Abstract
Muscle stem cells (MuSCs) are effective in treating inflammatory diseases driven by overactive innate immune responses, such as colitis and acute lung injury, due to their immunomodulatory properties. However, their potential in treating diseases driven by adaptive immune responses is still uncertain. When primed with inflammatory cytokines, MuSCs strongly suppressed T cell activation and proliferation in vitro in co-culture with activated splenocytes or peripheral blood mononuclear cells. Systemic administration of MuSCs from both mice and humans alleviated pathologies in mice with concanavalin A-induced acute liver injury, characterized by hyperactivated T lymphocytes. Importantly, MuSCs showed significant species-specific differences in their immunoregulatory functions. In mouse MuSCs (mMuSCs), deletion or inhibition of inducible nitric oxide synthase (iNOS) reduced their immunosuppressive activity, and absence of iNOS negated their therapeutic effects in liver injury. Conversely, in human MuSCs (hMuSCs), knockdown or inhibition of indoleamine 2,3-dioxygenase (IDO) eliminated their immunosuppressive effects, and loss of IDO function rendered hMuSCs ineffective in treating liver injury in mice. These results reveal significant species-specific differences in the mechanisms by which MuSCs mediate T cell immunosuppression. Mouse MuSCs rely on iNOS, while human MuSCs depend on IDO expression. This highlights the need to consider species-specific responses when evaluating MuSCs’ therapeutic potential in immune-related disorders.
Similar content being viewed by others
Introduction
Muscle stem cells (MuSCs), also known as satellite cells, are a distinct class of stem cells positioned between the muscle sarcolemma and the basal lamina of individual myofibers, and are essential to the processes of muscle development and regeneration. In response to muscle tissue damage resulting from injury, physical exertion, or diseases, these cell populations are roused from a quiescent state into an activated state, beginning their proliferation and differentiate into myoblasts, which then either merge with the existing damaged muscle fibers to rehabilitate them or amalgamate among themselves to forge new muscle fibers1,2. Apart from replacing damaged tissues through myogenic lineage differentiation, MuSCs can utilize their immunoregulatory abilities to promote inflammation resolution, thereby facilitating regeneration and healing3. For instance, MuSCs endow maturing macrophages with an oxidative phosphorylation (OXPHOS) preference by producing insulin-like growth factor 2 (IGF2), thereby shaping anti-inflammatory properties of maturing macrophages and thus ameliorating dextran sulfate sodium-induced inflammatory bowel disease4.
Moreover, preconditioning of MuSCs before their infusion by priming with inflammatory cytokines that mimic the inflammatory tissue microenvironment of injured sites would enhance their immunosuppressive properties and reinforce therapeutic efficacy for ulcerative colitis and acute lung injury in a TNF-stimulated gene 6 (TSG6)-dependent manner. On one hand, the kynurenine pathway initiated by indoleamine 2,3-dioxygenase (IDO), utilizes tryptophan to produce kynurenic acid, which functions as an endogenous aryl hydrocarbon receptor (AhR) ligand, thereby increasing TSG6 expression in activated MuSCs5. On the other hand, interleukin-4-induced-1 (IL4I1)-catalyzed indole generation serves as an alternate route of tryptophan catabolism in inflammatory cytokine-primed MuSCs, where indole-3-pyruvic acid and indole-3-aldehyde activate AhR, which directly binds to the TSG6 promoter to enhance its production6. The immunoregulatory properties of MuSCs on innate immune responses further expand their potential applications in immunological diseases beyond muscle-related diseases. However, whether MuSCs possess immunosuppressive functions on adaptive immune responses remains unknown.
The immune systems of humans and mice share several fundamental similarities, which render mice an invaluable model for studying human immunology and developing treatments for inflammatory diseases. However, researchers must consider notable differences when extrapolating findings from mice to humans, particularly in the field of stem cell research. Mesenchymal stem/stromal cells (MSCs) represent a paradigmatic example in immunomodulation. In this context, MSCs from different mammalian species exhibit distinct immunosuppressive mechanisms, with those from mice, rats, rabbits, and hamsters relying on inducible nitric oxide synthase (iNOS), while those from monkeys, pigs, and humans utilize IDO7. The selection of appropriate animal models for preclinical studies of MSC-based therapies is a critical concern8. Nevertheless, it remains unknown whether MuSCs employ differential mechanisms that account for adaptive immune regulation in different species.
In this study, systemic administration of MuSCs from mice and humans was found to dampen the pathological conditions in mice with concanavalin A (ConA)-induced acute liver injury caused by hyperactivated T lymphocytes. MuSCs primed with inflammatory cytokines demonstrated strongly suppressive effects on the activation and proliferation of T cells in in vitro co-culture systems. Interestingly, different immunosuppressive molecules were involved in the immunoregulatory functions of MuSCs in the two species. MuSCs derived from mice utilized iNOS for immunosuppression, while MuSCs from humans relied on IDO expression. Therefore, our study highlights species variation in the mechanisms of MuSC-mediated immunosuppression affecting T cells.
Materials and methods
Animals
C57BL/6J mice were obtained from SLAC Laboratory Animal Co., LTD (Shanghai, China) and maintained under a specific pathogen-free barrier facility of the Laboratory Animal Center of Soochow University. All mice used for tissue harvest or experimental procedures were males aged between 10 and 12 weeks (20 ~ 25 g). These mice were housed in ventilated cages at 21 °C to 23 °C with a 12 h light-dark cycle. The relative humidity in the animal facility was between 40% and 60%. Irradiated food purchased from Double Lion Experimental Animal Feed Technology Co., LTD (Suzhou, China) and sterile water were provided ad libitum. This study was reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, and approved by the Ethics Committee of Soochow University (Approval Number: SUDA20210916A02). All methods were performed in accordance with the relevant guidelines and regulations.
Musc isolation and culture
mMuSCs and hMuSCs were isolated and expanded as previously described9,10,11,12. For mMuSC isolation, hind limb muscles of C57BL/6J mice were dissected. For hMuSC isolation, surgical specimens of the gastrocnemius, debrided tissues from patients with orthopedic trauma, were obtained with the informed consent of the patients in accordance with the Ethics Committee of the First Affiliated Hospital of Soochow University (Approval Number: ECSU-201900214). These muscle tissues from different sources were gently and rapidly minced with scissors and then incubated with collagenase II (800 U/mL, from Invitrogen, Carlsbad, CA) solution prepared in wash medium containing Ham’s F-10 supplemented with 10% fetal bovine serum (FBS, from Gibco, San Francisco, CA) and 1× penicillin-streptomycin (Gibco) for 1 h at 37 °C on a shaker (60~70 r.p.m.). Digested tissues were then washed once with cold wash medium and centrifuged at 500g for 5 min at 4 °C. Second incubation was then performed by adding collagenase II (100 U/mL, from Invitrogen) and Dispase (11 U/mL, from Invitrogen) solution for 30 min at 37 °C on a shaker (60~70 r.p.m.). Digested tissues were then filtered through a 40µm nylon cell strainer to generate a mononucleated cell suspension ready for an antibody staining. Resuspended cells were stained using the following antibodies: PE-conjugated rat anti-mouse CD31 (Cat# 160203), PE-conjugated rat anti-mouse CD45 (Cat# 103105), APC-conjugated rat anti-mouse Sca1 (Cat# 108111) and Pacific Blue™-conjugated rat anti-mouse VCAM1 (Cat# 105722, all from BioLegend, San Diego, CA). All antibodies were used at ~ 1 µg per 107 cells. The staining samples were placed on a nutating rocker and incubated with antibodies for 40 min at 4 °C. mMuSCs from mouse hind limb muscles marked as VCAM1+CD31−CD45−Sca1− population were obtained by fluorescence-activated cell sorting. hMuSCs were characterized as CD29+CD56+CD31−CD34−CD45− through fluorescence-activated cell sorting.
Plastic tissue culture dishes and plates were precoated with extracellular matrix (ECM) gel (Sigma-Aldrich, St Louis, MO) before culture. mMuSCs were then serially expanded every 2 days in mouse myogenic cell proliferation medium containing Ham’s F-10 supplemented with 20% FBS, 5 ng/ml IL-1α, 5 ng/ml IL-13, 10 ng/ml IFN-γ and 10 ng/ml TNF-α, 2.5 ng/ml bFGF (all from Peprotech, Cranbury, NJ) and 1× penicillin-streptomycin. hMuSCs were serially cultured in human myogenic cell growth medium containing 1:1 mixture of DMEM and MCDB 131 supplemented with 20% FBS, 1% insulin-transferrin-selenium (Gibco), 1% penicillin-streptomycin and 10 µM p38 MAPK inhibitor (SB203580, from Selleck, Houston, TX), which functions to prevent spontaneous differentiation of hMuSCs as well as enhance their self-renewal and engraftment potential ex vivo11. In addition, all cultured MuSCs were differentiated in myogenic cell differentiation medium containing DMEM supplemented with 5% horse serum (Gibco) and 1× penicillin-streptomycin for 3 days. All cell culture experiments were performed under 5% CO2 and atmospheric oxygen at 37 °C in a humidified incubator without mycoplasma contamination. All details regarding the confirmation of the myogenicity of cultured mMuSCs and hMuSCs were showed in the Supplementary Figs. 1 and 2.
IDO knockdown
To achieve IDO knockdown, hMuSCs were transfected with IDO-targeting short hairpin RNA (shRNA) carried on a lentivirus vector (PGLV3/H1/GFP/Puro, from GenePharma, Shanghai, China) and incubated with polybrene (5 µg/mL, from GenePharma) for 12 h. The shRNA target sequence for IDO was 5’-GCAGACTGTGTCTTGGCAAAC-3’. The scrambled shRNA target sequence was 5’-TTCTCCGAACGTGTCACGT-3’. Puromycin (5 µg/mL, from Gibco) was added to the culture medium to screen successfully transfected cells.
T cell proliferation assay
The proliferation of T cells in splenocyte populations and peripheral blood mononuclear cell (PBMC) populations was detected using carboxyfluorescein diacetate succinimidyl ester (CFSE) staining and flow cytometry13. Briefly, freshly isolated splenocytes and PBMCs in 1 ml of PBS containing 0.1% bovine serum albumin (BSA, from Sangon Biotech, Shanghai, China) were stained with 5 µM CFSE dye (Invitrogen) for 10 min in a 37 °C water bath, with incessant mixing every 2 min. The reaction was stopped by adding 1 ml of cold FBS. After 5 min, cells were washed with 5% FBS in PBS. Cells were activated with purified anti-mouse CD3ε antibody (clone: 145-2C11, Cat# 100301, from BioLegend) or purified anti-human CD3 SF antibody (clone: OKT3, Cat# 317353, from BioLegend) and respectively co-cultured with mMuSCs or hMuSCs. After 72 h, cells were collected and the remaining cell-associated CFSE fluorescence was analyzed by flow cytometry.
Generation and analysis of murine acute liver injury model
Male C57BL/6J mice were intravenously injected with ConA (Sigma-Aldrich) in PBS at 15 mg/kg to induce acute liver injury. mMuSCs (1 × 106) cultured with cytokine cocktail or hMuSCs (5 × 105) primed with human IFN-γ (10 ng/ml) and TNF-α (10 ng/ml) for 24 h were then intravenously administrated into mice that have been treated with ConA for 30 min. After 12 h, mice were euthanized through 70% (VDR/min) carbon dioxide and subsequent cervical dislocation, and serum and liver tissues were sampled. Serum alanine transaminase (ALT) and aspartate transaminase (AST) activity were respectively determined by an ALT detection kit (Cat. No.: C009-2-1) and an AST detection kit (Cat. No.: C010-2-1, from Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Additionally, serum IFN-γ and IL-12 levels were respectively determined by the mouse IFN-γ ELISA kit (Cat. No.: PI508) and the mouse IL-12 ELISA kit (Cat. No.: PI530, from Beyotime, Shanghai, China).
Histological analysis
Liver tissue samples were fixed in 4% paraformaldehyde for 2 days, sequentially dehydrated through treatment with 75% ethanol (overnight), 85% ethanol (1 h), 95% ethanol (1 h), and 100% ethanol (1 h). The samples were treated with xylene for 20 min twice before embedded in paraffin and cut into 3µm sections by rotary microtome (Leica, Wetzlar, Germany). Histological analysis was performed using the standard hematoxylin and eosin (H&E) staining process to show centrilobular necrosis in liver.
Isolation of liver mncs and flow cytometric analysis
The liver samples were excised from mice suffering acute liver injury, weighed and digested in DMEM medium containing 10% FBS, collagenase I (1 mg/mL), collagenase II (1 mg/mL) and DNAase (0.1 mg/ml, from Gibco) at 37 °C for 1 h on a shaker (60~70 r.p.m.). Digested liver tissues were then passed through a 70µm cell strainer to generate a single cell suspension ready for 70%/40% percoll separation. Finally, collected cells for flow cytometric analysis were pre-incubated with purified anti-mouse CD16/32 antibody (Cat# 101301, from BioLegend) to block Fc receptors, and then stained with APC-conjugated anti-mouse CD45 antibody (Cat# 103137, from BioLegend) and FITC-conjugated anti-mouse CD3ε antibody (Cat# 100305, from BioLegend) at 4 °C for 20 min. Then, total cell numbers of T cells infiltrated in liver were determined through the Cytoflex Flow Cytometer (Beckman Coulter, Brea, CA).
Quantitative real time polymerase chain reaction
Total RNA from each sample was extracted using RNAfast200 total RNA rapid extraction kit (Feijie, Shanghai, China) and reverse-transcribed using PrimeScript™ RT Master Mix (TaKaRa, Dalian, China) following the manufacturers’ instructions. Gene expression was measured by QuantStudio™ 6 Flex System (Applied Biosystems, Foster City, CA) using PowerUp SYBR Green Mater Mix (Applied Biosystems). Total amount of mRNA was normalized to endogenous mouse β-actin mRNA or human β-ACTIN mRNA. The primers of the target genes were designed and listed as shown in Supplementary Table 1 of supplementary information.
Western blotting analysis
Cells were washed twice with cold PBS, harvested and lysed in the RIPA buffer (Beyotime) containing a cocktail of protease inhibitors (Roche, Nutley, NJ) and PMSF (Beyotime) for 30 min on ice. Lysates were collected by centrifugation at 14,000g for 20 min and heated in sodium dodecyl sulfate sample buffer at 95 °C for 10 min. Protein concentration of the supernatant was determined by Pierce™ BCA Protein Assay Kits (Thermo Scientific™, Shanghai, China). Protein samples were separated on a polyacrylamide gel, transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Temecular, CA) and incubated for 1 h in 5% nonfat dry milk dissolved in TBST (150 mM NaCl, 50 mM Tris-HCl, 0.05% Tween 20, pH 7.5) at room temperature. According to the molecular weight of the target proteins, the PVDF membranes were severally cut and incubated with primary antibodies overnight at 4 °C. The primary antibodies included anti-mouse iNOS antibody (Cat# 13120), anti-mouse β-actin antibody (Cat# 8457), anti-human IDO antibody (Cat# 86630), anti-human iNOS antibody (Cat# 20609) and anti-human β-ACTIN antibody (Cat# 4970, all from Cell Signaling Technology, Danvers, MA). The membrane was then extensively washed in TBST, incubated with anti-rabbit IgG, horseradish peroxidase (HRP)-linked antibody (Cat# 7047, from Cell Signaling Technology) for 2 h at room temperature, and washed again with TBST. The blotting membranes were developed with chemiluminescent detection and imaged using the FluorChem E Multi-Imager System from ProteinSimple or the Touch Imager Multi-Imager System from E-BLOT. Unprocessed original images of gels and western blots were provided in the supplementary information.
NO measurement
NO was measured using a modified Griess reagent (Sigma-Aldrich). Briefly, all NO was converted into NO2 by nitrate reductase, and total NO2 was detected by the Griess reaction.
Statistical analysis
All data were represented as the mean and standard error of the mean (mean ± SEM) as specified in the figure legends. Statistical comparisons were made using the GraphPad Prism software (Version 8.0, GraphPad Prism Software Inc., San Diego, CA). The number of each treatment group was indicated as “n” in the figure legends. The statistical analyses used were indicated in the corresponding figure legends. P values were shown in the corresponding figures. Notably, P values less than 0.05 were considered statistically significant.
Results
Mmuscs demonstrate therapeutic efficacy for acute liver injury
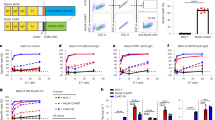
To explore whether MuSCs could orchestrate adaptive immune responses, we employed an in vitro co-culture system, in which mouse MuSCs (mMuSCs) were co-cultured with homogeneous splenocytes at graded ratios (mMuSC-to-splenocyte) for 3 days. First, we activated splenocytes with anti-CD3 antibody (clone: 145-2C11), which specifically activated T cells through binding to T cell-specific CD3ε receptor. Subsequently, the proliferation of T cells was measured by the carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay (Fig. 1A). This anti-CD3 antibody activated splenocyte system has been extensively utilized to verify the immunosuppressive effects of MSCs in our previous studies13,14. As shown in Fig. 1B, mMuSCs markedly inhibited the proliferation of splenocytes in a cell density-dependent manner. We next sought to determine whether their immunosuppressive ability was instrumental for the treatment of inflammatory diseases driven by excessive adaptive immunity. ConA-induced liver injury is a well-recognized in vivo model of hepatocyte apoptosis resulting from acute adaptive immune responses, where hyperactivated T lymphocyte proliferation is the major contributor15,16. In this context, mMuSCs dramatically alleviated liver damage, leading to substantial reductions in serum alanine transaminase (ALT) and aspartate transaminase (AST) levels, proinflammatory mediators, and centrilobular necrosis (Fig. 1C-F). Detailed analyses of immune cells in the liver revealed that the infiltration of mononuclear cells (MNCs) and CD3+ T cells was significantly reduced in mice treated with mMuSCs (Fig. 1G). Collectively, these data suggest that mMuSCs possess immunosuppressive functions on adaptive immune responses.
Mouse muscle stem cells (mMuSCs) alleviate acute liver injury. (A) Splenocytes stained with CFSE were co-cultured with mMuSCs (2.5 × 104 cells per well in 48-well plates) at graded ratios (mMuSC : splenocyte) for 72 h in the presence of anti-mouse CD3 antibody (1 µg/ml). CFSE fluorescence intensity reduction of splenocytes was detected by flow cytometry. (B) The proliferation of splenocytes was measured by the CFSE dilution assay (n = 4). (C) Mice were intravenously injected with ConA (15 mg/kg) to induce acute liver injury; 0.5 h later, mMuSCs (1 × 106) were transfused to suffering mice in the same way. After 12 h, serum and liver tissues were sampled. (D) Serum levels of AST and ALT were measured. (E) IFN-γ and IL-12 levels in the serum were assayed by ELISA. (F) Representative H&E-stained liver sections and percentages of tissue necrosis (Control: n = 5, ConA + PBS: n = 5, ConA + mMuSC: n = 5). Scale bar, 250 μm. (G) Absolute numbers of MNCs in liver tissues were calculated. Absolute numbers of CD3+ T cells were determined by flow cytometry (Control: n = 3, ConA + PBS: n = 6, ConA + mMuSC: n = 5). Values are presented as mean ± SEM. Statistical analysis was performed by Mann-Whitney test.
Inflammatory priming induces high expression of several chemokines and inos in myogenic cells
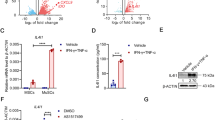
Activated T cells can secrete a large array of inflammatory factors, notably interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). They activate MSCs and induce the secretion of ligands for CC-chemokine receptor 5 (CCR5) and CXC-chemokine receptor 3 (CXCR3), including CC-chemokine ligand 2 (CCL2), CCL5, CXC-chemokine ligand 9 (CXCL9), CXCL10 and CXCL11, which recruit T cells to the proximity of MSCs. Subsequently, MSCs suppress the proliferation and activities of T cells in their vicinity by expressing iNOS, particularly in murine MSCs17,18. To determine whether the immunoregulative functions of mMuSCs depend on the chemokine-iNOS axis, we utilized myogenic C2C12 cells. Although C2C12 cells do not exhibit all the features of mMuSCs, they nonetheless retain several shared immunological characteristics. As expected, IFN-γ/TNF-α-primed mMuSCs expressed very high levels of several leukocyte chemokines, most notably Ccl2, Ccl5, Cxcl9, Cxcl10, and Cxcl11, which are well-known chemoattractants for T cells (Fig. 2A-E). These C2C12 cells preconditioned with inflammatory cytokines also exhibited substantial iNOS expression, resulting in the production of large amounts of nitric oxide (NO) that could further inhibit the proliferation of T cells (Fig. 2F-H). These results demonstrate that inflammatory cytokines can initiate the immunoregulative capacities of myogenic cells on adaptive immune responses.
Inflammatory priming provokes the expression of high levels of several chemokines and iNOS by C2C12 cells. (A-F) Expression levels of chemokines and iNOS in C2C12 cells stimulated with murine IFN-γ and TNF-α (10 ng/ml each) for 24 h were assayed by qRT-PCR (n = 3). (G) The protein expression of iNOS and β-actin (loading control) were determined by immunoblotting in C2C12 cells stimulated with murine IFN-γ and TNF-α (10 ng/ml each) for 24 h (n = 3). The original images of blots are in the Supplementary Information. (H) C2C12 cells were stimulated with murine IFN-γ and TNF-α (10 ng/ml each) for 24 h and the supernatants were assayed for nitrate by a modified Griess reagent (n = 3). Values are presented as mean ± SEM. Statistical analysis was performed by two-tailed unpaired t test.
Mmusc-mediated immunosuppression requires iNOS expression
To verify whether mMuSCs exert immunosuppressive effects on the proliferation of activated T cells by producing high concentrations of NO through the expression of iNOS, we employed a specific inhibitor, NG-monomethyl-L-arginine (L-NMMA), in the mMuSC co-culture system with activated splenocytes, which selectively inhibits the activity of iNOS in catalyzing arginine to produce NO19. While the proliferation of activated splenocytes was markedly inhibited by mMuSCs, this inhibition was partially reversed upon the addition of L-NMMA (Fig. 3A). As further evidence of the pivotal role of iNOS, we found that mMuSCs derived from iNOS-deficient mice (iNOS−/−) were incapable of suppressing anti-CD3 antibody-induced splenocyte proliferation in the absence of NO (Fig. 3B-D). We next employed the ConA-induced acute liver injury model to further validate the mechanism of mMuSC-mediated immunomodulation in vivo. In this context, a single intravenous injection of mMuSCs from wild-type (WT) mice significantly alleviated liver damage, as evidenced by a dramatic reduction in serum ALT and AST levels, proinflammatory mediators, and centrilobular necrosis. Following the intravenous administration of WT mMuSCs, there was a diminished infiltration of MNCs and CD3+ T cells at the damaged sites. However, these beneficial effects were not observed in mice treated with iNOS−/− mMuSCs (Fig. 3E-I), suggesting that the therapeutic efficacy of mMuSCs in the inflammatory disease is dependent on iNOS. Taken together, these data demonstrate that iNOS is a key mediator of T cell immunosuppression by mMuSCs.
The beneficial effects of mMuSCs on acute liver injury require iNOS. (A) Splenocytes stained with CFSE were co-cultured with mMuSCs (2.5 × 104 cells per well in 48-well plates) at a ratio of 1 : 20 (mMuSC : splenocyte) for 72 h in the presence of anti-mouse CD3 antibody (1 µg/ml) with or without the iNOS inhibitor L-NMMA (1 mM). CFSE fluorescence intensity reduction of splenocytes was detected by flow cytometry to measure the proliferation of splenocytes (n = 4). (B) The protein expression of iNOS and β-actin (loading control) were determined by immunoblotting in WT mMuSCs and iNOS−/− mMuSCs (n = 3). The original images of blots are in the Supplementary Information. (C) The supernatants of WT mMuSCs and iNOS−/− mMuSCs were assayed for nitrate by a modified Griess reagent (n = 3). (D) Splenocytes stained with CFSE were co-cultured with WT mMuSCs or iNOS−/− mMuSCs (2.5 × 104 cells per well in 48-well plates) at graded ratios (mMuSC : splenocyte) for 72 h in the presence of anti-mouse CD3 antibody (1 µg/ml). CFSE fluorescence intensity reduction of splenocytes was detected by flow cytometry to measure the proliferation of splenocytes (n = 4). (E) Mice were intravenously injected with ConA (15 mg/kg) to induce acute liver injury; 0.5 h later, WT mMuSCs or iNOS−/− mMuSCs (1 × 106) were transfused to suffering mice in the same way. After 12 h, serum and liver tissues were sampled. (F) Serum levels of AST and ALT were measured. (G) IFN-γ and IL-12 levels in the serum were assayed by ELISA. (H) Representative H&E-stained liver sections and percentages of tissue necrosis (Control: n = 4, ConA + PBS: n = 5, ConA + WT mMuSC: n = 5, ConA + iNOS−/− mMuSC: n = 5). Scale bar, 250 μm. (I) Absolute numbers of MNCs in liver tissues were calculated. Absolute numbers of CD3+ T cells were determined by flow cytometry (Control: n = 4, ConA + PBS: n = 5, ConA + WT mMuSC: n = 4, ConA + iNOS−/− mMuSC: n = 5). Values are presented as mean ± SEM. Statistical analysis was performed by one-way analysis of variance test (A),two-tailed unpaired t test (C) or Mann-Whitney test (F-I).
Hmuscs demonstrate therapeutic efficacy for acute liver injury
To investigate whether MuSCs from other mammalian species can modulate adaptive immune responses, we utilized an in vitro co-culture system, where human MuSCs (hMuSCs) were co-cultured with human peripheral blood mononuclear cells (PBMCs) at graded ratios (hMuSC-to-PBMC) for 3 days. Initially, we stimulated PBMCs with anti-CD3 antibody (clone: OKT3), which selectively activated T cells by binding to the T cell-specific CD3 receptor. Subsequently, the proliferation of T cells was measured by the CFSE dilution assay (Fig. 4A). This anti-CD3 antibody-stimulated PBMC system has been extensively employed to confirm the immunosuppressive effects of human MSCs in our previous research20. As depicted in Fig. 4B, hMuSCs significantly suppressed the proliferation of PBMCs in a manner dependent on cell density. Next, we aimed to ascertain whether their immunosuppressive capacity was beneficial for treating inflammatory diseases driven by excessive adaptive immunity. In this regard, hMuSCs significantly ameliorated liver damage, resulting in substantial decreases in serum levels of ALT and AST, proinflammatory mediators, and centrilobular necrosis (Fig. 4C-F). Detailed analysis of immune cells in the liver revealed a significant reduction in the infiltration of MNCs and CD3+ T cells in mice treated with hMuSCs (Fig. 4G). Overall, these findings demonstrate that hMuSCs possess immunosuppressive capabilities against adaptive immune responses.
Human muscle stem cells (hMuSCs) alleviate acute liver injury. (A) PBMCs stained with CFSE were co-cultured with hMuSCs (2.5 × 104 cells per well in 48-well plates) at graded ratios (hMuSC : PBMC) for 72 h in the presence of anti-human CD3 antibody (1 µg/ml). CFSE fluorescence intensity reduction of PBMCs was detected by flow cytometry. (B) The proliferation of PBMCs was measured by the CFSE dilution assay (n = 4). (C) Mice were intravenously injected with ConA (15 mg/kg) to induce acute liver injury; 0.5 h later, hMuSCs (5 × 105) stimulated with human IFN-γ and TNF-α (10 ng/ml each) were transfused to suffering mice in the same way. After 12 h, serum and liver tissues were sampled. (D) Serum levels of AST and ALT were measured. (E) IFN-γ and IL-12 levels in the serum were assayed by ELISA. (F) Representative H&E-stained liver sections and percentages of tissue necrosis. Scale bar, 250 μm. (G) Absolute numbers of MNCs in liver tissues were calculated. Absolute numbers of CD3+ T cells were determined by flow cytometry (Control: n = 5, ConA + PBS: n = 5, ConA + hMuSC: n = 5). Values are presented as mean ± SEM. Statistical analysis was performed by Mann-Whitney test.
Inflammatory priming induces high expression of several chemokines and IDO in hmuscs
In response to inflammatory cytokines, human MSCs produce a large amount of IDO and chemokines, which are key participants in MSC-mediated immunomodulation. The chemokines attract T cells into close proximity to activated MSCs, where IDO-mediated tryptophan depletion leads to the suppression of immune responses by directly inhibiting T cell proliferation21,22. To determine whether the immunoregulatory functions of hMuSCs depend on the chemokine-IDO axis, we preconditioned hMuSCs with human IFN-γ and TNF-α. As expected, IFN-γ/TNF-α-primed hMuSCs expressed very high levels of several leukocyte chemokines, notably CCL2, CCL5, CXCL9, CXCL10, and CXCL11, which are well-known chemoattractants for T cells (Fig. 5A-E). These hMuSCs preconditioned with inflammatory cytokines also exhibited substantial expression of IDO (Fig. 5F&G). The mRNA and protein expression of iNOS were not detectable in IFN-γ/TNF-α-primed hMuSCs (Fig. S3A&B). These results demonstrate that inflammatory cytokines can initiate the immunoregulatory capacities of hMuSCs on adaptive immune responses.
Inflammatory priming provokes the expression of high levels of several chemokines and IDO by hMuSCs. (A-F) Expression levels of chemokines and IDO in hMuSCs stimulated with human IFN-γ and TNF-α (10 ng/ml each) for 24 h were assayed by qRT-PCR (n = 3). (G) The protein expression of IDO and β-ACTIN (loading control) were determined by immunoblotting in hMuSCs stimulated with human IFN-γ and TNF-α (10 ng/ml each) for 24 h (n = 3). The original images of blots are in the Supplementary Information. Values are presented as mean ± SEM. Statistical analysis was performed by two-tailed unpaired t test.
Hmusc-mediated immunosuppression requires IDO expression
To verify whether hMuSCs exert immunosuppressive effects on the proliferation of activated T cells through IDO-mediated tryptophan metabolism, we conducted the chemical inhibition of IDO with 1-methyl-tryptophan (1-MT), in the hMuSC co-culture system with activated PBMCs. While the proliferation of activated PBMCs was markedly inhibited by hMuSCs, this inhibition was partially reversed upon the addition of 1-MT (Fig. 6A). To further elucidate the importance of IDO for immunomodulation by hMuSCs, we used lentivirus-loaded shRNA to reduce IDO expression in inflammatory cytokine-primed hMuSCs (Fig. 6B). IDO shRNA-treated hMuSCs, in which tryptophan catabolism was impaired, were incapable of suppressing anti-CD3 antibody-induced PBMC proliferation (Fig. 6C). We next employed the ConA-induced acute liver injury model to further validate the mechanism of hMuSC-mediated immunomodulation in vivo. In this context, a single intravenous injection of Scrambled-shRNA hMuSCs significantly alleviated liver damage, as evidenced by a dramatic reduction in serum ALT and AST levels, proinflammatory mediators, and centrilobular necrosis. There was a diminished infiltration of MNCs and CD3+ T cells at the damaged sites. However, these beneficial effects were not observed in IDO-shRNA hMuSC-treated mice (Fig. 6D-H), suggesting that the therapeutic efficacy of hMuSCs in the inflammatory disease is dependent on IDO. Taken together, these data demonstrate that IDO is a key mediator of T cell immunosuppression by hMuSCs.
The beneficial effects of hMuSCs on acute liver injury require IDO. (A) PBMCs stained with CFSE were co-cultured with hMuSCs (2.5 × 104 cells per well in 48-well plates) at a ratio of 1 : 20 (hMuSC : PBMC) for 72 h in the presence of anti-human CD3 antibody (1 µg/ml) with or without the IDO inhibitor 1-MT (0.5 mM). CFSE fluorescence intensity reduction of PBMCs was detected by flow cytometry to measure the proliferation of PBMCs (n = 4). (B) Efficiency of shRNA-mediated IDO knockdown in hMuSCs (n = 3). The original images of blots are in the Supplementary Information. (C) PBMCs stained with CFSE were co-cultured with Scrambed-shRNA hMuSCs or IDO-shRNA hMuSCs (2.5 × 104 cells per well in 48-well plates) at graded ratios (hMuSC : PBMC) for 72 h in the presence of anti-human CD3 antibody (1 µg/ml). CFSE fluorescence intensity reduction of PBMCs was detected by flow cytometry to measure the proliferation of PBMCs (n = 4). (D) Mice were intravenously injected with ConA (15 mg/kg) to induce acute liver injury; 0.5 h later, Scrambled-shRNA hMuSCs or IDO-shRNA hMuSCs (5 × 105) were transfused to suffering mice in the same way. After 12 h, serum and liver tissues were sampled. (E) Serum levels of AST and ALT were measured. (F) IFN-γ and IL-12 levels in the serum were assayed by ELISA. (G) Representative H&E-stained liver sections and percentages of tissue necrosis. Scale bar, 250 μm. (H) Absolute numbers of MNCs in liver tissues were calculated. Absolute numbers of CD3+ T cells were determined by flow cytometry (Control: n = 5, ConA + PBS: n = 5, ConA + Scrambled hMuSC: n = 5, ConA + IDO-shRNA hMuSC: n = 5). Values are presented as mean ± SEM. Statistical analysis was performed by one-way analysis of variance test (A), two-tailed unpaired t test (B) or Mann-Whitney test (E-H).
Discussion
The interplay between MuSCs and immune cells is mutually influential. While MuSCs not only respond passively to inflammatory signals from immune cells that promote their proliferation and differentiation, they also actively release immunomodulatory factors that assist in mitigating inflammation3. For instance, MuSCs can facilitate the polarization of macrophages from a proinflammatory phenotype to an anti-inflammatory phenotype through IGF2-mediated metabolic reprogramming towards OXPHOS4. Furthermore, tryptophan metabolites of the IDO-initiated kynurenine pathway and IL4I1-catalyzed indole metabolism in inflammatory cytokine-primed MuSCs serve as endogenous ligands to activate AhR, resulting in an increased expression of TSG6 and thus reducing the infiltration of macrophages, monocytes, and neutrophils into inflammation sites5,6. Utilizing their immunomodulatory properties, MuSCs have been successfully applied in treating inflammatory diseases characterized by hyperactivated innate immune responses, such as inflammatory bowel disease and pneumonia. However, the therapeutic efficacy of MuSCs for other inflammatory diseases characterized by hyperactivated adaptive immune responses is still to be determined. In this study, the systemic administration of MuSCs from mice and humans proved effective in alleviating the pathological conditions in mice with ConA-induced acute liver injury due to hyperactivated T lymphocytes. MuSCs primed with inflammatory cytokines exhibited significant suppressive effects on the activation and proliferation of T cells in in vitro co-culture systems. Notably, different immunosuppressive molecules participated in the immunoregulatory functions of MuSCs in the two species. MuSCs derived from mice utilized iNOS for immunosuppression, while those from humans relied on IDO expression. Therefore, our study underscores species-specific variations in the mechanisms of MuSC-mediated immunosuppression affecting adaptive immunity.
High concentrations of NO are recognized for their ability to suppress T cell activity23. Although NO rapidly disperses from its point of origin, the concentration of its active state rapidly diminishes at distances exceeding approximately 100 millimeters24. Consequently, the influence of NO is restricted to the immediate vicinity of its producing cells25. Given the limited diffusion and short half-life of NO, the action of iNOS on T cells necessitated the coordinated release of significant quantities of chemokines by mMuSCs primed with inflammatory cytokines, including CCL2, CCL5, CXCL9, CXCL10, and CXCL11, which targeted CXCR3 and CCR5 on T cells. This enabled the recruitment of T cells into close proximity with mMuSCs, thereby facilitating NO-mediated suppression of T cell proliferation. In a similar vein, through paracrine mechanisms, hMuSCs exerted substantial immunoregulatory functions, capable of inhibiting the activation and proliferation of human T cells in the hMuSC-PBMC co-culture system. Specifically, leukocyte chemokines produced by hMuSCs brought T cells into close proximity. The distinction lies in that tryptophan depletion in the local microenvironment, attributed to high IDO expression in hMuSCs primed with IFN-γ and TNF-α, resulted in an energy deficit originally utilized for T cell proliferation, thereby diminishing mitosis in activated T cells. These immune cascades underscore the immunoregulatory mechanisms of MuSCs in adaptive immunity.
Actually, the use of human-derived stem cells in mouse models not only enhances our understanding of their mechanisms of action in vivo but also provides critical preliminary data essential for future clinical translation. These studies contribute to the development of novel stem cell-based therapies for various human inflammatory diseases, including colitis26, psoriasis27, and systemic sclerosis28. Such preclinical models allow for a more accurate assessment of the safety and efficacy of stem cells, thereby advancing their clinical applications29. Moreover, it is important to note that mouse inflammatory cytokines often do not effectively induce the expression of known immunosuppressive molecules, such as IDO, in human stem cells. This suggests that human stem cells may exert their immunosuppressive functions in diseased mice based on the immunoregulatory capabilities ‘licensed’ by human inflammatory cytokines30. In our studies exploring the immunoregulatory mechanisms of MuSCs, we found that hMuSCs primed with human IFN-γ and TNF-α significantly ameliorated symptoms of inflammatory bowel disease and acute lung injury by rectifying aberrant innate immune responses5,6. Although pharmacological or genetic inhibition of IDO only partially reduced T cell proliferation in in vitro co-culture systems and MuSC-induced immunosuppression in vivo in mice with acute liver injury, these results underscore the critical role of IDO in the immunomodulatory functions of hMuSCs. However, additional mechanisms are likely to contribute to MuSC-mediated immunomodulation. To explore whether the immunoregulatory functions of hMuSCs depend on iNOS expression, we preconditioned hMuSCs with human IFN-γ and TNF-α. Our results showed that the mRNA and protein expression of iNOS were not detectable in IFN-γ/TNF-α-primed hMuSCs. This indicates that hMuSCs do not rely on iNOS to exert their immunosuppressive effects. Prostaglandin E2 (PGE2) is known to act as a potent mitogen for MuSCs by binding to the EP4 receptor on their surface, triggering the intracellular cAMP/phospho-CREB pathway, and activating the proliferation-inducing transcription factor Nurr1, thereby promoting MuSC expansion31. However, its role as an immunosuppressive factor and its potential to inhibit T cell proliferation in hMuSCs remain unclear. Given the importance of species differences in rodent-based studies as models of human disease, future translational studies should also focus on developing a novel humanized mouse model to more accurately investigate MuSC-mediated immunomodulation.
Differences between mMuSCs and hMuSCs are influenced by their respective muscle architectures, cellular behaviors during growth and regeneration, and myogenic potentials. hMuSCs exhibit varying regenerative abilities across different muscles, mediated by a subset of cells that fulfill the criteria of adult stem cells. mMuSCs play a prominent role in muscle regeneration, yet exhibit differences in expression profiles and regeneration outcomes compared to humans32. Moreover, the response of MuSCs to muscle loading and hypertrophy significantly differs between mice and humans33. mMuSCs readily proliferate and differentiate in response to muscle loading, thus contributing to hypertrophy. In humans, although MuSCs are activated, the extent and nature of their contribution to muscle hypertrophy vary and are dependent on factors such as age, muscle type, and specific physiological conditions34. Concerning immune function, our study reveals distinct mechanisms underpinning the immunoregulatory abilities of MuSCs derived from these two mammalian species, with reliance on iNOS in mice and IDO in humans. These differences emphasize the extensive use of mouse models in research but also illustrate why findings from such studies may not directly apply to human conditions. The functional specializations in mice provide valuable insights into MuSC biology but require careful consideration when extrapolating data to humans.
Conclusions
This study has identified a gap in understanding the different modalities of MuSC action in immunosuppression across species, providing crucial insights to guide future research. Specifically, MuSCs derived from mice utilize iNOS for immunosuppression, whereas MuSCs from humans rely on IDO expression. We believe that the deeper understanding gained from this study will contribute to developing more precise in vitro potency assays that can better correlate the critical quality attributes of MuSCs with their therapeutic function in future preclinical and clinical studies.
Data availability
Data are available from the corresponding authors upon reasonable request.
References
Fu, X., Zhuang, C. L. & Hu, P. Regulation of muscle stem cell fate. Cell Regen. 11, 40 (2022).
de Morree, A. & Rando, T. A. Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity. Nat. Rev. Mol. Cell Biol. 24, 334–354 (2023).
Fang, J. et al. Redressing the interactions between stem cells and immune system in tissue regeneration. Biol. Direct 16, 18 (2021).
Fang, J. et al. Skeletal muscle stem cells confer maturing macrophages anti-inflammatory properties through insulin-like growth factor-2. Stem Cells Transl. Med. 9, 773–785 (2020).
Zhang, S. et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res. Ther. 12, 50 (2021).
Zuo, M. et al. IL4I1-catalyzed tryptophan metabolites mediate the anti-inflammatory function of cytokine-primed human muscle stem cells. Cell Death Discov. 9, 269 (2023).
Wang, Y., Fang, J., Liu, B., Shao, C. & Shi, Y. Reciprocal regulation of mesenchymal stem cells and immune responses. Cell Stem Cell 29, 1515–1530 (2022).
Han, Y. et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal. Transduct. Target. Ther. 7, 92 (2022).
Liu, L., Cheung, T. H., Charville, G. W. & Rando, T. A. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat. Protoc. 10, 1612–1624 (2015).
Fu, X. et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 25, 655–673 (2015).
Charville, G. W. et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Rep. 5, 621–632 (2015).
Judson, R. N. et al. Inhibition of methyltransferase Setd7 allows the in vitro expansion of myogenic stem cells with improved therapeutic potential. Cell Stem Cell 22, 177-190e177 (2018).
Fang, J. et al. NAD(+) salvage governs the immunosuppressive capacity of mesenchymal stem cells. Cell Mol. Immunol. 20, 1171–1185 (2023).
Han, X. et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 21, 1758–1768 (2014).
Muratori, L., Lohse, A. W. & Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ 380, e070201 (2023).
Terziroli Beretta-Piccoli, B., Mieli-Vergani, G. & Vergani, d. Autoimmmune hepatitis. Cell Mol. Immunol. 19, 158–176 (2022).
Ren, G. et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 (2008).
Wang, Y., Chen, X., Cao, W. & Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 15, 1009–1016 (2014).
Zhang, S., Wu, H. & Liu, C. Inhibition of lymphocyte proliferation: An ability shared by murine mesenchymal stem cells, dermal fibroblasts and chondrocytes. Transpl. Immunol. 47, 55–61 (2018).
Su, J. et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 21, 388–396 (2014).
Shi, Y. et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 14, 493–507 (2018).
Wang, G. et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 25, 1209–1223 (2018).
Niedbala, W., Cai, B. & Liew, F. Y. Role of nitric oxide in the regulation of T cell functions. Ann. Rheum. Dis. 65(Suppl 3), iii37–40 (2006).
Garcia-Ortiz, A. & Serrador, J. M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol. Med. 24, 412–427 (2018).
Silberman, D. et al. CD28 ligation increases macrophage suppression of T-cell proliferation. Cell Mol. Immunol. 9, 341–349 (2012).
Li, Y. et al. SOD2 promotes the immunosuppressive function of mesenchymal stem cells at the expense of adipocyte differentiation. Mol. Ther. 32, 1144–1157 (2024).
Ding, Y. et al. Mesenchymal stem/stromal cells primed by inflammatory cytokines alleviate psoriasis-like inflammation via the TSG-6-neutrophil axis. Cell Death Dis. 13, 996 (2022).
Gong, P. et al. Mesenchymal stem cells alleviate systemic sclerosis by inhibiting the recruitment of pathogenic macrophages. Cell Death Discov. 8, 466 (2022).
Levy, O. et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 6, eaba6884 (2020).
Du, L. et al. IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 29, 1363-1375e1368 (2019).
Ho, A. T. V. et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc. Natl. Acad. Sci. U.S.A. 114, 6675–6684 (2017).
Xu, X. T. et al. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Rep. 5, 419–434 (2015).
Fukada, S. I., Higashimoto, T. & Kaneshige, A. Differences in muscle satellite cell dynamics during muscle hypertrophy and regeneration. Skelet. Muscle 12, 17 (2022).
Bareja, A. et al. Human and mouse skeletal muscle stem cells: Convergent and divergent mechanisms of myogenesis. Plos One 9, (2014).
Funding
This study was supported by grants from the National Natural Science Foundation of China (82202032) and Special Fund for Scientific Research of Jiangsu Medical Association (SYH-3201140-0093(2023040)).
Author information
Authors and Affiliations
Contributions
Shisong Liu and Pengbo Hou designed experiments, made figures and wrote original manuscript. Weijia Zhang, Muqiu Zuo, Zhanhong Liu, Tingting Wang and Yipeng Zhou performed cell experiments and data analysis. Wangwang Chen and Chao Feng performed animal experiments and data analysis. Bo Hu and Jiankai Fang supervised experiments, provided experimental feedback, and edited the manuscript. All authors read and approved the final manuscript. Notably, Shisong Liu and Pengbo Hou acted as first authors and contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Hou, P., Zhang, W. et al. Species variations in muscle stem cell-mediated immunosuppression on T cells. Sci Rep 14, 23410 (2024). https://doi.org/10.1038/s41598-024-73684-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73684-2