lungs remained unaffected upon comparison between Toxoplasma gondii infected and uninfected animals (Table 5).
Data analysis revealed that the Superoxide Dismutase levels in kidney, liver, heart and lungs varied remained unaffected (P > 0.05) when compared between Toxoplasma gondii infected and uninfected Rattus norvegicus trapped from various sampling sites during present study (Table 6). Catalase levels were significantly elevated in lungs (P = 0.01) while Malondialdehyde levels were decreased in kidney of Toxoplasma gondii infected Rattus norvegicus. While catalase and Malondialdehyde levels remained unaffected in other analyzed organs when compared between parasite positive and negative Rattus norvegicus during present investigation (Table 6).
Analysis of oxidative stress marker data indicated non-significant variations (P > 0.05) for all the studied parameters from kidney, liver and heart when compared between Toxoplasma gondii infected and uninfected Rattus rattus trapped during present investigation (Table 7).
Discussion
Rodents are often found living in areas close to human dwelling and they are already established carriers for number vector-borne parasites with zoonotic potential14. To date, only a couple of reports have been documented in literature regarding the molecular prevalence of Toxoplasma gondii in wild rodents from Pakistan. Hence the present study was designed to report the molecular prevalence of this pathogen in the blood samples of four rodent species that were collected from six districts in two provinces of Pakistan.
During the present study, overall 18% of the enrolled Pakistani wild rodents were found infected with Toxoplasma gondii (Table 1). Prior to this study, there is only one report from Pakistan where PCR was used to document in the presence of Toxoplasma gondii in Pakistani rodents. Recently, Rizwan et al.8 had captured 236 rats including Rattus rattus and Rattus norvegicus from three districts of the Sahiwal division in Punjab (Pakistan) and reported the presence of Toxoplasma gondii in the brain of 5.9% rodents. The only other study available in literature on this topic from Pakistan was documented by Ahmad et al.9 as they had screened rodents and human samples (300 each) from Lahore in Punjab by using Latex Agglutination Test and reported the presence of this parasite in 58.57% of Rattus rattus, 36.66% of Mus musculus and 11.33% of enrolled humans. This limited data from Pakistan clearly indicates large scale screening of this parasite in local rodents in order to prevent its zoonotic transmission. A number of studies from various parts of the World have reported the presence of Toxoplasma gondii in a variety of rodent species. Ode et al.15 had reported that 76% central rock rats in Nigeria were Toxoplasma gondii infected. Hosseini et al.5 had reported 56% ELISA based prevalence of this parasite in Rattus rattus of Iran. In another study from Iran, Nazari et al.16 had found 35% prevalence of Toxoplasma gondii in three rodent species. Elamin17 reported the presence of this parasite in 11% of Rattus rattus captured from Riyadh in Saudi Arabia. Ruffolo et al.18 found 8% prevalence in Rattus rattus and Mus musculus trapped from Brazil. Kalmár et al.19 had reported that 7.3% of enrolled animals from Romania, including 9 rodent species, were infected by this protozoan parasite. Normaznah et al.20 reported 5.9% infection rate of Toxoplasma gondii in five rodent species from Malaysia. Mikhail et al.21 had reported 4% prevalence rate in Rattus norvegicus and Rattus rattus captured from Egypt. Manabella Salcedo et al.22 found 3.6% infection rate in Mus musculus that were captured from poultry farms in Argentine. These diverse findings underscore the global prevalence of Toxoplasma gondii in wild rodent populations and highlight the importance of ongoing surveillance and research.
The genetic diversity of Toxoplasma gondii in rodents has been cited in the literature but it has never been investigated in Pakistani rodents. Hence, we have we used the ITS-1 gene sequences of Toxoplasma gondii amplified during current study for the phylogenetic analysis. Nuclear internal transcribed spacer one (ITS-1) gene is among the common molecular targets that are in use for phylogenetic studies as its variability is relatively high and it the amplification is convenient23. BLAST analysis of the partial sequences confirmed that the rodents were infected with Toxoplasma gondii and the phylogenetic study revealed that the DNA sequence amplified during this investigation were genetically diverse as three of them (OR797081, OR797082 and OR797084) clustered together with Toxoplasma gondii sequences from quails (Accession number OR727859.1, unpublished data) and large ruminants (Accession number MW885251.124), from Pakistan, chicken and pigs from Malaysia (Accession numbers OP49060325) and from rams in Brazil (Accession numbers MH79350326), While the fourth Toxoplasma gondii haplotype amplified in this study (OR797083) clustered with the ITS-1 sequences of Toxoplasma gondii deposited from goats (Accession number FJ17623327), chicken (Accession number JF81094328), and wild birds (Accession number OL32310829), from Brazil, stray cats (Accession numbers JX45645730), and pork (Accession number MH553292, unpublished data) from China, dog from Sweden (Accession number U1616131), captive Parma Wallaby from Japan (Accession number LC722483, unpublished data), from Australia (Accession number L4939032), cats from Germany (Accession number EU02502533), rodents from Italy (Accession number HG79339434), chicken from Tunisia (Accession numbers ON072255 and ON514614, unpublished data), livestock milk from Mongolia (Accession number MH423902, unpublished data), cat feces from Thailand (Accession number KP89587235) from Iraq (Accession numbers OR672854, unpublished data) (Fig. 2). This extensive genetic diversity underscores the widespread distribution of Toxoplasma gondii and its potential transmission across diverse host species and geographical locations.
Analysis of risk factors indicated the prevalence of Toxoplasma gondii varied significantly among the analyzed rodents and Rattus norvegicus was the most susceptible rodent species to this parasitic infection followed by Rattus rattus and Mus musculus (Table 1). It was observed that this parasitic infection varied significantly between the sampling sites for Rattus norvegicus as well as for Rattus rattus but remained unaffected for Mus musculus (Table 2). We also observed that Toxoplasma gondii infection was not associated with the particular sex of all four rodent species enrolled in this study (Table 3). Our results are in agreement with a recent study from Pakistan where Rizwan et al.8 has reported that Toxoplasma gondii infection rates were significantly higher in Rattus norvegicus than in Rattus rattus. Similar trend has been reported from the other parts of the world as well. Kalmár et al.19 had also reported significant variation in Toxoplasma gondii infection among the nine rodent species enrolled from Romania. Rizwan et al.8 reported that rodent species, gender, location, season and habitat type were not associated with Toxoplasma gondii prevalence in wild rodents from Sahiwal division in Pakistan. Similar observations were documented by Kalmár et al.19 from Romania and Mikhail et al.21 from Egypt. On the other hand, Hosseini et al.5 had reported that Toxoplasma gondii infection was not restricted to a particular sex of rat but adult rats and those captured from rural areas were more prone to this parasitic infection than juvenile rats and those captured from urban areas in northern Iran. Nazari et al.16 had also reported that Toxoplasma gondii infection was not limited to a particular rodent species in Iran but males had higher rate of infection than females. Normaznah et al.20 had reported a significant variation in Toxoplasma gondii prevalence in rodent species that were trapped from various locations in Malaysia. These contrasting findings underscore the complexity of factors influencing Toxoplasma gondii infection dynamics among rodent species including geographical location, age and sex necessitating further research for a better understanding of these patterns.
Clinical pathology evaluation is common in rodents during the studies where effect of parasites or toxicants is to be determined. These analyses also help to identify disease in specific target organ as well as provide a general health profile of the individual animal36. Complete blood count analysis is among the most commonly used clinical pathology tools despite of the fact that acquisition and analysis of blood samples from rodents’ mice is problematic as their body size are not large and small sample volumes are usually obtained from a single animal37. Analysis of our complete blood count data revealed that Toxoplasma gondii infection had disturbed the Lymphocyte count, mean corpuscular volume, mean corpuscular hemoglobin concentration and mean platelet volume in Rattus norvegicus and Mus musculus but the overall blood profile of Rattus rattus remained unaffected when compared between parasite positive and negative animals (Table 4). The decreased lymphocyte count observed in this study is probably due to deficient regulation of inflammatory response due to Toxoplasma gondii mediated infection which is reported to be capable of tissue damage in inflammatory response38. The decreased mean platelet volume observed in infected rodents during this study is in line with the findings of by El-Henawy et al.39 as they had reported a significant decrease in platelet counts and associated parameters in Toxoplasma gondii infected human subjects. The disturbed hemoglobin associated parameters in Toxoplasma gondii infected rodents observed in this investigation are confirming the finding of Wang et al. 40 who had reported that Toxoplasma gondii-infected mice exhibited anemia due to a decrease in both erythropoiesis and survival time of red blood cells in the circulation. In a recent report, Ode et al.15 have documented a significant decrease in platelet count while an increased granulocyte: lymphocyte ratio and increased lymphocyte: monocyte ratio in Toxoplasma gondii infected wild rats (Zyzomys pedunculatus) from Nigeria as compared to uninfected animals. Similarly, Zoghroban et al.41 had also observed significant differences in the neutrophil, lymphocytes, monocyte, eosinophil and red blood cell counts in Swiss albino mice that were experimentally infected with Toxoplasma gondii as compared to uninfected animals. As several rodent species are preferred animal models, a few studies has reported the effect of Toxoplasma gondii infection on their complete blood count under laboratory conditions but there is no such data available in literature from wild rodents. Hence, the data generated in this study is adding to the existing knowledge regarding the effect of this one of the prevalent protozoan parasites on the blood cell count and associated parameters of three Pakistani wild rodent species.
Parasites are among the biotic factors of the environment that regulates the wild animals and they are known to affect their host by several ways. For example, they may reduce host’s growth, prevent them from reproducing or change their behavior42. Parasitic activities result in the stimulation of the host’s immune system that led to the generation of toxic oxidants that should counteracted by the antioxidant system of the host otherwise a state of oxidative stress may occur43. These oxidants are either produced due to increased metabolic process to combat the infection or they are generated by the cytotoxic cells to kill the pathogens44. Parasites themselves are known to release oxidants by the degradation of their own metabolic products. These toxic oxidants have the potential to damage host tissues. These oxidative damages may lead to degenerative pathologies that shorten the lifespan of host45. Defenses against reactive ions mediated damage include the enzymes catalase and as well as superoxide dismutase. Superoxide dismutases are metalloenzymes that are found in all life forms and are considered as first line of defense against the oxidative stress as they catalyze the dismutation of superoxide anion free radical (O2−) into molecular oxygen and hydrogen peroxide (H2O2)46. Catalase is an extremely efficient enzyme with the ability to break down millions of H2O2 molecules per second and convert them into water and oxygen in an energy-efficient manner 47. The free radicals generated in a cell following the parasitic activity attacks the lipids in the membranes, especially polyunsaturated fatty acids, and convert them into peroxide and hydroperoxide that trigger further reactions that lead to DNA and protein modifications: the process known as lipid peroxidation48. One of the end products of lipid peroxidation is malondialdehyde, an extremely toxic compound, which is usually determined to estimate the catastrophy of the membrane49.
Toxoplasma gondii is known to invade a number of the tissues of their diverse hosts including wild rodents but little has been reported regarding the response of host tissues to this infection through their antioxidant system5,50. Hence, we have compared the Superoxide dismutase, catalase (antioxidants) and Malondialdehyde (marker of oxidative stress) levels between Toxoplasma gondii infected and uninfected rodents. Analysis of the results indicated that the three rodent species should different response to Toxoplasma gondii infection as far as the markers of oxidative strength was concerned (Tables 5, 6 and 7). Like complete blood count analysis, the antioxidant profile from the vital organs of Rattus rattus remained unaffected when compared between Toxoplasma gondii infected and uninfected animals (Table 7). Infected Mus musculus had elevated malondialdehyde in kidney and liver while kidney of infected Rattus norvegicus had also significantly higher levels of this indicator of lipid peroxidation indication sever membrane damage following parasitic infection. Kidney and heart of Toxoplasma gondii infected Mus musculus and lungs of infected Rattus norvegicus had disturbed catalase concentrations that may lead to the accumulation of H2O2 in these cells that can trigger catastrophy leading to disturbed physiology. Prior to this data, there is only one report available in literature where et al. Nazarlu et al.51 had reported a significant decrease in superoxide dismutase, catalase, glutathione and total antioxidant capacity while the concentration of malondialdehyde was increased in the testes of Toxoplasma gondii infected male. Our results are indicating the markers if oxidative stress must be studies in all the tissues of rodents to get a better understanding of the pathophysiology of toxoplasmosis.
In conclusion, we are reporting a relatively higher Toxoplasma gondii infection in four Pakistani rodent species. Parasite prevalence varied between the rodent species and Rattus norvegicus had the highest rate of infection where more than one species members were found infected. Toxoplasma gondii infection disturbed the red, white blood cells and platelet associated parameters as well as markers of oxidative stress in liver, kidney and lungs of infected rodents. We recommend that similar and large-scale studies to be conducted in various geo-climatic regions of Pakistan to report the prevalence of Toxoplasma gondii in rodents for their effective control in order to prevent the zoonotic spread of this parasite through rodents.
Data availability
The datasets generated and/or analysed during the current study are available in the GenBank repository, with Accession numbers OR797081, OR797082, OR797083, OR797084 (https://www.ncbi.nlm.nih.gov/nuccore/OR797082.1https://www.ncbi.nlm.nih.gov/nuccore/OR797082.1https://www.ncbi.nlm.nih.gov/nuccore/OR797084.1 https://www.ncbi.nlm.nih.gov/nuccore/OR797083.1.
References
Rabiee, M. H., Mahmoudi, A., Siahsarvie, R., Kryštufek, B. & Mostafavi, E. Rodent-borne diseases and their public health importance in Iran. PLoS Negl. Trop. Dis.12 (4), e0006256. https://doi.org/10.1371/journal.pntd.0006256 (2018).
Khan, W. et al. Zoonotic and non-zoonotic helminths in black rats of rain-fed and irrigated areas of Swat, Khyber Pakhtunkhwa, Pakistan. Saudi J. Biol. Sci.28 (4), 2285–2290 (2021).
Naveed, A. et al. Seroprevalence and risk factors of Toxoplasma Gondii in Wild Birds of Punjab Province. Pakistan J. Wildl. Dis.55 (1), 129–135. https://doi.org/10.7589/2017-09-228 (2019).
Galal, L. et al. Diversity of Toxoplasma gondii strains shaped by commensal communities of small mammals. Int. J. Parasitol.49 (3–4), 267–275. https://doi.org/10.1016/j.ijpara.2018.11.004 (2019).
Hosseini, S. A. et al. Seroprevalence of Toxoplasma Gondii in Wild rats (Rattus rattus) in Northern Iran. Vet. Med. Int. 6655696. https://doi.org/10.1155/2021/6655696 (2021).
Ahmad, G. et al. Molecular prevalence, phylogeny and hematological impact of Toxoplasma Gondii and Plasmodium spp. in common quails from Punjab, Pakistan. PLOS ONE. 19 (5), e0304179. https://doi.org/10.1371/journal.pone.0304179 (2024).
Tokiwa, T. et al.ToxopGondiigondii infection in Amami spiny rat on Amami-Oshima Island, Japan. Int. J. Parasitol. Parasit. Wildlife9, 244–247 (2019).
Fredebaugh, S. L., Mateus, P. N. E., McAllister, M., Warner, R. E. & Weng, H. Y. Prevalence of antibody to Toxoplasma Gondii terrestrial wildlife in a natural area. J. Wildl. Dis.47, 381–392 (2011).
Rizwan, M., Ali, S., Javid, A. & Rashid, M. I. Molecular detection of Toxoplasma Gondii among commensal rodents from the Sahiwal division. Punjab Pakistan Parasitol. Res.122, 299–306. https://doi.org/10.1007/s00436-022-07729-8 (2023).
Ahmad, M. S., Maqbool, A., Mahmood-ul-Hassan, M., Mushtaq-ul-Hassan, M. & Anjum, A. A. Prevalence of Toxoplasma gondii antibodies in human beings and commensal rodents trapped from Lahore. Pakistan J. Anim. Plant. Sci.22 (1), 51–53 (2012).
Smithers, R. H. N. Land Mammals of Southern Africa: A Field Guide (MacMillan South Africa Pty Ltd., 1986).
Halov´, D. et al.ToxopGondiigondii in Ireland: seroprevalence and novel molecular detection method in sheep, pigs, deer and chickens. Zoon Pub. Health60(2), 168–173 (2012).
Hussain, M. F., Ashiq, M. N., Gulsher, M., Akbar, S. & Iqbal, F. Exposure to variable doses of nickel oxide nanoparticles disturbs serum biochemical parameters and oxidative stress biomarkers from vital organs of albino mice in a sex specific manner. Biomark. 25 (8), 719–724 (2020).
Selmi, R. et al. Zoonotic vector-borne bacteria in wild rodents and associated ectoparasites from Tunisia. Infect. Genet. Evol.95, 105039 (2021).
Ode, S. et al. High prevalence of Toxoplasma Gondii in Nigerian wild rats by molecular detection. Vet. Parasitol. Reg. Stud. Rep.35, 100776. https://doi.org/10.1016/j.vprsr.2022.100776 (2022).
Nazari, N. et al. Serological survey of Neospora Caninum and Toxoplasma Gondii Co-infection in rodents in Northwestern Iran. Iran. J. Parasitol.15 (2), 253–258 (2020).
Elamin, M. H. Genotyping of Toxoplasma gondii from rats (Rattus rattus) in Riyadh, Saudi Arabia. Kor J. Parasitol.52 (3), 257–261. https://doi.org/10.3347/kjp.2014.52.3.257 (2014).
Ruffolo, B. B. et al. Isolation and genotyping of Toxoplasma Gondii in seronegative urban rats and presence of antibodies in communicating dogs in Brazil. Rev. Inst. Med. Trop. Sao Paulo. 58, 28. https://doi.org/10.1590/S1678-9946201658028 (2016).
Kalmár, Z. et al. ToxopGondiigondii in small mammals in Romania: the influence of host, season and sampling location. BMC Vet. Res.19 (1), 177. https://doi.org/10.1186/s12917-023-03729-7 (2023).
Normaznah, Y. et al. Seroprevalence of Toxoplasma Gondii in rodents from various locations in Peninsular Malaysia. Southeast. Asia J. Tropic Med. Pub. Health46(3), 388–395 (2015).
Mikhail, M. W., Hasan, A. H., Allam, A. & Mohammed, N. M. K., Seroprevalence of Toxoplasma Gondii among commensal rodents from Giza Governorate. Egypt. J. Egypt. Soc. Parasitol. 47(1), 145–150 (2017).
Manabella Salcedo, I. et al. Role of Mus musculus in the transmission of several pathogens in poultry farms. Int. J. Parasitol. Parasit. Wildl.14, 130–136. https://doi.org/10.1016/j.ijppaw.2021.01.007 (2021).
Tippery, N. P. & Les, D. H. Phylogenetic analysis of the internal transcribed spacer (ITS) region in Menyanthaceae using predicted secondary structure. Mol. Phylogenet Evol.49 (2), 526–537 (2008).
Taalay, I. et al. Molecular survey of Toxoplasma Gondii in cattle and buffaloes and phylogenetic position of Pakistani isolates based on ITS-1 gene. Comp. Immunol. Microbiol. Infect. Dis.84, 101782. https://doi.org/10.1016/j.cimid.2022.101782 (2022).
Leong, S. D., Hassan, L., Sharma, R. S. K., Toung, O. P. & Musa, H. I. Prevalence and haplotypes of Toxoplasma gondii in native village chickens and pigs in Peninsular Malaysia. Vet. Sci.10(5), 334. https://doi.org/10.3390/vetsci10050334 (2023).
de Oliveira Koch, M. et al. Detection of Neospora Caninum and Toxoplasma Gondii in Semen of naturally infected rams. Acta Scient Veterinar. 47(1). https://doi.org/10.22456/1679-9216.92090 (2019).
Silva, M. S. et al. Detection of Hammondia heydorni and related coccidia (Neospora Caninum and Toxoplasma Gondii) in goats slaughtered in Bahia, Brazil. Vet. Parasitol.162 (1–2), 156–159 (2009).
Gonçalves, I. N. et al. Molecular frequency and isolation of cyst-forming coccidia from free ranging chickens in Bahia State, Brazil. Vet. Parasitol.190 (1–2), 74–79 (2012).
Liano, H. A. B. et al. Molecular screening for Sarcocystidae in muscles of wild birds from Brazil suggests a plethora of intermediate hosts for Sarcocystis falcatula. Int. J. Parasitol. Parasit. Wildl.17, 230–238. https://doi.org/10.1016/j.ijppaw.2022.03.002 (2022).
Wang, Q. et al. Prevalence of Toxoplasma gondii antibodies, circulating antigens and DNA in stray cats in Shanghai. China Parasit. Vect. 5, 190. https://doi.org/10.1186/1756-3305-5-190 (2012).
Holmdahl, O. J. & Mattsson, J. G. Rapid and sensitive identification of Neospora caninum by in vitro amplification of the internal transcribed spacer 1. Parasitol. 112 (Pt 2), 177–182. https://doi.org/10.1017/s0031182000084742 (1996).
Payne, S. & Ellis, J. Detection of Neospora caninum DNA by the polymerase chain reaction. Int. J. Parasitol.26 (4), 347–351. https://doi.org/10.1016/0020-7519(96)00030-6 (1996).
Schares, G., Vrhovec, M. G., Pantchev, N., Herrmann, D. C. & Conraths, F. J. Occurrence of Toxoplasma Gondii and Hammondia Hammondi oocysts in the faeces of cats from Germany and other European countries. Vet. Parasitol.152 (1–2), 34–45. https://doi.org/10.1016/j.vetpar.2007.12.004 (2008).
Zanzani, S. A. et al. Parasitic and bacterial infections of Myocastor coypus in a Metropolitan Area of Northwestern Italy. J. Wildl. Dis.52 (1), 126–130. https://doi.org/10.7589/2015-01-010 (2016).
Chemoh, W., Sawangjaroen, N., Nissapatorn, V. & Sermwittayawong, N. Molecular investigation on the occurrence of Toxoplasma Gondii oocysts in cat feces using TOX-element and ITS-1 region targets. Vet. J.215, 118–122 (2016).
Wiedmeyer, C. E., Ruben, D. & Franklin, C. Complete blood count, clinical chemistry, and serology profile by using a single tube of whole blood from mice. J. Amr Assoc. Lab. Anim. Sci.46 (2), 59–64 (2007).
McClure, D. E. Clinical pathology and sample collection in the laboratory rodent. Vet. Clin. North. Am.2, 565–589 (1999).
Chan, L. et al. The roles of neutrophils in cytokine storms. Virus. 13 (11), 2318. https://doi.org/10.3390/v13112318 (2021).
El-Henawy, A. E. et al. Is toxoplasmosis a potential risk factor for liver cirrhosis? Asian Pac. J. Trop. Med.8 (10), 784–791 (2015).
Wang, Z., Zhang, D. X. & Zhao, Q. Infection-stimulated anemia results primarily from interferon gamma-dependent, signal transducer and activator of transcription 1-independent red cell loss. Chin. Med. J. 128 (7), 948–955. https://doi.org/10.4103/0366-6999.154303 (2015).
Zoghroban, H. S. et al. Novel insights on the therapeutic effect of levamisole on the chronic toxoplasmosis in mice model. Exp. Parasitol. 248. https://doi.org/10.1016/j.exppara.2023.108515 (2023).
Borgsteede, F. H. The effect of parasites on wildlife. Vet. Quart.18 (Suppl 3), S138–S140 (1996).
Dowling, D. K. & Simmons, L. W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745 (2009). https://doi.org/10.1098/rspb.2008.1791
Demas, G. E., Chefer, V., Talan, M. I. & Nelson, R. J. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. Reg.273, R1631–R1637 (1997).
Janske, V. D. C., Richardson, D. S., Koltz, A. M., Hutchings, K. & Komdeur, J. Parasitic infection and oxidative status are associated and vary with breeding activity in the Seychelles warbler. Proc. R. Soc. B. 2791466–1476 (2012).
Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci.12 (3), 88–93 (2018).
Xu, D., Wu, L., Yao, H. & Zhao, L. Catalase-like nanozymes: classification, catalytic mechanisms, and their applications. Small18, 2203400. https://doi.org/10.1002/smll.202203400 (2022).
Ayala, A., Muñoz, M. F. & Argüelles, S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidat. Med. Cell. Longevit. 360438. https://doi.org/10.1155/2014/360438 (2014).
Moldovan, L. & Moldovan, N. I. Oxygen free radicals and redox biology of organelles. Histochem. Cell. Biol.122 (4), 395–412 (2004).
Szewczyk-Golec, K., Pawłowska, M., Wesołowski, R., Wróblewski, M. & Mila-Kierzenkowska, C. Oxidative stress as a possible target in the treatment of Toxoplasmosis: perspectives and ambiguities. Int. J. Mol. Sci.22 (11), 5705. https://doi.org/10.3390/ijms22115705 (2021).
Nazarlu, H. A., Matini, Z., Bahmanzadeh, M. & Foroughi-Parvar, F. Toxoplasma gondii: A possible inducer of oxidative stress in reproductive system of male rats. Iran. J. Parasitol.15(4), 521–529. https://doi.org/10.18502/ijpa.v15i4.4857 (2020).
Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol.16, 111–120 (1980).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol.35, 1547–1549 (2018).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R197), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
FI and AdK had designed and supervised this study. AUK and SU collected the rodent samples and epidemiological data. MI performed the wet lab experiments. AdK and AfK performed the phylogenetic analysis. SI, BS and YABJ performed the statistical analysis. All authors contributed to the writing of manuscript and approved the final version for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All methods and experiments were approved by the ethical review committee of Bahauddin Zakariya University Multan, Pakistan (BZU/Ethics/Zool- 29-2022). Importantly, all methods were performed in accordance with the relevant guidelines and regulations.
ARRIVE guidelines
All the methods were performed in accordance with ARRIVE guidelines laws and regulations.
Informed consent
There are no human subjects in this article and informed consent is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ijaz, M., Khan, A.U., Ullah, S. et al. Toxoplasma gondii infection affects the complete blood count and disturbs the markers of oxidative stress from the vital organs of wild rodents. Sci Rep 14, 22716 (2024). https://doi.org/10.1038/s41598-024-73265-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73265-3
Keywords
This article is cited by
-
Unveiling Hidden Threats: Molecular Detection and Genetic Diversity of Hepatozoon spp. and Toxoplasma Gondii in Wild Rodents of Saudi Arabia and Their Ectoparasites
Acta Parasitologica (2026)
-
First report of Hepatozoon and Lankesterella spp. infections in wild rodents from Pakistan, and their potential impact on blood parameters and oxidative stress markers in vital organs
Veterinary Research Communications (2025)
-
Molecular prevalence and genetic diversity of Toxoplasma gondii in free ranging Asil hen (Gallus gallus domesticus)
Tropical Animal Health and Production (2025)