Abstract
Secondary metabolites play important physiological roles being bioactive as defences against other organisms, or attractive signals used for various purposes, including reproduction. Their production and the emission in the environment may be viewed as an adaptive feature subjected to evolutionary selection. They were demonstrated to be useful for applications in various biotechnological fields, such as pharmaceutical, nutraceutical and cosmeceutical. Sponges and microalgae, including diatoms, are the most promising sources of bioactive compounds from the sea. We aimed at detecting the ecotoxicological effects of crude extracts and fractions obtained from three marine sponges, Geodia cydonium, Haliclona (Halichoclona) vansoesti and Agelas oroides and two benthic diatoms, Nanofrustulum shiloi and Cylindrotheca closterium on model marine organisms. We tested their effects on the Mediterranean purple sea urchin, Paracentrotus lividus, and on two diatoms, Phaeodactylum tricornutum and Cylindrotheca closterium, chosen because they are considered standard indicators for assessment of ecological impacts. Our results showed that extracts and fractions from both sponges and diatoms may be harmful for model invertebrates. However, eggs appeared “protected” from sponge allelochemicals when still unfertilized. The majority of sponge fractions exhibited noticeable impacts during the post-fertilization treatments. In contrast, fractions from diatoms notably increased the rate of malformations compared to the control, both in pre- and post-fertilization treatments.
Similar content being viewed by others
Introduction
Researches in the field of drug discovery are leading to the characterization of compounds from several marine organisms1,2. In fact, their secondary metabolites exhibited various physiologic roles, and demonstrated an allelopathic activity when involved in defence and predation. Some of them have been applied to biotechnologies as antifouling and antimicrobial substances3. They may be, as well, involved in spawning and in symbiotic relationship and, in this case, they may be applied to medical and nutraceutical biotechnologies. The life competition, as well as various environmental pressures, pushed towards a wide chemical biodiversity during the evolution, that characterizes all marine environments, and it has no counterpart in the terrestrial environments. Among marine organisms, microalgae (mainly diatoms) and sponges represent the most challenging sources of bioactive compounds for biotechnological applications in pharmacological, nutraceutical and cosmeceutical fields4,5. Still, although some research has been done on the actual effects of secondary metabolites derived from diatoms and sponges on marine model organisms, currently, the available data is insufficient. Some research examples include the pioneering work by Cariello et al.6, which showed that compounds isolated from an ethanolic extract of the sponge Dysidea avara were toxic for the egg development of the sea urchin Sphaerechinus granularis, causing delayed development and block of the cell division. Three main compounds were indicated to be responsible for this activity, viz. the Avarol (sesquiterpenoid hydroquinone) and other two chemically correlated to avarol compounds obtained with butanol extraction, named DA and DB6. The extracts from the sponges Rossella fibulata, Rossella sp. and Isodictya verrucosa displayed toxic effect on the embryos of Sterechinus neumayeri at low concentrations (1 mg/mL and 0.05 mg/mL, respectively)7. The highest concentration triggered block of embryo development prior to reach the blastula stage. In the same study, extracts from Iophon sp. and Mycale acerate demonstrated toxigenic activity on the sea urchin sperm at low concentrations (1, 0.5 and 0.05 mg/mL, respectively), causing inhibition of the sperm mobility. Another study8 demonstrated that two compounds, Mycalosides A and G, extracted from the marine sponge Mycale laxissima, inhibited the fertilization of the sea urchin Strongylocentrotus nudus eggs, acting as spermostatics.
Remarkably, sponges host a number of microorganisms responsible for the synthesis of bioactive compounds. Regueiras et al.9 tested aqueous and organic extracts from twelve cyanobacteria associated to several sponges from Portugal on P. lividus embryos. The most active organic extract derived from a cyanobacterial strain ascribed to Chroococcales (6MA13ti), associated to the sponge Tedania ignis. Sea urchin embryos exposed to this organic extract exhibited complete arrest of development, and were unable to reach the pluteus stage. Similarly, aqueous extracts from Synechoccales cyanobacteria (LEGE11384) and Phormidium spp. (25J1tp) isolated from Polymastia sp. and Tedania pilarriosae, respectively, induced a reduced number of embryos reaching the pluteus stage9.
The effect of sponge extracts on algae has been less explored. In 2002 Tsoukatou et al.10 demonstrated that extracts from three sponges belonging to the genus Ircinia inhibited the growth of several diatoms (Amphora coffeaformis, P. tricornutum and Cylindrotheca closterium). More recently, the extract of Ircinia oros was demonstrated to inhibit the growth of the diatom P. tricornutuum11. In addition, sponge-derived polybrominated diphenyl ether (3,5-dibromo-2-(2’,4’-dibromophenoxy)-phenol A) exhibited antifouling activity on the diatom A coffeaeformis12. Other three compounds extracted from the marine sponge Semitaspongia bactriana (i.e., 7E,12E,20Z-variabilin, cavernosolide, lintenolide A) showed efficient antifouling properties towards the diatom Nitzschia closterium13. The anti-fouling activity against a species of the genus Chlorella sp. can be due to compounds produced by micro-organisms associated to sponges, as in the case of the strain SS05 of Bacillus cereus, associated to the sponge Sigmadocia sp14. Another extract from sponge-associated bacteria (Bacillus pumilus) inhibited the growth of N. closterium15 .
In parallel, diatom-derived extracts were demonstrated to influence the physiology of sea urchin embryos. The incubation of embryos of the sea urchin P. lividus with crude extract of the diatom Thalassiosira rotula led to a disorganization of tubulin and impairment of the mitotic spindle16. The end-products of the lipoxygenase/hydroperoxide lyase metabolic pathway of planktonic diatoms (primed by wounding of cells, as done by grazers) caused malformations and cell cycle arrest on embryos of the sea urchin P. lividus. These compounds, mainly represented by Polyunsaturated Fatty Acids (PUFAs)17, Polyunsaturated Aldehydes (PUAs)18 and hydroxyacids19 are grazing deterrents. Gudimova et al.20 demonstrated that even the simple exposure of embryos of Strongylocentrotus droebranchiensis and Echinus acutus to intact cells of various diatoms arrested embryonic development. Skeletonema marinoi resulted to be the most effective, priming acute mortality in S. droebachiensis embryos after four hours, as well as Thalassiosira gravida, which caused acute mortality after 24 h of exposure.
Taking into account these data, we aimed at detecting the ecotoxicological effects and the possible biotechnological applications of total extracts and fractions (according to Cutignano et al.21 and Nuzzo et al.22) of three marine sponges, G. cydonium, H. (H.) vansoesti and A. oroides and two benthic diatoms N. shiloi and C. closterium, on marine model organisms. In particular, they were tested on the Mediterranean sea urchin P. lividus, extensively used for ecotoxicological studies in response to natural and anthropogenic toxins, because of its easy manipulation in laboratory23,24. Two diatom species were also adopted as targets for sponge and diatom metabolites: i.e. P. tricornutum, a well-established and standardized bioindicator, widely recognized for its sensitivity to environmental stressors and commonly employed in ecotoxicological assessments; ii. C. closterium, a cosmopolitan diatom quite common in the Mediterranean Sea, in order to study local strains in their native environments.
Results
Sea urchin bioassay
Overall, sea urchin bioassays evidence contrasting results between pre- and post- fertilization exposures. Post-fertilization exposure to various extract and fractions lead to a different range of observable effects, while pre-fertilization generally did not cause detectable effects in the same magnitude and were mostly limited to plutei malformations.
In particular, when eggs were exposed after fertilization to extracts and fractions obtained from the sponges G. cydonium and H. (H.) vansoesti, the first mitotic division of the fertilized eggs was blocked at all the tested concentrations (see Supplementary Tables S1 and S2), and several delayed embryos were detected still at the gastrula stage, with evident apoptotic signals, very similar to those reported by Ruocco et al.25(Supplementary figures S1-S2). Moreover, several embryos reaching the pluteus stage showed morphological malformations, mainly consisting in alterations of arms, spicules and apices, as reported in Varrella et al.18. The impact was proportional to the tested concentrations, becoming more statistically significant at the highest concentration only for malformed plutei, as demonstrated by statistical analysis (see Supplementary Table S3-S4).
Different results were obtained when embryos were exposed to extracts and fractions from the sponge A. oroides. The most active one was AORO 2D that both at the concentrations C3 (0.500 mg/L) and C2 (0.250 mg/L) triggered embryo malformations (75% and 70%, respectively), whereas at the lowest concentration C1 (0.125 mg/L), it showed antimitotic activity, leading to a high percentage (73%) of fertilized eggs not entering the first mitotic division (Fig. 1). In the fractions AORO 2B and 2 C tested at concentrations C3 and C2 (0.5 and 0.250 mg/L, respectively), the malformed plutei in the fraction 2B were 42% and 43% (respectively at C3 and C2), whereas the percentage of the malformed plutei in the fraction AORO 2 C were and 51% and 44% of the total number of embryos for the highest (C3) and intermediate concentration (C2), respectively. Also in this case, the effect of the extract and its fractions were dose-dependent and more evident at the highest concentration (Supplementary Table S5; for statistical analysis see Supplementary Table S6).
In contrast, when eggs were exposed before fertilization to the extract and the fractions from all the three sponges (Supplementary figures S3-S4 and Supplementary Tables S7-S8-S9) no effects were detected, with the only exception of the fraction AORO 2 C, which induced embryo malformations similar to the ones observed by Varrella et al.18, mainly affecting arms, spicules and apices. The percentages of malformed plutei prompted by this fraction were 64%, 40% and 23% at the highest (C3), medium (C2) and lower (C1) concentrations, respectively (Fig. 2; for statistical analysis see Supplementary Table S10-11-12).
In the case of the two diatoms, the total extract and the fraction NSHII 2D obtained from N. shiloi, induced a statistically significant percentage of malformed plutei in the pre-fertilization treatment accounting for 51% at the higher concentration (C3) and 43% both at the medium (C2) and the low concentration (C1). (Fig. 3 and Supplementary Table S13; see also Supplementary Table S14 for statistical analysis). This fraction caused malformation in the plutei, also when embryos were exposed after fertilization, accounting for 44% at C3, 40% at C2 and 41% at C1, respectively (Supplementary Figure S5 and Supplementary Table S15 and S16 for statistical analysis). In contrast, no effects were prompted by C. closterium extract and fractions, with the only exception of the fraction 2D (Fig. 4 and Supplementary figure S6), showing low percentage of malformed plutei in the pre-fertilization treatment, equal for all three concentrations (23%), similar to the ones obtained in the post-fertilization treatment (26%, 24% and 24% respectively at C3, C2 and C1). All percentages of malformations are reported in the Supplementary Tables S17-S18 (see also Supplementary Tables S19-S20 for statistical analysis).
Algal growth bioassay
The tests on the diatoms P. tricornutum and C. closterium exposed to sponge extracts, specifically the fractions from H. (H.) vansoesti (HVAN), A. oroides (AORO) and G. cydonium (GCYD), yielded complex patterns of results. Short- term preliminary tests provided with a stimulatory effect as compared to the control group at the same three tested concentrations. Figure 5 showed the results expressed as the percentage of growth stimulation at the highest concentration, where short-term preliminary tests indicated a significant bio-stimulatory effect for the three sponge extracts (AORO, HVAN, GCYD), on both diatoms. Specifically, AORO and HVAN consistently exhibited a notable bio-stimulatory effect, with values indicating moderate or strong stimulation on both P. tricornutum and C. closterium. GCYD displayed a varied response at this concentration, with some results showing a weaker growth stimulation compared to other extracts. However, a block of the growth was prompted by both diatoms, upon a longer-term exposure.
Short-term preliminary tests at the highest concentration (C3 = 0.500 mg/L) of sponge extracts AORO, HVAN, and GCYD on (A) P. tricornutum and (B) C. closterium. Values indicate growth stimulation ± standard deviation. Different letters (a-c) represent statistically different values according to Tukey’s post hoc test.
The growth responses of the two diatoms exposed to diatom extracts (NSHI and CCLO) is shown in Figs. 6 and 7. P. tricornutum exhibited an exponential growth pattern when exposed to NSHI extracts, characterized by an initial lag phase and followed by an exponential increase in the cell density, particularly at the concentration C3. In contrast, C. closterium displayed a linear increase of the growth when exposed to diatom extracts, tough at a lower rate than P. tricornutum. When subjected to CCLO extracts, both species exhibited concentration-dependent growth increases, yet the growth patterns remained distinct. However, statistical analysis revealed no significant differences between the growth responses within the same species across the different concentrations of extracts.
Discussion
Effects of sponge bioactive compounds
Contrasting results were obtained when P. lividus eggs were treated with extracts and fractions of sponges before and after fertilization. Only in the case of exposure after fertilization all sponge extracts affected the first mitotic division and caused death of gastrulae, whereas in both cases malformed plutei were present. It must be considered that sea urchin eggs have a different permeability to molecules before and after the fertilization, as it has been demonstrated in previous studies where a decrease in the electrical resistance of sea-urchin eggs following fertilization leaded to permeability increase to water and solutes26,27. In relationship to this phenomenon, less permeable plasma membrane from unfertilized eggs could exert a protection mechanisms from sponge allochemicals.
The majority of sponge fractions exhibited noticeable impacts during the post-fertilization treatment. For example, all sponge subfraction 2B, which according the extraction protocol21 contained nucleosides, induced plutei malformation and inhibited the initial mitotic division. As shown in previous investigations, the intake of exogenous nucleosides in the sea urchins P. lividus and S. purpuratus increases after fertilization28,29. Specifically, the fertilized eggs of P. lividus are capable of intake about 20 times more nucleosides just one hour post fertilization, as compared to their unfertilized counterparts, and these exogenous components are actively used in the embryonic metabolism28. It was demonstrated that some nucleoside analogues have cytotoxic effect and are used as anticancer drugs, due to their effect as competitors of nucleotides and eventually, interaction with intracellular targets to induce cytotoxicity30,31.
Exposing embryos to fractions 2D resulted in similar effects when derived from G. cydonium and H. (H.) vansoesti, leading to an increase in malformed plutei and fertilized but undivided eggs. However, the most potent impact was observed when embryos were treated with the subfraction AORO 2D obtained from A. oroides. In fact, this fraction caused malformations (at C3 and C2) or block of the first mitotic division (at C1) of the fertilized eggs. These results demonstrated that the classes of compounds present in this fraction (mainly sterols and free fatty acids according Cutignano et al.21) could penetrate in the embryos and interfere with the larval embryogenesis. As also demonstrated for diatoms-derived fractions, particularly those from N. shiloi, this class of compounds was toxic both for adults and their larval stage. Hence, it is likely that that sterols and free fatty acids deriving from sponges are as toxic as the ones present in diatoms. Embryos exposed to fractions 2E, which mainly includes triglycerides21, exhibited similar impacts, resulting in malformed plutei, apoptotic gastrulae and eggs that did not undergo the first mitotic division across all three tested concentrations in the post-fertilization treatment. Embryos treated with fraction 2 C (containing mainly glycolipids and phospholipids, according to Cutignano et al.21) showed similar effects in the post-fertilization assays regardless of the sponge from which this fraction was obtained. However, in the pre-fertilization treatment, the fraction 2 C was more potent when derived from A. oroides, causing a higher percentage of malformed plutei than the control. It is worth-noting that fatty acids and sterols have been also shown to be important nutrients during larval development of several organisms, from nematodes to fish32, but their effects as allochemicals are scarcely investigated33. The activity of these fractions of A. oroides is in agreement with the results obtained on cancer cell lines, showing a strong cytotoxic activity (unpublished data).
The long-term exposure of the model marine diatoms (P. tricornutum and C. closterium) to sponge extracts showed complete block of the growth. According to various studies4,34, sponges produce unique compounds retarding the formation of biofilms on their surfaces. Additionally, sponge extracts directly tested on diatoms were effective and limited the fouling adhesion10,35. Nevertheless, these findings do not exclude the possibility that the same sponge extracts and fractions could be effective if tested on other diatoms.
Effects of diatoms as producers of bioactive compounds
P. lividus embryos and eggs treated with extracts from N. shiloi (NSHI) and C. closterium (CCLO) yielded similar results. Fraction 2D obtained from both diatoms and used both for pre- and post-fertilization treatments notably prompted an increase of malformations than the control. Nevertheless, this effect is still less potent than the one obtained with sponge extracts. The efficacy of fraction 2D was in line with the results of Ruocco et al.36, showing the effect on adult P. lividus fed on N. shiloi and C. closterium for one month. The study demonstrated a toxigenic impact on embryos obtained from eggs produced by sea urchin females fed on these benthic diatoms. Within the same study, a chemical examination indicated an exclusive production of polyunsaturated aldehydes by N. shiloi, while both diatoms exhibited notable production of various oxylipins, known for their cytotoxic effects on grazers and cancer cell lines36,37. Moreover, sterols and fatty acids are contained in the fraction D according to the extraction protocol by Cutignano et al.21. Our data are with previous findings showing the toxicity of diatom-derived secondary metabolites.
Apparently, extracts and fractions from diatoms seem to be natural occurring supplements because they triggered increased growth. A slight concentration-dependent stimulation was found within the replicates treated with the same extract. There was a difference in the pattern among the P. tricornutum and C. closterium growth enhancement pattern thus indicating specie-specific variations in growth dynamics response to the same natural extracts. Scarce information is available about the chance of diatoms extract used as growth supplements, although it could appear scarcely useful to culture diatoms to prime the growth of other microalgae. Anyway, diatoms were mainly studied as sustainable sources of nutritious compounds for humans38 and the effect of diatoms herein demonstrated might be simply ascribed to the addition of organic materials, which are composed by bacteria and produce nutrients for other microalgae. Diatom extracts and their purified compounds, however, find large exploitation in the bio-pharmacological and nutraceutical field5.
Moreover, P. tricornutum genome is well-characterized, and there is a rich toolbox of engineering tools available for straightforward gene manipulation of the algae39.
Conclusions
Summarizing our findings showed how the effects of extracts/fractions was strictly linked to the moment in which they came into contact, therefore strictly linked to the physiological state of the eggs. In addition, the effect probably depended on the different metabolites present in the extracts and fractions from the three sponges and two diatoms, as well as on the sensitivity of the model system used. Further experiments will be necessary to chemically characterize the extracts and fractions of sponges and diatoms, in order to identify the metabolites present responsible for the effect found. This could be useful to identify for example possible anti-mitotic compounds, which could be applied in the pharmacological field for cancer treatment. It is noteworthy that findings on algae may introduce research towards genetic modifications aimed at enhancing the production of specific compounds with various industrial applications. The integration of diatom extracts with genetic engineering methodologies holds the potential for sustainable and versatile solutions within the fields of biotechnology and bioengineering, further underscoring the remarkable promise of natural diatom-derived supplements. On the other hand, sponge extracts exerted inhibiting activity on the same microalgae being valid antifouling candidates.
Methods
Experimental design and sample collection
Our experimental design comprised: (i) collection and culture of organisms; (ii) extraction and fractionation of cultured biomasses of sponges and diatoms; (iii) replicated tests of extracts and fractions produced on the survival rates and malformations of sea urchin eggs and embryos, as well as on the survival and growth of two target diatoms (see Fig. 8), compared to controls.
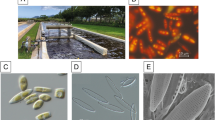
Sponge samples were collected as follows: G. cydonium in Secca delle Fumose, Parco Sommerso di Baia (40°49ʹN, 14°5ʹE) and A. oroides in Punta San Pancrazio (Ischia Island, 40°42ʹN, 13°57ʹE) in the Gulf of Napes; H. (H.) vansoesti in The Faro Lake (Sicily; 38° 16′ 07′′ N, 15° 38′ 13′′E) (see Bertolino et al.40). The two benthic diatoms N. shiloi and C. closterium were previously isolated from the leaves of Posidonia oceanica and identified using both morphological and molecular approaches37.
Chemical extraction and solid-phase fractionation of methanol extracts
After lyophilization, 35 g of dried sponges and 2 g of dry diatoms were sonicated and extracted with MeOH (3 × 100 mL). The organic phase was decanted and dried under vacuum. The obtained extracts were weighed and 60 mg of sponges and 30 mg of diatoms were further fractioned using a vacuum manifold CHROMABOND® HR-X cartridges (6 mL/500 mg), according to the method reported in Cutignano et al.21 and Nuzzo et al.22 (Supplementary figure S7), to obtain 5 enriched fractions (A-E). Briefly, after a washing step with water to remove salts (fraction A), the organic fraction were eluted with CH3OH/H2O 50:50 (fraction B) to CH3CN/H2O 70:30 (fraction C), 100% CH3CN (fraction D) and, finally, CH2Cl2/CH3OH 90:10 (fraction E) (see Table 1 for extracts and fractions obtained for sponges and diatoms). All fractions were analysed by Thin Layer Chromatography (TLC) on KieselGel 60 F254 plates (Merck, Darmstadt, Germany) using developing solvent eluent and revealed by spraying with a Ce(SO4)2 acidic solution, followed by plate heating.
Sea urchin exposure
Adult sea urchins were hand collected by scuba divers in the Gulf of Naples at a depth of about 10 m. Collected individuals had a size between 4 and 6 cm (diameter of tests) and they were immediately stored in a cool-box and transported to the laboratory to be reared in aerated recirculating tanks for ten days. A chemical stimulation was performed to collect their gametes, by injecting 1 mL of 0.5 M KCl through their peribuccal membrane41. The gamete donors were stored in aerated recirculating tanks immediately after the collection of sperms and eggs. Sperm was collected dry from males with a plastic pipette and kept undiluted at 5 °C until the fertilization. Eggs were collected from three females in glass dishes filled with filtered sea water (FSW) and then washed-up several times to remove faecal pellets and contaminants. Pools of 120 eggs from each female were treated according two experimental procedures: (i) eggs were incubated for 10 min with sponge or diatom extracts and fractions (obtained as described in paragraph 2.2; see also Table 1), at three different concentrations (C1 = 0.125 mg/L; C2 = 0.250 mg/L; C3 = 0.500 mg/L) and then fertilized; (ii) eggs were fertilized and then incubated with the extracts and fractions (same concentrations as above). The embryos produced were incubated in a thermostatic chamber at 18 °C with a 12/12 h light/dark cycle, and their development was monitored, from the fertilization to the first mitotic division, until forty-eight hours post-fertilization (hPF), normally corresponding to the pluteus stage. The embryos were fixed with 0.5% glutaraldehyde and morphological observations were performed to evaluate and record the percentage of normal plutei (N.P.), malformed plutei (M.P.), apoptotic gastrulae (A.G.) and fertilized eggs exhibiting no first mitotic division (N.D.). All the experiments were performed in triplicates.
Algal growth assays
Axenic cultures of marine diatoms, P. tricornutum and C. closterium, were cultivated in artificial seawater medium supplemented with nutrients (ISO 10253:2016, a method for the determination of growth of the unicellular marine algae Skeletonema sp. and P.tricornutum by substances and mixtures contained in sea water or by environmental water samples). Cultures were kept at a temperature of 22 ± 1 °C under a light-dark cycle of 16:8 h with a light intensity of 6,700 lx. To assess algal growth, a 72-hour test was conducted according to the guidelines of ISO 10253:2016 and ISO 8692:2012 (a method for the determination of the growth inhibition of unicellular green algae by substances and mixtures contained in water or by waste water). 24-well plates were used for the test. For each concentration, three wells were filled with 2,250 µL of each solution (using spiked sponge extract solutions in synthetic culture medium), 125 µL of a culture medium, and 125 µL of inoculum consisting of microalgae collected during the exponential growth phase (20 *10 4 cell/mL). The prepared wells were then moved on a horizontal shaker, at a speed of 50 rpm, for 72 h at 22 ± 1 °C under a continuous light intensity of 6,700 lx. The solutions spiked with sponge extracts correspond to the three concentrations C1 = 0.125 mg/L, C2 = 0.250 mg/L and C3 = 0.500 mg/L. After 72-hour of exposure, spectrophotometric measurements at 670 nm of the samples were performed using a DR5000-SC UV-Vis Laboratory Spectrophotometer (ach Srl). These measurements yielded the cell density according to the Eq. (1):
Where, y is the optical density and x is the corresponding cell density.
The R2 coefficient for this equation was 0.9802, indicating a strong correlation between optical density and cell density. The growth rates were compared to the control, following the guidelines of ISO 2016 and 2012. Additional algal growth tests were conducted with P. tricornutum, and C. closterium, extending the test duration to 7 days and modifying the standard protocol, in order to be conducted under static conditions. All of the aforementioned tests were conducted in triplicate, to ensure the accuracy of the results. The use of ISO guidelines ensured that the tests were standardized, to assure reliable and comparable results.
Statistical analysis
The two-way analysis of variance (ANOVA) was performed using GraphPad Prism 8.4.2 to assess the differences between the effects induced from the extracts and the four fractions from the sponges and diatoms on the embryonic development of the sea urchin P. lividus. A one-way analysis of variance (ANOVA) was performed using GraphPad Prism 8.4.2 to assess the differences in growth stimulation across the different sponge extracts (AORO, HVAN, GCYD) for each diatom species (P. tricornutum and C. closterium). To further investigate these differences, Tukey’s post hoc test was conducted. Additionally, a t-test was employed to compare the cell density curves between treatments in the long-term exposure test.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Proksch, P. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon. 32 (6), 639–655 (1994).
Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 8 (2), 83 (2016).
Protopapa et al. Of antifouling potential and ecotoxicity of secondary metabolites derived from red algae of the genus Laurencia. Mar. Drugs. 17 (11), 646 (2019).
Esposito, R. et al. Bioactive compounds from Marine sponges and Algae: effects on Cancer Cell Metabolome and Chemical structures. Int. J. Mol. Sci. 23 (18), 10680 (2022).
Nieri, P., Carpi, S., Esposito, R., Costantini, M. & Zupo, V. Bioactive molecules from marine diatoms and their value for the nutraceutical Industry. Nutrients. 15 (2), 464 (2023).
Cariello, L., Giudici, M. D. N. & Zanetti, L. Developmental aberrations in sea-urchin eggs induced by avarol and two cogeners, the main sesquiterpenoid hydroquinones from the marine sponge, Dysidea avara. Comp. Biochem. Physiol. Part. - C: Toxicol. Pharmacol. 65 (1), 37–41 (1980).
Figuerola, B., Taboada, S., Monleón-Getino, T., Vázquez, J. & Avila, C. Cytotoxic activity of Antarctic benthic organisms against the common sea urchin Sterechinus Neumayeri. Oceanography. 1 (2), 1–9 (2013).
Antonov, A. S. et al. Mycalosides B – I, eight New Spermostatic Steroid Oligoglycosides from the Sponge Mycale Laxissima. J. Nat. Prod. 66 (8), 1082–1088 (2003).
Regueiras, A., Pereira, S., Costa, M. S. & Vasconcelos, V. Differential toxicity of cyanobacteria isolated from marine sponges towards echinoderms and crustaceans. Toxins. 10 (7), 297 (2018).
Tsoukatou, M., Hellio, C., Vagias, C., Harvala, C. & Roussis, V. Chemical defense and antifouling activity of three Mediterranean sponges of the genus Ircinia. Z. Naturforsch C J. Biosci. 57 (1–2), 161–171 (2002).
De Marchi, L. et al. Potential anti-fouling properties of extracts from the Mediterranean sponge Ircinia oros: An ecotoxicological screening. Preprint at: (2021). https://www.researchsquare.com/article/rs-513755/v1
Ortlepp, S., Pedpradap, S., Dobretsov, S. & Proksch, P. Antifouling activity of sponge-derived polybrominated diphenyl ethers and synthetic analogues. Biofouling. 24 (3), 201–208 (2008).
Stewart, M., Depree, C. & Thompson, K. J. Antifouling sesterterpenes from the New Zealand marine sponge Semitaspongia Bactriana. Nat. Prod. Commun.4 (3), 1934578X0900400306 (2009).
Satheesh, S., Soniamby, A. R., Shankar, S. & Josephine Punitha, C. V. M. Antifouling activities of marine bacteria associated with sponge (Sigmadocia Sp). Ocean. Univ. China. 11, 354–360 (2012).
Xin, X. et al. Potential antifouling compounds with antidiatom adhesion activities from the sponge-associated bacteria, Bacillus pumilus. J. Adhes. Sci. Technol. 31 (9), 1028–1043 (2017).
Buttino, I., Miralto, A., Ianora, A., Romano, G. & Poulet, S. A. Water-soluble extracts of the diatom Thalassiosira rotula induce aberrations in embryonic tubulin organisation of the sea urchin Paracentrotus lividus. Mar. Biol. 134, 147–154 (1999).
Romano, G., Miralto, A. & Ianora, A. Teratogenic effects of diatom metabolites on sea urchin Paracentrotus lividus embryos. Mar. Drugs. 8 (4), 950–967 (2010).
Varrella, S. et al. Molecular response to toxic diatom-derived aldehydes in the sea urchin Paracentrotus lividus. Mar. Drugs. 12 (4), 2089–2113 (2014).
Varrella, S. et al. First morphological and molecular evidence of the negative impact of diatom-derived hydroxyacids on the sea urchin Paracentrotus lividus. Toxicol. Sci. 151 (2), 419–433 (2016).
Gudimova, E., Eilertsen, H. C., Jørgensen, T. Ø. & Hansen, E. In vivo exposure to northern diatoms arrests sea urchin embryonic development. Toxicon. 109, 63–69 (2016).
Cutignano, A. et al. Development and application of a novel SPE-method for bioassay-guided fractionation of marine extracts. Mar. Drugs. 13 (9), 5736–5749 (2015).
Nuzzo, G., Manzo, E., Gallo, C., d’Ippolito, G. & Fontana, A. Fractionation protocol of Marine metabolites. Mar. Genomics 307–313 (2022).
Marrone, V. et al. Defensome against toxic diatom aldehydes in the sea urchin Paracentrotus lividus. PLoS One. 7 (2), e31750 (2012).
Ruocco, N. et al. Diatom-derived oxylipins induce cell death in sea urchin embryos activating caspase-8 and caspase 3/7. Aquat. Toxicol. 176, 128–140 (2016).
Ruocco, N., Costantini, M. & Santella, L. New insights into negative effects of lithium on sea urchin Paracentrotus lividus embryos. Sci. Rep. 6, 32157 (2016d).
Lyon, E. P. & Shackell, L. F. On the increased permeability of sea urchin eggs following fertilization. Science. 32 (816), 249–251 (1910).
Adams, S. L., Kleinhans, F. W., Mladenov, P. V. & Hessian, P. A. Membrane permeability characteristics and osmotic tolerance limits of sea urchin (Evechinus Chloroticus) eggs. Cryobiology. 47 (1), 1–13 (2003).
Nemer, M. Characteristics of the utilization of nucleosides by embryos of Paracentrotus lividus. J. Biol. Chem. 237 (1), 143–149 (1962).
Killian, C. E. & Nishioka, D. Ribonucleoside uptake and phosphorylation during fertilization and early development of the sea-urchin, Strongylocentrotus purpuratus. Eur. J. Mol. Biol. Biochem. 165 (1), 91–98 (1987).
Galmarini, C. M., Mackey, J. R. & Dumontet, C. Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol. 3 (7), 415–424 (2002).
Galmarini, C. M., Popowycz, F. & Joseph, B. Cytotoxic nucleoside analogues: different strategies to improve their clinical efficacy. Curr. Med. Chem. 15 (11), 1072–1082 (2008).
El Kertaoui, N. et al. Key nutritional factors and interactions during larval development of pikeperch (Sander lucioperca). Sci. Rep. 9 (1), 7074 (2019).
Rodríguez, J. F., Dynarowicz-Latka, P. & Conde, J. M. How unsaturated fatty acids and plant stanols affect sterols plasma level and cellular membranes? Review on model studies involving the Langmuir monolayer technique. Chem. Phys. Lipids. 232, 104968 (2020).
Stowe, S. D. et al. Anti-biofilm compounds derived from marine sponges. Mar. Drugs. 9 (10), 2010–2035 (2011).
Dobretsov, S., Dahms, H. U. & Qian, P. Y. Antibacterial and anti-diatom activity of Hong Kong sponges. Aquat. Microb. Ecol.38 (2), 191–201 (2005).
Ruocco, N. et al. Toxigenic effects of two benthic diatoms upon grazing activity of the sea urchin: morphological, metabolomic and de novo transcriptomic analysis. Sci. Rep. 8(1), 5622 (2018).
Sansone, C. et al. Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS One 9(7), e101220 (2014).
Bhattacharya, T. et al. Nutraceuticals and bio-inspired materials from microalgae and their future perspectives. Curr. Top. Med. Chem. 21 (12), 1037–1051 (2021).
Butler, T., Kapoore, R. V. & Vaidyanathan, S. Phaeodactylum tricornutum: a diatom cell factory. Trends Biotechnol. 38 (6), 606–622 (2020).
Bertolino, M. et al. First certain record of Demospongiae class (Porifera) alien species from the Mediterranean Sea. Mar. Genomics. 63, 100951 (2022).
Gharbi, M. et al. Scale-Up of an Aquaculture Plant for Reproduction and Conservation of the Sea Urchin Paracentrotus lividus: development of Post-larval feeds. J. Mar. Sci. Eng. 11 (1), 154 (2023).
Acknowledgements
The axenic cultures of Phaeodactylum tricornutum and Cylindrotheca closterium at the Hygiene Laboratory of the Department of Biology of the University of Naples Federico II. SF received support through a Ph.D. fellowship co-funded by the Stazione Zoologica Anton Dohrn (Naples, Italy) and the University of Genoa. This work was partially funded by the National Biodiversity Future Centre (NBFC) Program, Italian Ministry of University and Research, PNRR, Missione 4 Componente 2 Investimento 1.4 (Project: CN00000033).
Author information
Authors and Affiliations
Contributions
S.F. performed the ecotoxicological tests on the sea urchin, as well as on two diatoms, with A.S., M.S. M.G. R.E., N.R., G.N. A.C. performed chemical extractions. M.B. identified the sponges. M.G., M.P., M.C. and V.Z. were involved in the supervision of S.F. S.F., A.S. M.C. and V.Z. contributed in writing original draft of the manuscript. All the authors contributed in review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Federico, S., Siciliano, A., Esposito, R. et al. Toxigenic effects of sponges and benthic diatoms on marine invertebrates and their possible biotechnological applications. Sci Rep 14, 25325 (2024). https://doi.org/10.1038/s41598-024-74100-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74100-5