Abstract
Primary mRNA vaccination against COVID-19 typically involves three doses for immunocompromised individuals, including hematopoietic stem cell transplantation (allo-HSCT) recipients. However, optimal subsequent boosting strategies remain unclear. This study aimed to assess the immunogenicity of a booster dose using the most recently updated vaccine (Comirnaty Omicron XBB.1.5) among long-term allo-HSCT survivors having previously received multiple mRNA vaccine doses, in median 4 (2–6). Thirty-four allo-HSCT recipients were enrolled at Sahlgrenska University Hospital, and peripheral blood samples were collected immediately before and four weeks after booster. Antibodies against the receptor-binding domain (anti-RBD) of spike 1 (S1) and nucleocapsid, as well as S1-specific ex vivo T-cell responses, were evaluated. Adverse events were monitored. Despite a median of 13 months since the prior vaccine dose, both humoral and T-cell responses against S1 were present in the pre-booster samples in all but two participants, who suffered from severe chronic Graft-versus-host disease. Notably, 62% of participants had a previously confirmed COVID-19 infection. Significantly higher pre-booster antibody levels were observed in women than men (p = 0.003). Booster dosing strengthened specific antibody and T cell responses and equalized pre-booster gender differences, although responses remained significantly lower among those receiving immunosuppressive treatment (p = 0.041). In a population of long-term allo-HSCT survivors, the majority of whom had a prior confirmed COVID-19 infection, both pre- and post-booster immune responses were robust. However, patients undergoing immunosuppressive treatment for GvHD exhibited significantly weaker responses.
Similar content being viewed by others
Introduction
Multiple studies have examined both immunogenicity and clinical efficacy of mRNA COVID-19 vaccines among recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT), confirming their pivotal role in preventing COVID-19 associated morbidity and mortality among fully vaccinated individuals1,2 despite lack of detectable seroconversion in 5–26% of recipients after three vaccine doses3,4,5. The emergence of the less virulent Omicron variants and the development of effective antiviral treatments have further ameliorated the course of COVID-19. Breakthrough infections, i.e., infection despite full immunization, are often mild6, but deaths have been reported among allo-HSCT recipients with poor humoral vaccine responses7. The primary vaccine schedule, according to guidelines from the European Conference for Infections in Leukemia (ECIL) and the Centers for Disease Control and Prevention (CDC) includes three doses, starting at 3–6 months post-transplantation8,9,10, but subsequent boosting strategies remain to be elucidated.
This study thus aimed at assessing the immunogenicity of a COVID-19 booster dose using the most recently updated vaccine (Comirnaty Omicron XBB.1.5) among long-term allo-HSCT survivors having previously received multiple mRNA vaccine doses, in median 4 doses (2–6).
Methods
Study population and design
This study was a sub-study within the DurIRVac study, conducted at Sahlgrenska University Hospital in Gothenburg, Sweden between March 2021 and November 2023. Allo-HSCT recipients were identified using local transplant registries. The cohort mainly consisted of long-time allo-HSCT survivors (baseline characteristics in Table 1), with a median time elapsed since transplant of 9 (range 1–18) years. The majority of patients (n = 30) were enrolled early in the pandemic, before the first vaccine dose, and four more patients were newly recruited. Thirteen patients (38%) had chronic Graft-versus-host disease (cGvHD) at the visit prior to vaccination, and 6 (18%) were receiving immunosuppressive therapy at the time of vaccination. A majority, 62%, had earlier COVID-19 infection, details in Table 1. Enrolled transplant recipients fulfilled the following predetermined criteria at inclusion (i) to be at least three months post allo-HSCT (ii) not to have received rituximab the last six months and, (iii) to be without uncontrolled acute GvHD (grade III to IV). All according to EBMT-criteria at study start (version 2.0, December 21, 2020).
Patients were invited to receive the booster at Sahlgrenska University Hospital in November 2023. Peripheral blood was collected immediately before and 4 weeks after the booster immunization. Currently, all participants received the most recently updated Pfizer-BioNTech mRNA vaccine, Comirnaty Omicron XBB.1.5. All allo-HSCT recipients completed a standardized questionnaire following vaccination regarding side effects, which were categorized per the Common Terminology Criteria for Adverse Events standards. Information about GvHD using the National Institutes of Health global severity score was assessed through the medical notes from the visit prior to vaccination.
Analysis of IgG against the RDB within S1 of SARS-CoV-2 (anti-RBD-S1 IgG) and nucleocapsid of SARS-Cov-2
Chemiluminescent microparticle immunoassays (CMIAs) were performed using the automated Alinity system for analysis of IgG antibodies against the receptor-binding domain (RBD) (SARS-CoV-2 IgG II Quant, Abbott, Illinois, USA) within the spike protein (anti-RBD IgG). Levels were reported in the WHO international standard binding antibody units (BAU)/mL (detection range of 14-5680 BAU/mL). Samples above 5680 BAU/mL were diluted and reanalyzed. Antibodies against nucleocapsid (SARS-CoV-2 IgG, Abbott, Abbott Park, Illinois, USA) were also evaluated (nIgG).
Assay of peptide-induced cytokine release in whole blood
Whole blood was stimulated with 1 µg/mL/peptide of 15-mer peptides with 11-amino acid overlap spanning the N-terminal S1 domain of the SARS-CoV-2 surface glycoprotein (S1; product number: 130-127-041, Miltenyi Biotec). After incubation at 37 °C plasma and 5% CO2 for two days, plasma was recovered for analysis of IFN-γ and IL-2 using ELISA (3420–1 H-20 and 3445-1 H-20; Mabtech AB). The S1-induced IFN-γ and IL-2 production is presented with levels in PBS-stimulated samples subtracted. The limit of detection was 10 pg/mL. All methods were performed in accordance with the relevant guidelines and regulations.
Documentation of COVID-19
Previous COVID-19 infection was defined as one or several of the following: detectable SARS-CoV-2 RNA, reactive COVID-19 antigen test, and/or presence of antibodies against the nucleocapsid. Twenty-one participants (62%) had a documented COVID-19 infection at some point during the study period before pre-booster sample.
Statistical analysis
Continuous variables were described as median and range, that is, min-max values, as applicable. Categorical data were described with contingency tables including frequency and percent. The association between continuous parameters was determined using Spearman’s correlation. Statistical comparisons between pre- and post-booster samples were calculated by Wilcoxon matched-pairs signed-rank test. The association between various parameters in independent groups was performed by Mann-Whitney U-test. Statistical analyses were performed using SPSS statistical software package (version 28) or GraphPad Prism software (version 9). P-values are designated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001. All indicated P-values are 2-sided.
Ethical considerations and trial registration
All participants gave written informed consent prior to enrollment. The DurIRVac study was approved by the Swedish Ethical Review Authority (permit nos. 2020–03276, 2021 − 00374, and 2021 − 00539) and by the Swedish Medical Products Agency (Dnr: 5.1-2021-11118) and has been registered at the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT no. 2021-000349-42).
Results
Humoral booster immune responses
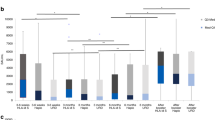
Following mRNA vaccine boosting, anti-RBD IgG levels increased from a median of 4123 (0-21291) to 9718 (26-44436) BAU/mL (p < 0.0001; Fig. 1). Patients with ongoing immunosuppression had significantly lower pre-booster (median 278 vs. 4513 BAU/mL; p = 0.019) as well as post-booster anti-RBD concentrations (median 4513 vs. 9780 BAU/mL; p = 0.041; Fig. 2). Two patients lacked detectable antibody levels pre-booster, both of whom were receiving immunosuppressive treatment for severe chronic GvHD, but all had quantifiable concentrations post-booster. Clinical details on the seronegative patients are provided in Table 2. Female allo-HSCT recipients had significantly higher pre-booster (median 6403 vs. 2430 BAU/mL for female vs. male participants respectively; p = 0.003) but not post-booster anti-RBD IgG antibody levels (p = 0.3; Fig. 3) in comparison to male allo-HSCT recipients. In contrast, the number of previous vaccine doses, previous COVID-19 infection, time elapsed since transplantation, time since last vaccine dose, and age were not significantly associated with pre- or post-booster humoral responses, although this may be secondary to the relatively small sample size.
Booster vaccination induces enhanced serological and specific T-cell responses to spike 1 of SARS-CoV-2 in allo-HSCT recipients. (A) shows serum levels of immunoglobulin G (IgG) against the receptor binding domain (RBD) of spike 1. (B) shows IL-2 and interferon-γ (IFN-γ) production in supernatant plasma following stimulation of whole blood with spike 1 peptides, reflecting the reactivity of SARS-Cov-2 specific T-cells. Median with 95% confidence interval. Statistical comparisons by Wilcoxon matched-pairs signed-rank test. P-values are two-sided.
Reduced spike 1 specific immune responses in allo-HSCT recipients on immunosuppressive treatment. (A) shows serum levels of immunoglobulin G (IgG) against the receptor binding domain (RBD) of spike 1 in patients on immunosuppression (IS) compared to patients not receiving immunosuppression (non-IS). (B) shows interferon-γ (IFN-γ) and (C) shows IL-2 production in plasma supernatant following stimulation of whole blood with spike 1 peptides patients on immunosuppression compared to patients not receiving immunosuppression. Median with 95% confidence interval. Statistical comparisons by Mann-Whitney U-test. P-values are two-sided.
Reduced serological response to spike 1 in male allo-HSCT recipients prior to booster vaccination. Serological response (immunoglobulin G (IgG) against the receptor binding domain (RBD) in spike 1 before and after a booster mRNA COVID-19 vaccine dose delivered to allo-HSCT recipients comparing responses in males and females. Median with 95% confidence interval. Statistical comparisons by Mann-Whitney U-test. P-values are two-sided.
T cell immune booster responses
S1-specific IL-2 levels significantly increased from a median of 143 (0-5079) to 269 (0-12304) pg/mL (p = 0.038), while IFN-γ release was less affected by the booster (30 (0-418) to 35 (0-352); p = 0.83; Fig. 1). Neither the number of previous vaccine doses, previous COVID-19 infection, time since transplantation, time since last vaccine dose or age were significantly associated with pre-booster or post-booster ex vivo T-cell responses.
Correlation between humoral and T cell immune booster responses
Pre-booster anti-RBD IgG level was correlated with pre-booster T-cell reactivity, both with regards to S1-specific IL-2 levels (rho = 0.39, p = 0.023) and IFN-γ production (rho = 0.53, p = 0.001). Post-booster anti-RBD IgG antibody concentrations were correlated with post-booster IFN-γ production (rho = 0.39, p = 0.045) but not with S1-specific IL-2 levels (rho = 0.26, p = 0.19).
Pre-booster anti-RBD IgG was also correlated with post-booster anti-RBD IgG (p = 0.0015), post-booster IL-2 (p = 0.003) and post-booster IFN-γ (rho = 0.5, p = 0.09). Post-booster anti-RBD IgG was also correlated with pre-booster levels of IFN-γ (rho = 0.49, p = 0.007).
Impact of recent COVID-19 infection and immune responses
Antibodies against nucleocapsid (nIgG) were noted in 12/34 (35%) allo-HSCT recipients, indicating probable exposure to SARS-CoV-2 within the past year11, but did not impact on pre- or post-booster anti-RBD IgG levels, ex vivo T-cell responses, need for hospitalization nor documented respiratory illness the past 6 months.
Adverse events
Fourteen of 25 (56%) patients reported side effects, of whom 7/14 (50%) reported only local side effects and 7/12 (50%) reported systemic side effects, mainly fever and malaise. No severe adverse events or worsening of GvHD were reported.
Discussion
The main finding in our study was that among these long-term allo-HSCT survivors, both humoral B-cell and ex vivo T-cell responses were robust in the pre-booster samples, despite almost a year having elapsed since the last vaccine dose. This robustness is likely attributable to the fact that most patients had previously contracted COVID-19, with 62% having a confirmed prior SARS-CoV-2 infection. It is probable that an even larger proportion had subclinical infections, with nucleocapsid antibodies waning below the detection threshold prior to sampling. Additionally, booster dosing with Comirnaty Omicron XBB.1.5 further strengthened virus specific immune responses, though responses were significantly lower both pre- and post-boosting among those receiving immunosuppressive therapy.
There was no correlation between earlier confirmed SARS-CoV-2 infection and antibody levels or T-cell responses, which might be explained by the very high frequency of previous COVID-19 and an acquired infection-vaccine hybrid immunity, which has been documented to enhance immune responses also among immunocompromised individuals12,13. During the first waves of the pandemic, SARS-CoV-2 PCR was available to the public through self-testing and both in- and outpatient were extensively tested, however, after late 2022 SARS-CoV-2 testing has mainly been limited to individuals at risk for severe disease and early intervention or already hospitalized. 35% of participants in this study had antibodies against the nucleocapsid (nIgG), which is in line with reports from the Swedish Public Health Agency suggesting periods of high transmission during 202314, although with substantially attenuated clinical implications as compared to the early phases of the pandemic among allo-HSCT recipients15.
An increase in IL-2 following booster vaccination was noted, however, there was no significant change in IFN-γ production following booster. This finding is congruent with other studies investigating the cytokine profile following COVID-19 mRNA vaccination, where IL-2 is the dominant cytokine elicited by SARS-CoV-2 specific CD4 + cells post-immunization16.
A significant gender difference was also noted in pre-booster humoral immune responses among long-term allo-HSCT survivors, with female participants exhibiting higher antibody levels compared with their male counterparts, despite no differences in the number of previous doses, the time elapsed since the last dose or presence of cGvHD. This observation aligns with a growing body of research suggesting that gender can influence immune response to vaccinations17.
The Swedish Public Health Agency recommends booster COVID-19 mRNA vaccine doses for immunocompromised patients once to twice annually but acknowledge that this population is heterogeneous, and that the responsible physician is to advise on the boosting strategy. Some centers have opted to check serum antibody levels after the primary vaccination schedule to guide the need for subsequent reiterative doses. Tailored vaccination schemes, though promising, pose challenges due to the absence of clearly delineated thresholds regarding correlates of protection against severe COVID-19. Furthermore, the implementation of immune-guided vaccination strategies necessitates consensus on sampling schedules. Additionally, considerations regarding the inclusion of comorbidities, planned immunosuppressive treatments or the emergence of novel SARS-CoV-2 variants complicate decision-making processes in this regard. To this end, the finding in this study of high pre-booster immune responses, despite considerable time since the previous vaccine dose, in most patients is encouraging. The exception is long-term allo-HSCT survivors with ongoing immunosuppressive treatment, among who the COVID-19 specific immune responses were reduced.
Strengths with the current study include the parallel monitoring of aspects of virus-specific B- and T-cell–mediated immunity and sampling of participants right before the booster and at four weeks after. The study sample size was relatively small and, of presumable importance for outcomes, the median time from transplantation was nine years. With these precautions, we believe that our findings are applicable to long-term allo-HSCT survivors. There was no apparent impact of GvHD, but patients on immunosuppressive treatment, in our study mainly low doses of prednisone combined with ruxolitinib, had significantly lower immune responses.
One patient was on Ig replacement, this patient also had previous COVID-19 confirmed with both PCR and nIgG. He had high antibody levels, both in the pre booster and post booster sampling, but had a fivefold increase in sIgG after vs. before booster and a three-fold increase in IL-2, suggesting that his Ig replacement did not substantially confound results.
In conclusion, boosting strategies in immunocompromised patients remains challenging given the heterogenicity of this population. Among these long-term allo-HSCT survivors, most of whom had previously contracted COVID-19, the majority exhibited strong immune responses both pre- and post-booster. However, an exception was observed in allo-HSCT recipients undergoing immunosuppressive treatment, who had reduced COVID-19-specific immune responses before and after the booster.
Data availability
For original data, please contact the corresponding author. As per Swedish law, individual participant data will not be shared.
References
Chen, P. et al. Real-world assessment of immunogenicity in immunocompromised individuals following SARS-CoV-2 mRNA vaccination: A one-year follow-up of the prospective clinical trial COVAXID. EBioMedicine. 94, 104700 (2023).
Wu, T. Y. et al. Clinical manifestations and risk factors for COVID-19 and its severity in patients with hematological malignancies. J. Microbiol. Immunol. Infect. (2024).
Einarsdottir, S. et al. Reduced immunogenicity of a third COVID-19 vaccination among recipients of allogeneic hematopoietic stem cell transplantation. Haematologica. 107(6), 1479–1482 (2022).
Watanabe, M. et al. A third dose COVID-19 vaccination in allogeneic hematopoietic stem cell transplantation patients. Vaccines (Basel); 10(11). (2022).
Rubin, M. et al. Efficacy of SARS-CoV-2 vaccine doses in allogeneic hemopoietic stem cell recipients: a systematic review and Meta-analysis. Asian Pac. J. Cancer Prev. 25(2), 393–399 (2024).
Piñana, J. L. et al. SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transplant. :1–14. (2023).
Chevallier, P. et al. Effectiveness of a third dose of BNT162b2 anti-SARS-CoV-2 mRNA vaccine over a 6-month follow-up period in allogenic hematopoietic stem cells recipients. Hematol. Oncol. 40(5), 1097–1099 (2022).
Bordat, J., Maury, S. & Leclerc, M. Allogeneic hematopoietic stem cell transplantation in the COVID-19 era. Front. Immunol. 14, 1100468 (2023).
Cesaro, S. et al. Update of recommendations for the management of COVID-19 in patients with haematological malignancies, haematopoietic cell transplantation and CAR T therapy, from the 2022 European Conference on Infections in Leukaemia (ECIL 9). Leukemia. ;37(9):1933-8. (2023).
CDC. (2024). https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
Krutikov, M. et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 3(1), e13–e21 (2022).
Al-Dury, S. et al. Catch-up antibody responses and hybrid immunity in mRNA vaccinated patients at risk of severe COVID-19. Infect. Dis. (Lond). 55 (10), 744–750 (2023).
Nazaruk, P. et al. Hybrid immunity provides the best COVID-19 humoral response in immunocompromised patients with or without SARS-CoV-2 infection history. Vaccines (Basel); 11(8). (2023).
Agency) FtSPH. (2023). https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/bekraftade-fall-i-sverige/).
Sharma, A. et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 8(3), e185–e93 (2021).
Li, L. et al. In-depth analysis of SARS-CoV-2-specific T cells reveals diverse differentiation hierarchies in vaccinated individuals. JCI Insight; 7(7). (2022).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16(10), 626–638 (2016).
Acknowledgements
This work was supported by the Swedish Medical Research council, Vetenskapsrådet, Diarenr 2021–04779, ALF funds from Sahlgrenska University hospital, Diarienr ALFGBG-438371 and a grant from the Gothenburg Medical Society (GLS).
Funding
Open access funding provided by University of Gothenburg.
Author information
Authors and Affiliations
Contributions
S.E. included all patients and was responsible for conducting the study, extracting and analyzing data, interpreting results, writing the manuscript, updating reference lists, and creating tables; S.A-D., A.M., K.H., and M.L. were responsible for designing and writing the protocol, extracting and analyzing data, interpreting results, writing the manuscript, updating reference lists; J.W. participated in interpreting results, writing the manuscript, performed statistical analyses and created the figures; J.R., L.D and H.W. were responsible for T-cell analyses and participated in extracting and analyzing data and interpreting results; E.F, A.M, J.W. and S.S. helped organize the vaccinations and blood sampling, participated in extracting and analyzing data and interpreting results. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Einarsdottir, S., Al-Dury, S., Fridriksson, E. et al. Immunogenicity of Comirnaty Omicron XBB.1.5 booster COVID-19 mRNA vaccine in long-term survivors after allogeneic hematopoietic stem cell transplantation. Sci Rep 14, 24749 (2024). https://doi.org/10.1038/s41598-024-74712-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74712-x