Abstract
Sepsis-associated encephalopathy (SAE) is a frequent and severe complication in septic patients, characterized by diffuse brain dysfunction resulting from systemic inflammation. Accurate prediction of long-term mortality in these patients is critical for improving clinical outcomes and guiding treatment strategies. We conducted a retrospective cohort study using the MIMIC IV database to identify adult patients diagnosed with SAE. Patients were randomly divided into a training set (70%) and a validation set (30%). Least absolute shrinkage and selection operator regression and multivariate logistic regression were employed to identify significant predictors of 1-year mortality, which were then used to develop a prognostic nomogram. The model’s discrimination, calibration, and clinical utility were assessed using the area under the receiver operating characteristic curve (AUC), calibration plots, and decision curve analysis, respectively. A total of 3,882 SAE patients were included in the analysis. The nomogram demonstrated strong predictive performance with AUCs of 0.881 (95% CI: 0.865, 0.896) in the training set and 0.859 (95% CI: 0.830, 0.888) in the validation set. Calibration plots indicated good agreement between predicted and observed 1-year mortality rates. The decision curve analysis showed that the nomogram provided greater net benefit across a range of threshold probabilities compared to traditional scoring systems such as Glasgow Coma Scale and Sequential Organ Failure Assessment. Our study presents a robust and clinically applicable nomogram for predicting 1-year mortality in SAE patients. This tool offers superior predictive performance compared to existing severity scoring systems and has significant potential to enhance clinical decision-making and patient management in critical care settings.
Similar content being viewed by others
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection1. The nervous system is often the first organ system to exhibit functional impairment due to sepsis, leading to sepsis-associated encephalopathy (SAE)2. SAE manifests as diffuse brain dysfunction stemming from the systemic inflammatory response to sepsis, with clinical presentations ranging from mild delirium to severe coma, and is associated with increased mortality and long-term physical, mental, and cognitive impairments3,4,5.
The severity of SAE correlates with short-term mortality, with more severe consciousness disorders indicating a poorer prognosis3. Notably, even mild mental status changes are independently linked to an increased risk of short-term mortality3. Beyond short-term outcomes, acute sepsis-related neurological disorders are the organ dysfunction most closely associated with long-term mortality and are a key mediator of adverse long-term outcomes following sepsis6. Among sepsis survivors who experienced acute neurological dysfunction, the 1-year mortality rate is increased by an absolute 6% compared to those without such dysfunction6. Long-term prognosis is a crucial outcome, reflecting the overall burden of the disease. Accurate prediction of long-term mortality rates enhances doctor-patient communication and informs sustainable medical management decisions7.
As the number of sepsis survivors rises5,8, identifying risk factors associated with the long-term prognosis of SAE patients, particularly those that are conventional, readily available, and cost-effective, is vital for developing optimized treatment strategies and assessing patient outcomes. This study aims to identify predictive factors for 1-year mortality in SAE patients and to establish and validate a 1-year mortality prediction model in the form of a nomogram, thereby providing clinicians with a valuable tool for evaluating patient prognosis.
Methods
Database and study design
The study utilized the Medical Information Mart for Intensive Care IV (MIMIC IV, Version 2.1) database, which contains de-identified health data from over 40,000 patients admitted to the Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, from 2008 to 20199. MIMIC IV includes comprehensive information on patient demographics, vital signs, laboratory test results, medications, procedures, and clinical outcomes, making it a valuable resource for conducting epidemiological and clinical research in critical care settings7.
The MIMIC IV database is de-identified in accordance with the Health Insurance Portability and Accountability Act, thereby safeguarding patient privacy. Consequently, its utilization for research does not necessitate additional patient consent. Researchers obtained access to the MIMIC IV data by completing the requisite data use agreement and the Collaborative Institutional Training Initiative Program’s course on “Data or Specimens Only Research.” The Institutional Review Board of BIDMC reviewed and approved the processes for patient data collection and the establishment of research resources, granting exemptions for informed consent and endorsing data-sharing protocols. Given the retrospective design of this study, and to uphold patient confidentiality while ensuring compliance with authorized use of the MIMIC IV database, no further ethical approval was required. All methods were performed in accordance with relevant guidelines and regulations.
In alignment with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines10, this study aimed to develop and validate a prognostic model for 1-year mortality in patients with SAE. The study involved screening adult patients with SAE from the MIMIC IV database, ensuring the exclusion of encephalopathy attributable to non-septic causes. Patients were randomly assigned to either a training set or a validation set. Least absolute shrinkage and selection operator (LASSO) regression and multivariate binary logistic regression analyses were conducted on the training set to identify significant risk factors for 1-year mortality. These identified risk factors were then utilized to construct a mortality prediction model, represented as a nomogram. The model’s performance was rigorously assessed by evaluating its discrimination, calibration, and clinical utility in both the training and validation cohorts.
Patient selection
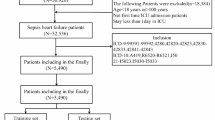
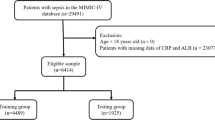
The study cohort comprised patients with SAE who were admitted to the intensive care unit (ICU) for the first time. The inclusion criteria were as follows: (1) Sepsis 3.0 criteria: patients diagnosed with or suspected of infection and an increase of at least 2 points in the Sequential Organ Failure Assessment (SOFA) score; (2) Diagnosis of SAE on the first day of ICU admission, defined by a Glasgow Coma Scale (GCS) score of < 15, a positive Confusion Assessment Method for the ICU, or corresponding International Classification of Diseases codes (2930, 2931, F05). The exclusion criteria included: (1) Any record of subsequent ICU admissions; (2) ICU stays shorter than 24 h; (3) Non-septic conditions; (4) Patients with clear consciousness and no delirium symptoms; (5) Underlying dementia; (6) Primary brain injuries such as traumatic brain injury, ischemic stroke, hemorrhagic stroke, intracranial infection, or epilepsy; (7) Hypoxic-ischemic encephalopathy; (8) Hypertensive encephalopathy; (9) Metabolic or toxic encephalopathy; (10) Mental and behavioral disorders due to alcohol, drugs, or psychoactive substances; (11) Severe electrolyte and metabolic imbalances, including hyponatremia (< 120 mmol/L), hyperglycemia (> 180 mg/dL), hypoglycemia (< 54 mg/dL), and partial pressure of carbon dioxide (PaCO2) ≥ 80 mmHg; (12) Patients aged 89 years or older. A total of 3882 patients meeting the inclusion criteria were selected for analysis (Fig. 1).
Selection Process and Exclusion Criteria for Research Subjects. CAM-ICU, Confusion Assessment Method for the ICU; GCS, Glasgow Coma Scale; MIMIC-IV, medical information mart for intensive care IV; ICD, International Classification of Diseases; ICU, intensive care unit; PaCO2, Partial pressure of CO2; SAE, Sepsis-Associated Encephalopathy.
Data extraction and preprocessing
Research data were extracted from the MIMIC IV database, encompassing variables such as age, gender, weight, marital status, race, underlying comorbidities, and the Charlson Comorbidity Index (CCI). Clinical data from the first day of ICU admission included vital signs, key laboratory parameters, the use of invasive mechanical ventilation, renal replacement therapy (RRT), vasoactive medications, albumin administration, the GCS score, and other critical care scoring indices. Vasoactive medications comprised norepinephrine, epinephrine, dopamine, dobutamine, vasopressin, and milrinone. For variables with multiple measurements, the MIMIC concept was utilized to systematically extract the minimum, maximum, and average values where applicable. Time-related indicators were extracted to calculate ICU mortality, in-hospital mortality, 28-day mortality, 1-year mortality, length of hospital stay (LOS), and ICU LOS. The primary outcome measure was the 1-year mortality, determined by the survival status one year post ICU admission.
Data processing were conducted using Stata software (version 17.0, Stata Corporation LLC, College Station, USA). Outliers were identified through histogram analysis and addressed using the Winsorization technique via the Winsor2 command. Missing data were imputed using multiple imputation methods to ensure robust and accurate analysis.
Study cohorts
Using R software (version 4.3.2, R Foundation for Statistical Computing, Vienna, Austria), the cohort of 3,882 patients with SAE was randomly divided into a training set (70%) and a validation set (30%) for the development and validation of predictive models, with the random seed set to 3684. The ‘gtsummary’ package was employed to perform univariate analyses on both the training and validation sets, assessing differences between the two groups and verifying the effectiveness of the randomization process. This approach ensures that both subsets are representative of the overall patient population, thereby supporting the robustness of the predictive models.
Model development
Following the implementation of the ‘glmnet’ R package, regression analysis was performed. LASSO regression was utilized on the training set to discern significant risk factors for 1-year mortality. This technique facilitates feature selection by constraining less significant coefficients to zero, thereby enhancing model interpretability. Subsequently, the variables identified through LASSO regression were further analyzed using multivariate binary logistic regression to determine independent risk factors for 1-year mortality in patients with SAE. The ‘car’ R package was employed to calculate the variance inflation factor. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for each risk factor to quantify their association with 1-year mortality. This process culminated in the development of a robust mortality prediction model based on these identified risk factors.
Nomogram construction and validation
Using the “rms” R package, a nomogram was developed from the final mortality prediction model based on logistic regression to visually represent the prediction model. This nomogram enables clinicians to estimate the probability of 1-year mortality for individual patients with SAE based on their specific clinical characteristics.
The discrimination, calibration, and clinical applicability of the mortality prediction model were assessed in both the training and validation sets. The “riskRegression” package was utilized to construct receiver operating characteristic (ROC) curves for the nomogram, SOFA, GCS score, Acute Physiology Score III (APS III), and Logistic Organ Dysfunction System (LODS), allowing evaluation of the model’s discriminative ability. Calibration curves were generated using the “val.prob” function to assess the agreement between predicted and observed outcomes. The “rmda” package facilitated decision curve analysis to evaluate clinical utility and ascertain the model’s net benefit across a range of threshold probabilities. Statistical significance was defined as a p-value < 0.05.
Results
Baseline characteristics
Comprehensive baseline characteristics of SAE patients in the entire cohort, as well as the training (n = 2751) and validation (n = 1131) subsets, are delineated in Table 1. Following extensive univariate analysis, no statistically significant disparities (P > 0.05) were observed across various demographic factors, comorbidities, disease severity indices, vital signs, essential laboratory parameters, or therapeutic interventions between the training and validation cohorts. Notably, the absence of significant discrepancies, particularly in 1-year mortality, underscores the rigorous adherence to scientific principles governing random allocation, thereby ensuring comparability across the cohorts. Patients with SAE were categorized into survival and death groups based on their 1-year survival outcomes. The supplementary materials provide a comprehensive comparison and analysis of the baseline characteristics of these two groups within both the training and validation sets.
Development of the nomogram
To identify predictors of 1-year mortality in patients with SAE, we evaluated numerous clinical variables. Using LASSO regression combined with 10-fold cross-validation, we initially screened for potential predictive factors (Fig. 2a and b). These preliminary factors were then subjected to binary multivariate regression analysis, resulting in the identification of 16 factors that were significantly associated with 1-year mortality in SAE patients (P < 0.05) (Fig. 3). Based on these predictive factors, a prognostic nomogram for 1-year mortality in SAE patients was subsequently developed (Fig. 4).
Feature Selection Process Using LASSO Regression and Tenfold Cross-Validation. (a) The graph illustrates the relationship between the coefficients of clinical features and the lambda values in LASSO regression. (b) The graph presents the tenfold cross-validation curve for LASSO regression, which aids in model selection. This figure provides a comprehensive view of the feature selection process and the criteria used for determining the optimal model parameters. LASSO, least absolute shrinkage and selection operator regression; λ, lambda.
Multivariate Binary Logistic Regression Analysis for Predicting 1-Year Mortality in SAE Patients. This forest plot displays the results of the multivariate binary logistic regression analysis, identifying independent predictors of 1-year mortality among SAE patients in the training set. Each predictor is represented with its corresponding odds ratio and 95% confidence interval, providing a clear visualization of the significant factors contributing to the 1-year mortality risk. PaCO2, Partial pressure of CO2; SAE, Sepsis-Associated Encephalopathy. *: Minimum recorded values of indicators during the first 24 h of ICU admission; **: Mean values of indicators during the first 24 h of ICU admission; ***: Maximum recorded values of indicators during the first 24 h of ICU admission.
Nomogram for Predicting 1-Year Mortality in Patients with SAE. The nomogram operates by assigning points to specific variables based on their respective values. The total points are then summed and the corresponding 1-year mortality rate is determined by referencing the total point axis. The red dots in the figure represent a specific case of an SAE patient with the following characteristics: no history of malignant tumors, a Charlson Comorbidity Index score of 5, weight of 86.2 kg, maximum heart rate of 114 beats per minute, average respiration rate of 18 breaths per minute, average body temperature of 36.7 degrees Celsius, first day urine output of 1621 mL, maximum hemoglobin value of 150 g/dL, anion gap of 18 mmol/L, maximum partial thromboplastin time of 29.2 s, minimum prothrombin time of 11.3 s, maximum lactate value of 0.9 mmol/L, minimum lactate value of 0.6 mmol/L, PaCO2 of 29 mmHg, and minimum Glasgow Coma Scale score of 14. The patient also received treatment with supplemental oxygen. The total score for this patient was calculated to be 625, corresponding to a 1-year mortality rate of 4.38%. PaCO2, Partial pressure of CO2; SAE, Sepsis-Associated Encephalopathy. *: Minimum recorded values of indicators during the first 24 h of ICU admission; **: Mean values of indicators during the first 24 h of ICU admission; ***: Maximum recorded values of indicators during the first 24 h of ICU admission.
Nomogram discrimination
The developed prognostic nomogram demonstrated robust discrimination performance, with an area under the receiver operating characteristic (ROC) curve of 0.881 (95% CI, 0.865 to 0.896) in the training set and 0.859 (95% CI, 0.830 to 0.888) in the validation set (Fig. 5). These results indicate a high level of accuracy in predicting 1-year mortality among patients with SAE. Importantly, the discrimination performance of the constructed nomogram exceeded that of the GCS score and other commonly utilized disease severity scoring systems, underscoring its superior prognostic utility (Fig. 5). This enhanced predictive capability can provide clinicians with a more reliable tool for risk stratification and individualized patient management, ultimately may improving clinical outcomes for patients with SAE.
Comparison of Model Accuracy in Predicting 1-Year Mortality in SAE Patients. The graph compares the accuracy of different models in predicting the 1-year mortality rate of patients with sepsis-associated encephalopathy (SAE) in the training set (a) and validation set (b). The established nomogram demonstrated the highest area under the receiver operating characteristic curve in both datasets, indicating superior predictive performance compared to other models. APS III, Acute Physiology Score III; AUC, area under the receiver operating characteristic curve; GCS, Glasgow Coma Scale; LODS, Logistic Organ Dysfunction System; SAE, Sepsis-Associated Encephalopathy; SOFA, Sequential Organ Failure Assessment.
Nomogram calibration
Calibration curves were drawn for the nomograms in both the training and validation sets to evaluate the accuracy of the prognostic nomogram. The analysis revealed a significant alignment between the predicted mortality rate and the actual 1-year mortality rate, underscoring the robust calibration performance of the model across both datasets (Fig. 6a, b). This consistency highlights the reliability of the nomogram in providing accurate mortality predictions for patients with SAE, thereby enhancing its clinical applicability for risk assessment and patient management.
Calibration Curve of the Established Nomogram. The graph displays the calibration curves for the established nomogram, illustrating the agreement between predicted and observed 1-year mortality rates in the training set (a) and validation set (b). The curves demonstrate good consistency, indicating the nomogram’s reliable predictive performance in both datasets.
Nomogram clinical utility
Decision curve analysis (DCA) was employed to assess the clinical utility of the developed nomogram. Our nomogram demonstrated superior clinical utility compared to both the GCS score and other commonly used disease severity scoring systems (Fig. 7). This analysis underscores the enhanced practicality of our nomogram in clinical settings, providing clinicians with a more effective tool for prognostic assessment and patient management.
Decision Curve Analysis (DCA) for Predicting 1-Year Mortality in SAE Patients. The graph presents the DCA for various models predicting 1-year mortality in patients with SAE in the training set (a) and validation set (b). The curves assess the clinical utility of the models. The established nomogram demonstrates significantly higher clinical utility compared to other models in both datasets, indicating its superior performance in practical application. APS III, Acute Physiology Score III; AUC, area under the receiver operating characteristic curve; GCS, Glasgow Coma Scale; LODS, Logistic Organ Dysfunction System; SAE, Sepsis-Associated Encephalopathy; SOFA, Sequential Organ Failure Assessment.
Discussion
In this study, we developed and validated a prognostic nomogram to predict 1-year mortality in patients with SAE using a comprehensive set of clinical variables. Our findings demonstrate that the nomogram exhibits robust discriminative ability and calibration performance, surpassing traditional scoring systems such as the GCS score and other common disease severity indices. By integrating easily obtainable clinical parameters, our model provides clinicians with a practical and reliable tool for risk stratification in SAE, facilitating more informed decision-making and personalized patient management. These results underscore the importance of incorporating multifaceted clinical data to enhance prognostic accuracy and improve outcomes in critically ill patients with sepsis.
Our research has made substantial advancements compared to previous studies on prognostic models for SAE. Earlier studies primarily concentrated on short-term outcomes and, although useful for immediate clinical decision-making, often lacked the ability to predict long-term outcomes, particularly for SAE patients11,12,13,14,15. For instance, using the MIMIC III database, a user-friendly nomogram was created to predict 30-day mortality risk in SAE patients, with an AUC of 0.763 [0.736–0.791]11, indicating moderate predictive performance. Another study developed a nomogram based on clinical data to predict in-hospital mortality in SAE patients12. However, this model included the Simplified Acute Physiology Score II (SAPS II) score as a predictive variable, necessitating its prior completion, thereby increasing the model’s complexity and limiting its practical application.
Moreover, sophisticated machine learning methods have been employed to construct mortality prediction models, effectively predicting the 30-day mortality rate or ICU mortality rate in SAE patients13,14. Among these models, the APS III score emerged as a significant predictor, yet its necessity for model use adds complexity, hindering clinical adoption. A notable effort involved a stacking ensemble model that achieved a high AUC (0.807) in the test set and 0.671 in external validation for predicting ICU mortality risk in SAE patients using common clinical variables15.
In contrast, our nomogram, based on logistic regression, leverages common clinical features as predictive factors, making it both interpretable and straightforward. This simplicity enhances its clinical applicability and ease of use. By focusing on easily obtainable clinical features and ensuring transparency in how these factors influence mortality, our model offers a significant improvement in both predictive performance and practical implementation, facilitating broader clinical adoption.
In our predictive model, key risk factors for 1-year mortality in SAE patients include a history of malignancy, higher CCI scores, elevated minimum lactate levels, lower mean body temperature, and decreased maximum lactate levels. Conversely, SAE patients receiving oxygen supplementation exhibited lower 1-year mortality rates. Cancer patients are more susceptible to sepsis than the general population, with sepsis being a leading cause of ICU admissions among these individuals16. Compared to non-cancer sepsis patients, those with cancer have a significantly higher risk of late mortality (OR = 2.46, 95% CI: 1.42–4.25, I²=99%)17. Cancer patients undergo complex immune alterations, with treatments often inducing local or systemic inflammation as a result of tissue damage and the death of cancer cells18. Both chronic host state dysregulation due to cancer and acute host response dysregulation due to sepsis mediate mortality in sepsis patients with pre-existing malignant cancer16.
The CCI is a well-established predictor of outcomes in sepsis19, with comorbidities being a significant determinant of infection-related in-hospital mortality20. Accumulation of comorbid conditions is closely linked to increased severity of acute organ dysfunction, underscoring the critical role of comorbidities in the clinical course and prognosis of septic patients21. Consequently, the CCI serves as a valuable tool in stratifying risk and guiding clinical decision-making in patients with sepsis, underscoring the necessity of comprehensive comorbidity assessment in improving prognostication and individualized patient care.
Body temperature is inversely correlated with the prognosis of patients with sepsis-associated encephalopathy (SAE), aligning with previous research findings. A systematic review of 42 studies reported mortality rates of 22.2% for septic patients with a fever > 38 °C, 31.2% for normothermic patients, and 47.3% for hypothermic patients (< 36.0 °C)22. Fever appears to enhance the innate immune response, and many bacteria exhibit reduced replication at higher temperatures23. Conversely, hypothermia is common in sepsis and is associated with increased mortality24. Therefore, interventions aimed at warming patients with hypothermic sepsis may improve prognosis.
The measurement of serum lactate levels is a critical component in the clinical management of critically ill patients, particularly those with sepsis or septic shock1. Various metabolic changes in sepsis can elevate blood lactate levels, such as increased glycolysis, heightened Na-K pump activity stimulated by catecholamines, alterations in pyruvate dehydrogenase activity, and decreased lactate clearance due to impaired liver perfusion25. Elevated lactate levels are recognized as an independent risk factor for mortality in sepsis patients26, whereas lower lactate concentrations are associated with better outcomes25. Lactate clearance, defined by the change in lactate levels between two time points, is an efficient and cost-effective parameter that holds promise as a target for quantitative recovery27. Early lactate clearance-guided therapy has been linked to reduced mortality in sepsis27. Our study indicates that lower maximum lactate levels are associated with an increased risk of long-term mortality. This finding may initially seem counterintuitive, as elevated lactate levels are typically associated with worse outcomes. However, the interpretation of lactate levels must consider the overall clinical context and trends rather than isolated values. In this study, the association between lower maximum lactate levels and higher minimum lactate levels as indicators of poor prognosis in SAE patients indeed suggests a complex relationship. Specifically, when both lower maximum and higher minimum lactate levels are observed, it implies that the peak lactate level on the first day of ICU admission is relatively close to the trough, which may indicate suboptimal lactate clearance. This pattern could be indicative of inadequate metabolic recovery or persistent underlying issues, even if initial improvements are apparent. Thus, in the presence of other indicators of poor clinical progression, lower maximum lactate levels might reflect more complex clinical conditions that contribute to increased mortality risk. Unfortunately, due to the variability in timing for lactate remeasurement among patients and the absence of standardized protocols, precise lactate clearance rate data are not available in the MIMIC database. This limitation prevents us from obtaining standard lactate clearance rates and further elucidating their direct relationship with SAE mortality. We acknowledge this gap and agree that future research is needed to investigate the exact relationship between standard lactate clearance rates and SAE mortality. Such studies could provide valuable insights into the role of lactate dynamics in predicting outcomes and guide more effective management strategies for SAE patients.
The clinical implications of this study are substantial. The developed nomogram, based on readily available clinical variables, provides a practical and reliable tool for predicting 1-year mortality in patients with SAE. By incorporating this predictive model into clinical practice, healthcare providers can more accurately stratify patients based on their risk, facilitating personalized treatment strategies and informed decision-making. This model aids in identifying high-risk patients who may benefit from intensified monitoring and therapeutic interventions, thereby potentially improving clinical outcomes. Furthermore, the nomogram’s superior performance compared to traditional scoring systems underscores its value in enhancing prognostic accuracy. The use of decision curve analysis further emphasizes the model’s clinical utility, demonstrating its ability to offer significant net benefits across a range of threshold probabilities. Ultimately, the adoption of this predictive tool in clinical settings could lead to improved resource allocation, better patient management, and enhanced communication between clinicians and patients regarding prognosis and care plans.
This study offers several advantages. Firstly, the use of the MIMIC IV database, which includes a large and diverse patient cohort, enhances the generalizability and robustness of our research findings. The combination of LASSO regression and multivariate logistic regression ensures rigorous selection of predictive factors, thereby ensuring the correlation and accuracy of identified risk factors. Moreover, the development of nomograms incorporates a range of readily available clinical variables, making them practical tools for use across various clinical settings. The validation of the nomograms in both the training and validation cohorts underscores their reliability and robustness. Additionally, the inclusion of decision curve analysis to assess clinical utility provides valuable insights into the practical benefits of nomograms, highlighting their superior performance in predicting 1-year mortality rates in patients with sepsis-associated encephalopathy compared to traditional scoring systems. Therefore, this study not only introduces a novel predictive model but also establishes a benchmark for future research aimed at enhancing prognostic tools for critically ill patients.
Several limitations should be noted in this study. Firstly, the retrospective nature of our analysis may introduce inherent biases, including selection and information biases, potentially affecting the generalizability of our findings. Prospective studies are warranted to further validate the established nomogram. Additionally, while the comprehensive use of the MIMIC IV database provides robust data, its restriction to a single healthcare system may limit the applicability of our results to broader populations and settings. Therefore, we plan to conduct external validation of our prediction model in diverse settings. Furthermore, although the nomogram demonstrates strong predictive performance, it relies on variables available within the database. During variable selection, we carefully considered factors such as the feasibility of data collection, accessibility, and economic considerations. Finally, while our study included a wide array of clinical variables, the MIMIC-IV database has inherent limitations, including the absence of certain potential predictive factors such as some biomarkers, genetic data, electroencephalography, and cranial imaging examinations. These limitations underscore the need for further research to explore these additional factors and enhance our understanding of SAE. Future prospective studies should address these limitations and validate our findings across various medical contexts and patient populations.
Conclusion
In conclusion, this study successfully developed and validated a robust nomogram for predicting 1-year mortality in patients with SAE. Utilizing a large and diverse cohort from the MIMIC IV database, our model demonstrated superior predictive performance compared to traditional scoring systems, underscoring its potential utility in clinical practice. The inclusion of easily obtainable clinical variables ensures the model’s practicality and accessibility, making it a valuable tool for risk stratification and personalized patient management. Furthermore, the decision curve analysis highlighted the clinical relevance and net benefit of the nomogram, affirming its potential to enhance prognostic accuracy and inform therapeutic decision-making. Future studies should focus on external validation and the incorporation of additional predictive factors to further refine and validate the model.
Data availability
The anonymised data collected are available as open data via the MIMIC data repository: https://physionet.org/content/mimiciv/2.1.
Abbreviations
- APS III:
-
Acute Physiology Score III
- AUC:
-
area under the receiver operating characteristic curve
- BIDMC:
-
Beth Israel Deaconess Medical Center
- CCI:
-
Charlson Comorbidity Index
- CI:
-
confidence interval
- DCA:
-
decision curve analysis
- GCS:
-
Glasgow Coma Scale
- ICU:
-
Intensive Care Unit
- LASSO:
-
Least Absolute Shrinkage and Selection Operator regression
- LODS:
-
Logistic Organ Dysfunction System
- LOS:
-
length of stay
- MIMIC-IV:
-
Medical Information Mart for Intensive Care IV
- OR:
-
odd ratio
- ROC:
-
receiver operating characteristic
- RRT:
-
Renal Replacement Therapy
- SAE:
-
Sepsis-Associated Encephalopathy
- SAPS II:
-
Simplified Acute Physiology Score II
- SOFA:
-
Sequential Organ Failure Assessment
- TRIPOD:
-
Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis
References
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med47, 1181–1247 (2021).
Piva, S., Bertoni, M., Gitti, N., Rasulo, F. A. & Latronico, N. Neurological complications of sepsis. Curr Opin Crit Care29, 75–84 (2023).
Sonneville, R. et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med43, 1075–1084 (2017).
Jin, G. et al. Identification of sepsis-associated encephalopathy risk factors in elderly patients: a retrospective observational cohort study. Turk J Med Sci52, 1513–1522 (2022).
Mostel, Z. et al. Post-sepsis syndrome - an evolving entity that afflicts survivors of sepsis. Mol Med26, 6 (2019).
Schuler, A. et al. The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med46, 843–849 (2018).
Jin, G., Hu, W., Zeng, L., Ma, B. & Zhou, M. Prediction of long-term mortality in patients with ischemic stroke based on clinical characteristics on the first day of ICU admission: an easy-to-use nomogram. Front Neurol14, 1148185 (2023).
Prescott, H. C. & Angus, D. C. Enhancing recovery from sepsis: a review. JAMA319, 62–75 (2018).
Johnson, A. et al. MIMIC-IV (version 2.1), https://doi.org/10.13026/rrgf-xw32 (2022).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ350, g7594 (2015).
Yang, Y. et al. Development of a nomogram to predict 30-day mortality of patients with sepsis-associated encephalopathy: a retrospective cohort study. J Intensive Care8, 45 (2020).
Zhao, L. et al. Development and validation of a nomogram for the prediction of hospital mortality of patients with encephalopathy caused by microbial infection: a retrospective cohort study. Front Microbiol12, 737066 (2021).
Peng, L. et al. Machine learning approach for the prediction of 30-day mortality in patients with sepsis-associated encephalopathy. BMC Med Res Methodol22, 183 (2022).
Guo, J. et al. Factor analysis based on SHapley Additive exPlanations for sepsis-associated encephalopathy in ICU mortality prediction using XGBoost - a retrospective study based on two large database. Front Neurol14, 1290117 (2023).
Liu, X., Niu, H. & Peng, J. Enhancing predictions with a stacking ensemble model for ICU mortality risk in patients with sepsis-associated encephalopathy. J Int Med Res52, 3000605241239013 (2024).
Williams, J. C., Ford, M. L. & Coopersmith, C. M. Cancer and sepsis. Clin Sci (Lond)137, 881–893 (2023).
Xiang, M. J. & Chen, G. L. Impact of cancer on mortality rates in patients with sepsis: a meta-analysis and meta-regression of current studies. World J Clin Cases10, 7386–7396 (2022).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell140, 883–899 (2010).
Emami-Razavi, S. H., Mohammadi, A., Alibakhshi, A., Jalali, M. & Ghajarzadeh, M. Incidence of post-operative sepsis and role of Charlson co-morbidity score for predicting postoperative sepsis. Acta Med Iran 54, 318–322 (2016).
Torvik, M. A. et al. Patient characteristics in sepsis-related deaths: prevalence of advanced frailty, comorbidity, and age in a Norwegian hospital trust. Infection51, 1103–1115 (2023).
Esper, A. M. et al. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med34, 2576–2582 (2006).
Rumbus, Z. et al. Fever is associated with reduced, hypothermia with increased mortality in septic patients: a meta-analysis of clinical trials. PLoS One12, e0170152 (2017).
Doman, M. et al. Temperature control in sepsis. Front Med (Lausanne)10, 1292468 (2023).
Baek, M. S., Kim, J. H. & Kwon, Y. S. Cluster analysis integrating age and body temperature for mortality in patients with sepsis: a multicenter retrospective study. Sci Rep12, 1090 (2022).
Vincent, J. L., Quintairos, E. S. A., Couto, L. Jr. & Taccone, F. S. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care20, 257 (2016).
Liu, Z. et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med27, 51 (2019).
Gu, W. J., Zhang, Z. & Bakker, J. Early lactate clearance-guided therapy in patients with sepsis: a meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med41, 1862–1863 (2015).
Acknowledgements
The authors express gratitude for the language refinement and assistance in drafting acknowledgments provided by ChatGPT. However, the scientific accuracy and integrity of the paper remain the sole responsibility of all contributing authors. Furthermore, the authors wish to extend their appreciation to HOME for Researchers (https://www.home-for-researchers.com) for their invaluable support in manuscript preparation and journal selection.
Funding
This study was supported by the grant from the Construction Fund of Medical Key Disciplines of Hangzhou (Grant: OO20200485), the Traditional Chinese Medicine Science and Technology Project of Zhejiang Province (Grant: 2024ZL750), the Science and Technology Development Project of Hangzhou (Grant: 202204A10), the National Health Commission Scientific Research Fund/Zhejiang Province Key Medical and Health Science and Technology Program Project (Grant: WKJ-ZJ-2315).
Author information
Authors and Affiliations
Contributions
Study design: G.J., M.Z., Y.D.; Data extraction: G.J., J.C.; Data curation: M.Z., Y.D., C.F.; Statistical analysis: G.J., J.C., J.W.; Writing—original draft preparation: M.Z., J.C., B.M., J.W., R.Y.; Writing—review and editing: G.J., M.Z., J.C., B.M., J.W., R.Y., C.F., W.H., Y.D.; Supervision and project administration: B.M., W.H., Y.D.; Resources and funding acquisition: G.J., W.H.; All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, G., Zhou, M., Chen, J. et al. Comprehensive risk factor-based nomogram for predicting one-year mortality in patients with sepsis-associated encephalopathy. Sci Rep 14, 23979 (2024). https://doi.org/10.1038/s41598-024-74837-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-74837-z