Abstract
Chronic liver disease is closely linked to dietary intake factors, such as high consumption of simple carbohydrates including sucrose. In this study, the influence of sucrose on the development of hepatocellular carcinoma (HCC), the most common primary liver malignancy, was explored. Using the hepatocarcinogen diethylnitrosamine (DEN) to induce HCC in the rat, we co-administered sucrose with DEN. The co-administration significantly modified body, liver and pancreas weight, as well as, serum fatty acids and triglycerides. DEN caused liver structural alteration, fibrosis, and tumor formation; surprisingly, co-administration with sucrose restored hepatic lipids, improved liver architecture, and reduced fibrosis and tumor development. Sucrose intake negatively regulated tumor markers and cell proliferation, and reduced the expression of genes associated with lipid metabolism and oxidative stress response. These findings highlight a hepatoprotective effect of sucrose during DEN-induced hepatocarcinogenesis, underlining an intriguing role of high sucrose consumption during HCC development and providing new insights as well as possible pathways of cellular protection under sucrose intake on hepatocarcinogenesis.
Similar content being viewed by others
Introduction
Over the past decade, the liver cancer incidence has increased by 25% and HCC is the most common type of cancer. Currently, it is the sixth most common malignancy and the third leading cause of cancer-related death worldwide1. In 2020, more than 900,000 cases were globally diagnosed and deaths exceeded 800,000 cases. Unfortunately, it has been estimated that around 1.4 million people will receive an HCC diagnosis between 2020 and 2040, which represents 55% increase of new cases and it is expected that mortality will increase by 56.4%2,3.
Current therapeutic approaches for HCC have evolved significantly, but challenges persist due to late diagnosis. Treatment options vary depending on the stage of the disease and include surgical resection, liver transplantation, and locoregional therapies such as radiofrequency ablation and transarterial chemoembolization for early and intermediate stages. In advanced HCC, systemic therapies are key, with drugs like sorafenib and lenvatinib along with emerging immunotherapies like pembrolizumab and nivolumab, improving outcomes. Additionally, combination of immunotherapies, like atezolizumab with bevacizumab show promising efficacy. However, the high rate of recurrence and resistance to therapies highlights the need for more effective, personalized treatments4,5,6.
Chronic inflammatory infections caused by hepatitis B (HBV) and hepatitis C (HCV) viruses, alcohol consumption, ingestion of aflatoxin-contaminated food, and non-alcoholic steatohepatitis related to obesity are the most common risk factors for HCC. Changes in alcohol consumption, the prevalence of obesity, and the treatment of HBV and HCV have contributed to modify the liver cancer epidemiology. However, these changes have not had a significant impact on the global burden of liver cancer1.
Excessive consumption of sucrose and high-fructose corn syrup in processed foods and beverages has significantly increased obesity and Metabolic Associated Fatty Liver Disease (MAFLD) rates in recent decades7. The contribution of excessive sugar consumption to the emerging epidemics of obesity and associated cardiometabolic disease has been well-documented, and the main strategies for managing MAFLD should focus on lifestyle interventions and behavioral adaptations8. For instance, fructose, a monosaccharide widely used in the food industry is added in high-calorie foods and beverages9,10.
MAFLD has been associated with varying percentages of HCC cases, ranging from 1 to 38%, depending on the geographic region and country1. Although the association between MAFLD and HCC has been established, and extensive research has explored the role of sucrose in the development of MAFLD, metabolic syndrome, and obesity, unmet gaps remain in clarifying the underlying mechanisms linking these liver diseases, particularly regarding the effects of high sucrose consumption on HCC development, which have yet to be investigated. The aim of this research was to determine the impact of the simultaneous administration of DEN, a highly reactive hepatocarcinogen, along with high sucrose consumption in the rat liver.
Materials and methods
Animals and ethical approval
Forty-five male Wistar rats, weighing 180–200 g, were procured from the Unit of Production and Experimentation of Laboratory Animals at the Center for Research and Advanced Studies of the National Polytechnic Institute (UPEAL-CINVESTAV-IPN, Mexico City, Mexico). All experimental procedures were performed according to protocol No. 0327-2, approved by the Committee for the Care and Use of Laboratory Animals (CICUAL) of CINVESTAV-IPN. Furthermore, the study conformed to the ARRIVE guidelines (AnimalResearch: Reporting of In Vivo Experiments). Rats were housed in well-ventilated cages kept in bio-bubbles protected by a double HEPA filtration system, and maintained in a stable environment of 12 h light/dark cycles and temperature. Animals had ad libitum access to filtered water, which was subjected to chlorination and ultrafiltration with a 9-ppm system, and a standard diet (PicoLab® Rodent Diet 20 5053). All animals underwent a 7-day acclimatization period before the experiments.
Induction of experimental hepatocarcinogenesis
From the established protocol for hepatocarcinogenesis11, we conducted the protocol described in Supplementary Fig. 1. Briefly, rats were randomly distributed into nine experimental groups. While DEN was weekly injected at 50 mg/kg via intraperitoneal (No 756-5; Sigma-Aldrich, Toluca, Mexico), sucrose was provided in drinking water ad libitum at 30% (w/v). Animal groups designated as DEN (8-DEN) and DEN plus Sucrose (8-D/S) received weekly injections of DEN for six weeks. Additionally, the groups Sucrose (8-S) and 8-D/S were provided with sucrose for 6 weeks. The control group (8-C) was subjected to six injections of vehicle (0.9% saline solution, w/v).
Animal groups designated as DEN (18-DEN), DEN plus Sucrose for six weeks (18-6-D/S), and DEN plus Sucrose for 18 weeks (18-18-D/S), were exposed to a 16-week period of DEN regimen. The 18-18-D/S groups also received sucrose alongside the 18-Suc group. The 18-6-D/S group received sucrose during the last 6 weeks of the experiment. The control group (18-C) was subjected to eighteen injections of vehicle (0.9% saline solution, w/v). All experimental groups were euthanized out 2 weeks following the last DEN injection.
Processing of biological material
Animal euthanasia was conducted via exsanguination under anesthesia with ketamine (100 mg/kg body weight) and xylazine (8 mg/kg body weight) following the experimental design (Supplementary Fig. 1). Blood samples were collected via cardiac puncture, mixed with heparin sodium [1000 UI/mL], (Laboratiorios PISA, S.A. DE C.V) and subsequently, samples were subjected to centrifugation at 300 rpm for 15 min. This process allowed for the separation of plasma from the cell components, which were then stored in 500 µL aliquots at − 70 °C.
Livers were excised, washed with cold PBS, weighed, photographed, and immediately split for different purposes. Some liver pieces for cryosection were rapidly frozen in cold 2-methyl butane contained in a container immersed for 10 min and stored at − 70 °C. Others liver pieces were fixed in 10% formalin, embedded in paraffin and subsequently, sectioned for further histological analyses.
Determination of free fatty acids and triglycerides in serum
Plasma triglycerides were measured using the Cobas c111 analyzer (Roche, Basel, Switzerland). Plasma free fatty acids were assessed following the manufacturer’s guidelines with the Free Fatty Acid Quantification Assay Kit (ab65341, Abcam, Cambridge, UK).
Histological analyses
For histological analyzes, liver tissues were embedded and sliced into 5 μm-thick sections and mounted on electrocharged slides. Then, sections were subjected to deparaffinization and rehydration steps before undergoing staining. Specifically, samples were stained using Hematoxylin and Eosin (H&E) method for histological examination. For Oil Red O (ORO) staining, 5 μm-thick fresh-frozen tissue sections were used, and the protocol outlined by Mehlem et al. was followed12.
AST and ALT determination in liver tissue
For the determination of AST and ALT in liver tissue, hepatic samples were homogenized in phosphate-buffered saline and centrifuged at 10,000 rpm for 5 min to obtain the supernatant. The enzyme activities were then measured using Activity Assay Kit Abcam (ab105134). Briefly, the supernatant was incubated with the respective substrates, and the reaction was monitored by measuring absorbance changes at specific wavelengths corresponding to AST and ALT activity. The results were normalized to the total protein content of the tissue extract to express enzyme activity as units per milligram of protein.
γ-Glutamyl transferase (GGT) histochemistry
This procedure was performed in 15 μm-tick fresh-frozen tissue sections. First, tissues were fixed by incubation in pure acetone for 10 min. Then, they were incubated with a solution consisting of 75 mg of L-glutamic acid γ-4-methoxy-β-naphthylamide (G0141; Sigma-Aldrich, St. Louis, MO, USA), 600 µg of glycylglycine (G1002; Sigma-Aldrich, St. Louis, MO, USA), and 150 µg of Fast-Blue (F3378; Sigma-Aldrich, St. Louis, MO, USA) at pH 7.4, for 12 min. GGT-positive tissue areas appeared as red-colored regions. The slides were then air-dried at room temperature (RT). Finally, the GGT-positive areas were captured using a ZEISS Axio-A1 microscope (Carl-Zeiss, Oberkochen, Germany).
Immunofluorescence analysis
For immunofluorescence analysis, antigen retrieval was performed using the Rodent Decloaker kit (BioCare Medical, CA, USA), followed by a 2 h tissue blocking step with 1% Bovine serum albumin (BSA) in PBS. Primary antibodies were Ki-67 (1:100; GTX16667 GeneTex, San Antonio, TX, US), KEAP1 (1:25; GTX54329, GeneTex, San Antonio, TX, US), NRF2 (1:25; sc-13032, Santa Cruz Biotechnology, Dallas, CA, USA) and CD36 (1:100, Novus, NB400-144). Tissues were incubated overnight at 4 °C with primary antibodies and then, they were incubated for 1 h at RT in the dark with Alexa Fluor 488 anti-rabbit antibody (1:300; ab150077, Abcam, MA, USA). Nuclear DNA was stained using DAPI (1:1000; 62247; Thermo Scientific, IL, USA) for 5 min at RT. Subsequently, tissues were fixed with Fluoroshield mounting medium (Thermo Scientific, IL, USA), and immunofluorescence images were captured using a ZEISS Axio-A1 Microscopy (Carl-Zeiss, Oberkochen, Germany).
Quantitative RT-PCR analysis
To assess gene expression in liver tissues samples, RNA extraction and RT-qPCR analysis were conducted. Liver tissue slices, weighing 20 mg, were precisely sectioned at − 16 °C using a cryostat and immediately protected in accordance with the manufacturer’s instructions using the RNeasy Mini Kit (Qiagen, 74106; NW; Germany). The purity and concentration of extracted RNA were determined using the NanoDrop One spectrophotometer (Thermo Fisher Scientific Inc; MA, USA), and RNA integrity was verified through agarose gel electrophoresis. Subsequently, cDNA reactions were prepared using 750 ng of total RNA with the High-Capacity Reverse cDNA transcription kit (4368814; Applied Biosystems; MA, USA). TaqMan® gene expression assays were used in the QuantStudio 7 Flex Real-time PCR system (Applied Biosystem, MA, USA) to evaluate the expression of the following rat genes, Ggt1 (Rn00587709_m1), Gstp1 (Rn00561378_gH), Keap1 (Rn00589292_m1), and Nfe2l2 (Rn00477784_m1), Ppargc1a (Rn00580241_m1), Ppargc1b (Rn00598552_m1), Pprc1 (Rn01411002_m1), Ppara (Rn00566193_m1), Ppard (Rn00565707_m1), Pparg (Rn00440945 _m1), Slc27a5 (Rn00577177_m1), Fabp1 (Rn00664587_m1), Cd36 (Rn00580728_m1) and Cav1 (Rn00755834_m1). Gene expression was normalized with that of 18 S rRNA (Rn0392899), which was used as an internal control. Reactions were carried out in according with the manufacturer’s recommendations. Relative gene expression values were calculated using the ΔΔCt method, using the control group as the sample reference.
Analysis of HCC disease-free progression using Kaplan–Meier curves
In this study, we also examined the relationship between the PPARGC1A, PPARGC1B, PPRC1, PPARA, PPARD, PPARG, SLC27A5, FABP1, CAV1 and CD36 genes, and disease-free progression in patients exclusively diagnosed with HCC. We used a sample group sourced from the TCGA PanCancer liver hepatocellular carcinoma dataset (available at: https://www.cbioportal.org/study/summary?id=lihc_tcga_pan_can_atlas_2018), comprising 345 patients. The cBioPortal platform (https://www.cbioportal.org/) was used to analyze the data and classified cases into groups with high gene expression alterations and those without any alterations. Finally, the logarithmic rank test (p < 0.05) was used to investigate the correlation between combined gene expression and HCC disease-free progression.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 8.0 Software (GraphPadTM Software, San Diego, CA, USA). Multiple comparisons were assessed via one-way ANOVA, followed by Tukey’s post hoc test. Statistical significance was defined as a p-value ≤ 0.05.
Results
Wistar rats treated with either DEN or DEN plus sucrose exhibited distinct patterns in energy consumption during HCC development. Total caloric intake, encompassing both sucrose and standard diet, was quantified (Fig. 1a; Supplementary Fig. 2). At 8 weeks, caloric intake was comparable across all experimental groups. However, by 18 weeks, the 18-18-D/S-F group displayed a significant increase in caloric intake compared to the 18-6-D/S-F group (Supplementary Fig. 1a). While no significant differences were observed in caloric intake from the standard diet between 8 and 18-week groups, differences became evident at 18 weeks, particularly among sucrose groups when compared to 18-C-F and 18-18-D/S-F groups (Supplementary Fig. 1b). Notably, the 8-Suc-F group showed an elevated total caloric intake, and at 18 weeks, the 18-DEN-F group had the highest total caloric intake, marking a significant difference between the included groups (Fig. 1a). These findings suggest that caloric intake from sucrose consumption may differentially impact the progression of DEN-induced HCC in rats.
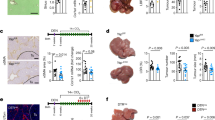
Caloric intake of the diet and the disparate alterations in the dynamics of body and organ mass in Wistar rats during the appearance of HCC induced by DEN and DEN with sucrose. Total caloric intake was determined by adding the caloric intake of both sucrose and the standard diet, which was calculated per 100 g of body weight (a). Body weight of experimental groups was measured at 8 and 18 weeks of treatment (b). Weight of liver (c), pancreas (d), and visceral adipose tissue (e) were also documented for comparative analyses. I Initial consumption, F Final consumption.
No significant differences in body weight were observed among animals subjected to DEN and DEN plus sucrose treatments at 8 weeks of HCC development (Fig. 1b). However, by 18 weeks, animals treated with DEN exhibited a significant decrease in body weight compared to the 18-C and 18-S groups. Liver weight remained unchanged across groups at 8 weeks; however, by 18 weeks, the 18-6-D/S group showed a reduction in liver weight compared to the 18-S group (Fig. 1c). Interestingly, pancreas weight differed between animals receiving DEN with or without sucrose, showing a reduction compared to the 18-S group. The 18-18-D/S group exhibited a recovery in pancreatic weight similar to the 18-C group, with a significant increase compared to the 18-DEN group (Fig. 1d).
Importantly, while sucrose treatment increased visceral adipose tissue weight compared to controls (8-C and 18-C), DEN treatment resulted in a significant decrease in adipose tissue weight at both 8 and 18 weeks (8-DEN and 18-DEN) compared to the 8-S and 18-S groups, respectively (Fig. 1e). In summary, DEN treatment significantly impacted body weight, pancreas weight, and visceral adipose tissue weight, whereas sucrose treatment primarily affected pancreas weight and visceral adipose tissue weight.
At 18 weeks, macroscopic analysis revealed significant differences in liver appearance among the groups. Livers from the 18-DEN group exhibited extensive macronodular cirrhosis and necrotic lesions, while those in the 18-6-D/S and 18-18-D/S groups showed fewer cirrhotic changes, resembling the normal appearance seen in the 18-C and 18-S groups (Fig. 2a). Histologically, the 18-DEN group demonstrated severe alterations in liver architecture, including tumor formation, cellular pleomorphism, nuclear atypia, and fibrous bridge development, leading to regenerative nodules (Fig. 2b,c). In contrast, the 18-6-D/S and 18-18-D/S groups displayed less disruption in liver structure, with reduced cellular abnormalities and fibrotic bridge formation. The quantification of collagen fibers also highlighted a significant reduction in fibrosis in the sucrose-treated groups, aligning closely with the levels observed in the control and sucrose-only groups (Fig. 2d). The levels of AST and ALT (Fig. 2f,e), although measured, did not show significant differences between the experimental groups, except for a significant increase in ALT levels in the 18-DEN group compared to the control group.
Sucrose consumption reduces histological alterations, fibrosis, cirrhosis, and liver enzyme levels in experimental HCC. Representative macroscopic liver images (a), H&E-stained sections (b), and Masson’s trichrome-stained sections (c). Fibrotic area quantification is shown as a percentage in (d). The DEN group differs significantly from all other groups in histological and fibrotic parameters.
Lipid profile alterations were assessed by measuring serum levels of free fatty acids (FFA), total triglycerides (TG), and neutral lipids stained with ORO (Fig. 3). At 8 weeks of treatment, no significant differences in FFA levels were observed. However, by 18 weeks, the 18-DEN group exhibited a significant decrease in FFA levels compared to the sucrose-treated groups (18-S, 18-6-D/S, and 18-18-D/S) (Fig. 3a). Histological analysis of ORO-stained sections, used to detect intracellular fat, was then conducted on tissues from animals treated with DEN, with and without sucrose, for 8 weeks (Fig. 3b). The highest ORO content was observed in the 8-S group, while the 8-DEN group showed a marked decrease in neutral lipids compared to the 8-C, 8-S, and 8-D/S groups. Interestingly, animals treated with both DEN and sucrose (8-D/S) did not show significant differences compared to the control group (8-C) (Fig. 3c).
Representative images of ORO staining are shown, alongside the quantification of neutral lipids in the liver after 18 weeks of treatment. The 18-DEN group displayed a zonal pattern of ORO staining; nodular areas (18-DEN_N) exhibited reduced ORO labeling, indicating a decrease in neutral lipids and altered lipid metabolism. In contrast, internodular areas (18-DEN_Int_N) showed partial recovery of ORO labeling, similar to nodular areas in the sucrose-treated groups (18-6-D/S_N and 18-18-D/S_N), suggesting that sucrose mitigated the reduced ORO labeling effects of DEN. Furthermore, internodular areas of the 18-6-D/S_Int_N and 18-18-D/S_Int_N groups presented ORO labeling levels comparable to the 18-C group, indicating a restoration of neutral lipid accumulation, potentially reflecting lipid metabolism normalization promoted by sucrose treatment (Fig. 3d and e).
Sucrose consumption mitigates the decrease in triglycerides and serum free fatty acids, restoring neutral lipids in hepatic parenchyma during preneoplastic and neoplastic HCC stages. Serum FFA and TG levels increased significantly in sucrose-treated groups vs. DEN (a). ORO staining shows reduced lipid content in the 8-DEN group, with patterns similar to 8-DEN in 8-D/S (b). Quantification of ORO-labeled areas during preneoplastic (c) and neoplastic stages (e) reveals sucrose preserves lipids in both tumor and inter-tumor regions (d). N Nodular, Int_N Internodular.
Building on these results, we investigated the potential antineoplastic effects of sucrose by evaluating the expression of Gstp1 and Ggt1, two established HCC markers. Sucrose-treated groups (18-6-D/S and 18-18-D/S) exhibited a significant decrease in Gstp1 and Ggt1 expression compared to the 18-DEN group (Fig. 4a). Histochemical quantification of gamma-glutamyl transferase (GGT)-positive areas revealed a reduction in nodules and hepatic lesions in sucrose-treated groups (Fig. 4b,c). Immunofluorescence analysis for the proliferation marker Ki-67 showed that the 18-DEN group had higher number of Ki-67 positive cells, indicating increased proliferative activity compared to the 18-6-D/S and 18-18-D/S groups (Fig. 4d). Quantitative analysis confirmed a significant reduction in Ki-67-positive cells in sucrose-treated groups (Fig. 4e). These findings suggest that sucrose treatment may inhibit the compensatory cell proliferation induced by DEN in the rat liver.
Sucrose consumption reduces neoplastic tumor development and attenuates Ki-67 proliferation marker in experimental HCC. Gstp1 and Ggt1 expression decreased in 18-6-D/S and 18-18-D/S groups (a). Representative GGT histochemistry images (b) show reduced GGT-positive areas with sucrose treatment (b,c). IF analysis indicates higher Ki-67 expression in the 18-DEN group (d), with quantification of Ki-67-positive cells (e). RE Relative expression.
To identify a potential protective pathway against HCC development associated with sucrose consumption, we examined the gene expression of kelch-like ECH-associated protein 1 (KEAP1) and nuclear factor erythroid 2-related factor 2 (NFE2L2), key regulators of oxidative stress response and glucose metabolism. No significant differences in Keap1 and Nfe2l2 expression were observed among all groups at 8 weeks. However, by 18 weeks, both 18-S and 18-DEN group showed significant upregulation of both Keap1 and Nfe2l2 compared to 18-C (Fig. 5a). In contrast, sucrose-treated groups (18-6-D/S and 18-18-D/S) exhibited notable downregulation of these genes (Fig. 5a). Immunofluorescence analysis revealed a significant reduction in cytoplasmic KEAP1-positive areas in the sucrose-treated groups compared to the 18-DEN group (Fig. 5b). Additionally, an increased number of NFE2L2-positive nuclei was observed in the 18-DEN group, which decreased in the sucrose-treated groups (Fig. 5c).
Impact of sucrose on KEAP1 and NRF2 in experimental HCC. The 18-DEN group upregulated Keap1 and Nfe2l2 gene expression, while sucrose significantly downregulated these genes (a). Representative images and quantification of KEAP1-positive areas and NRF2-positive nuclei by IF analysis (b,c). RE Relative expression.
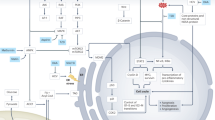
The results are further linked to our analysis of gene expression associated with oxidative stress response and lipid regulation13,14. A protein-protein interaction network was constructed, highlighting key proteins involved in these processes, including KEAP1 and NFE2L2, which are crucial for cellular homeostasis (Fig. 6a). mRNA expression analysis revealed that sucrose consumption in the 18-6-D/S group led to a significant increase in Pprc1 expression. Additionally, Ppara and Pparg expression significantly increased in the 18-18-D/S group compared to the 18-DEN group (Fig. 6b). For fatty acid transporter genes, co-administration of sucrose resulted in increased Fabp1 expression, while Cav1 and Cd36 expression was reduced compared to the 18-DEN group, highlighting the role of sucrose in regulating gene expression related to oxidative stress and lipid homeostasis. Furthermore, immunofluorescence (IF) analysis of the CD36 protein (Supplementary Fig. 3) revealed that sucrose consumption significantly downregulated CD36 expression in liver tissue compared to the 18-DEN group. This finding supports the impact of sucrose on lipid transport and metabolism, potentially mediated through the NRF2/KEAP1 pathway.
Genes related to oxidative stress response and lipid regulation. (a) Protein-protein interaction network illustrating key regulators of oxidative stress and lipid metabolism. (b) mRNA expression levels: Pgc1a decreased in all groups vs. 18-C, while Pgc1b showed no significant changes. PPRC1 significantly increased during the neoplastic period (18-18-D/S). Sucrose co-administration (18-6-D/S and 18-18-D/S) led to marked differences in Fabp1 and Cav1 expression vs. 18-DEN. (c) Disease-free progression in HCC patients: Alterations in genes such as PPARGC1A, PPARGC1B, PPRC1, PPARA, PPARD, PPARG, SLC27A5, FABP1, CAV1, and CD36 correlated with increased disease progression compared to patients without these gene alterations .FC Fold change.
Finally, disparities in disease-free progression in HCC patients were observed, with alterations in the expression of genes such as PPARGC1A, PPARGC1B, PPRC1, PPARA, PPARD, PPARG, SLC27A5, FABP1, CAV1, and CD36 being associated with worse disease progression (Fig. 6c). Data from TCGA (https://www.cancer.gov/tcga) indicate that patients with these genetic alterations experienced more pronounced deterioration compared to those without such changes, suggesting a significant link between gene expression and unfavorable HCC progression.
Discussion
Despite significant advances in HCC knowledge and the emergence of various treatment strategies, the mortality rate associated with this type of cancer remains extremely high. Here, our results suggest that sucrose co-administrated with DEN plays a crucial role in mitigating the HCC development and progression.
These findings challenge the prevailing misconception regarding the role of simple carbohydrates as cancer risk factors. Indeed, the current epidemiological evidence fails to substantiate such an association. Notably, a study by Fedirko V, concluded that the higher carbohydrate intake did not correlate with an elevated risk of liver or biliary tract cancer. This extensive investigation encompassed approximately 520,000 individuals across 23 centers in 10 European Union countries15. Similarly, Shun Liu Ya and colleagues conducted a comprehensive review of 43 meta-analyses, aggregating data from 281 individual studies involving 13,164,365 participants. Their findings failed to provide convincing evidence linking carbohydrate intake to cancer progression, except for a robust association with high carbohydrate consumption and an elevated risk of metabolic syndrome16.
The absence of a general epidemiological correlation suggests that carbohydrate consumption is not a significant cancer risk factor in the broader population. However, this does not preclude the possibility that, under specific experimental conditions, such as those utilized in our study, sucrose may exert a hepatoprotective effect on liver carcinogenesis.
In this context, numerous studies have assessed the effects of carbohydrate consumption, particularly sucrose, in various experimental models, with a focus on metabolic and hepatic health. These studies consistently demonstrate that chronic sucrose intake, whether alone or combined with other dietary factors like saturated fats or copper deficiency, tends to promote the development MAFLD, previously known as non-alcoholic fatty liver disease (NAFLD), insulin resistance, and general metabolic dysfunction17,18,19,20,21,22,23,24,25,26. These findings are consistent across rat and mouse models fed high-sucrose diets over extended periods, where the adverse effects include hepatic lipid accumulation, inflammation, fibrosis, and in some cases, progression to Metabolic Associated Steatohepatitis (MASH), previously referred to as non-alcoholic steatohepatitis (NASH)27,28,29,30,31.
However, this does not preclude the possibility that, under specific experimental conditions, such as those utilized in our study, sucrose may exert a hepatoprotective effect on liver carcinogenesis. This effect includes the restoration of hepatic lipid levels, improved liver architecture, and a reduction in fibrosis and tumor development. It is important to emphasize that these findings are specific to the context of chemically induced oncogenesis and should not be interpreted as beneficial in a long-term metabolic environment, as demonstrated by studies focused on MAFLD or MASH17,18,19,20,21,22,23,24,25,26,27,28,29,30,31.
The hepatoprotective results observed in our study should be considered within the exclusive context of experimental carcinogenesis, where sucrose may act as a metabolic modulator of liver microenvironment, possibly through the regulation of genes associated with lipid metabolism and oxidative stress response.
A constant feature of the studies that induce liver damage associated with non-alcoholic liver disease, is to promote liver alteration through the prolonged administration of these disaccharides. However, to the best of our knowledge, there has been no previous research showing the impact of a diet rich in sucrose on the development of HCC.
A well-defined correlation between the administration time and increasing doses of DEN on the decreased body weight has been previously established30. Cancer cachexia is a multifaceted syndrome characterized by the coexistence of inflammation, hormonal irregularities, insulin resistance, loss of skeletal muscle due to proteolysis, lipolysis, malnutrition, and weight reduction attributed to a hypermetabolic state associated with cancer32,33,34. Here, we found that sucrose consumption attenuates caloric intake and alterations in body and organ mass dynamics, especially at level of adipose tissue, during the progression of DEN-induced HCC.
Interestingly, the consumption of sucrose led to increased serum levels of TG and FFA compared with animals exclusively subjected to DEN administration (Fig. 4). These findings provide compelling evidence to strongly suggest that sucrose consumption interferes with the lipolysis process secondary to the hypermetabolic state associated with cancer in the rat. Moreover, our investigation revealed that sucrose consumption effectively preserved intrahepatic neutral lipid levels during both pre-neoplastic (8 weeks) and neoplastic stages (18 weeks). In contrast, animals exclusively subjected to DEN during pre-neoplastic stage exhibited a significant depletion of neutral lipids. Notably, during neoplastic stage, these animals displayed a notable heterogeneous pattern of neutral lipid distribution. The reduction of neutral lipids due to DEN administration is a sparsely studied phenomenon. In 2016, Ambade Aditya and colleagues reported that the steatosis induced by the Lieber–DeCarli diet containing 4% alcohol was mitigated by the continuous administration of DEN for 6 weeks35. The reduction in hepatic lipid accumulation observed in their study is consistent with our findings under the same course of DEN administration, which strongly suggests that DEN also can interferes on the effects of others hepatotoxic, such as high-fat diet and ethanol, as sucrose did it on DEN effects.
Excessive hepatic fibrogenesis represents a dynamic process at the molecular and cellular level, characterized by a substantial accumulation of extracellular matrix. This scar tissue replaces parenchyma causing profound alterations of liver function. This phenomenon arises from various chronic liver diseases, with the potential to progress to cirrhosis, HCC and liver failure36. Compared with human HCC development, where cirrhosis plays a critical role, our DEN-induced HCC model has the capability to induce intrahepatic fibrosis. Surprisingly, the administration of sucrose led to a significant decrease in liver fibrosis, cellular pleomorphism and nuclear atypia of hepatocytes in the experimental HCC induced by DEN. This observation is consistent with results from a liver injury model that uses thioacetamide, where a high-carbohydrate diet was effective in reducing fibrogenesis37.
In line with a reduction of HCC nodules and fibrosis, we also explored the potential impact of elevated sucrose consumption on DEN-induced tumorigenesis by analyzing markers reported for chemical hepatocarcinogenesis38. Surprisingly, sucrose resulted in a significant reduction in the expression HCC markers such as Gstp1 and Ggt1 at the mRNA level, as well as an in situ decrease of hepatocyte foci with GGT enzymatic activity. Furthermore, sucrose also diminished cell proliferation in the liver by assessing the Ki-67 proliferation marker using IF analysis. The KEAP1/NRF2 pathway is a pivotal regulator of cellular defense mechanisms against oxidative stress, playing a crucial role in maintaining metabolic homeostasis. For example, a high sucrose diet in mice led to progressive metabolic and oxidative disturbance including increased expression of antioxidant defense genes, such as NRF2 39. In the DEN-induced HCC model, a significant mechanism underlying tumor progression is the excessive production of reactive oxygen species (ROS)40. Given the central role of oxidative stress in liver cancer development, evaluating the activity of the KEAP1/NRF2 pathway is critical for elucidating the molecular basis of hepatocarcinogenesis41. The importance of this pathway is further emphasized by its involvement in various cancers, where NRF2 hyperactivation can lead to chemoresistance, while its suppression may increase vulnerability to oxidative damage. Importantly, our group has previously documented the elevation of KEAP1 and NRF2 in the context of DEN-induced hepatocarcinogenesis, underscoring its significance in this model.
Our research revealed an increase in both KEAP1 and NRF2 levels in the HCC group, while sucrose intake attenuated these levels at both the mRNA and protein levels. This phenomenon of elevated KEAP1 and NRF2 expression was extensively described in a previous study, where not only was there an increase in expression but also nuclear translocation of NRF2 in HCC cells, like the nuclear NRF2 marker observed in our work42. Moreover, NRF2 expression has been correlated with tumor differentiation, size, metastasis, and is associated with poor clinical prognosis43.
These findings strengthen our analysis of genes linked to oxidative stress and lipid regulation, showing that sucrose consumption significantly affects their expression. These key genes in lipid metabolism and oxidative stress directly influence HCC progression, highlighting the crucial role of sucrose in modulating gene expression related to oxidative stress and lipid homeostasis in HCC.
Conclusion
Our research represents the first exploration of the potential therapeutic advantages of sucrose consumption to counteract HCC (Fig. 7). Our results indicate that sucrose might exert its effects by interfering on carbohydrate and lipid metabolism, and potentially reducing oxidative stress; as a result, impacting the KEAP/NRF2 pathway. These pioneering findings challenge conventional assumptions about the role of simple carbohydrates in cancer development.
These findings not only illuminate previously unexplored aspects of dietary factors in HCC, but also open promising avenues for future research. It is crucial that research delve deeper into the complex interplay between dietary components and hepatocarcinogenesis. Thus, this research has the potential to lead to more advanced preventive and therapeutic strategies, providing optimism for a more effective approach to combat HCC.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Huang, D. Q. et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metabol. 34(7), 969–977e2. https://doi.org/10.1016/j.cmet.2022.05.003 (2022).
Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71(3), 209–249. https://doi.org/10.3322/caac.21660 (2021).
Rumgay, H. et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 77(6), 1598–1606. https://doi.org/10.1016/j.jhep.2022.08.021 (2022).
Fan, Y., Xue, H. & Zheng, H. Systemic therapy for hepatocellular carcinoma: current updates and Outlook. J. Hepatocell Carcinoma. 9, 233–263. https://doi.org/10.2147/JHC.S358082 (2022). Published 2022 Mar 30.
Lazzaro, A. & Hartshorn, K. L. A comprehensive narrative review on the history, current landscape, and future directions of hepatocellular carcinoma (HCC) systemic therapy. Cancers. 15(9), 2506. https://doi.org/10.3390/cancers15092506 (2023).
Zhang, H. et al. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark. Res. 10, 3. https://doi.org/10.1186/s40364-021-00350-4 (2022).
Sim Hellene Anjani Sigma. The effect and mechanism of sucrose consumption to liver disease – A systematic literature review. Biomol. Health Sci. J. 5(1), 47–53. https://doi.org/10.20473/bhsj.v5i1.35160 (2022).
Jeeyavudeen, M. S. et al. Management of metabolic-associated fatty liver disease: the diabetology perspective. World J. Gastroenterol. vol. 29(1), 126–143. https://doi.org/10.3748/wjg.v29.i1.126 (2023).
Federico, A. et al. The role of Fructose in non-alcoholic steatohepatitis: Old Relationship and New insights. Nutrients. 13(4), 1314. https://doi.org/10.3390/nu13041314 (2021).
Herman, M. A. & Birnbaum, M. J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metabol. 33(12), 2329–2354. https://doi.org/10.1016/j.cmet.2021.09.010 (2021).
Schiffer, E. et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 41(2), 307–314 (2015).
López-Torres, C. D. et al. Downregulation of indolethylamine N-methyltransferase is an early event in the rat hepatocarcinogenesis and is associated with poor prognosis in hepatocellular carcinoma patients. J. Gene Med.. 24, e3439. https://doi.org/10.1002/jgm.3439 (2022).
Castro-Gil, M. et al. Paulette The transcriptome of early GGT/KRT19-positive hepatocellular carcinoma reveals a downregulated gene expression profile associated with fatty acid metabolism. Genomics. 114(1), 72–83. https://doi.org/10.1016/j.ygeno.2021.11.035 (2022).
Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U. & Falkevall, A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8(6), 1149–1154. https://doi.org/10.1038/nprot.2013.055 (2013).
Fedirko, V. et al. Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in western europeans. Ann. Oncol. 24(2), 543–553 (2013).
Liu, Y. S. et al. Dietary carbohydrate and Diverse Health outcomes: Umbrella Review of 30 systematic reviews and Meta-analyses of 281 observational studies. Front. Nutr. 8, 670411 (2021).
Jensen, T. et al. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68(5), 1063–1075. https://doi.org/10.1016/j.jhep.2018.01.019 (2018).
Oliveira, C. P. M. S. et al. Saturated fatty acids and low carbohydrate diet are associated with the severity of liver damage in rats with non-alcoholic fatty liver disease. Eur. J. Clin. Investig. 44(7), 635–644 (2014).
Tallino, S. et al. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J. Nutr. Biochem. 26(10), 996–1006. https://doi.org/10.1016/j.jnutbio.2015.04.009 (2015).
Ragab, S. M. et al. Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: the ameliorative role of three natural compounds. Lipids Health Dis. 14, 83. https://doi.org/10.1186/s12944-015-0087-1 (2015).
Fernandes, M. R. et al. Animal models of obesity in rodents. An integrative review. Acta Cir. Bras. 31(12), 840–844. https://doi.org/10.1590/S0102-865020160120000010 (2016).
Corona-Pérez, A. et al. Interactive effects of chronic stress and a high-sucrose diet on nonalcoholic fatty liver in young adult male rats. Stress. 20(6), 608–617 (2017).
Castro, R. Q. et al. Dietary sucrose regulates the expression of the Cd36 gene in hepatic tissue of rats with obesity and non alcoholic fatty liver Disease (NAFLD). Biomed. Pap Med. Fac. Univ. Palacky Olomouc Czech Repub. 162(2), 99–106. https://doi.org/10.5507/bp.2018.016 (2018).
Plazas Guerrero, C. G. et al. Evaluation of sucrose-enriched diet consumption in the development of risk factors associated to type 2 diabetes, atherosclerosis and non-alcoholic fatty liver disease in a murine model. Int. J. Environ. Health Res. 31(6), 651–669. https://doi.org/10.1080/09603123.2019.1680817 (2021).
Baiges-Gaya, G. et al. Hepatic metabolic adaptation and adipose tissue expansion are altered in mice with steatohepatitis induced by high-fat high sucrose diet. J. Nutr. Biochem. 89, 108559. https://doi.org/10.1016/j.jnutbio.2020.108559 (2021).
Arroum, T. et al. High sucrose Diet-Induced Subunit I tyrosine 304 phosphorylation of cytochrome c oxidase leads to liver mitochondrial respiratory dysfunction in the Cohen Diabetic Rat Model. Antioxidants (Basel). 13(1), 19. https://doi.org/10.3390/antiox13010019 (2023).
al-Nagdy, S., Miller, D. S. & Yudkin, J. Changes in body composition and metabolism induced by sucrose in the rat. Nutr. Metab. 12(4), 193–219 (1970).
Lanaspa, M. A. et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287(48), 40732–40744 (2012).
Sanchez-Lozada, L. G. et al. Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr. 49(1), 1–9 (2010).
Roncal-Jimenez, C. A. et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 60(9), 1259–1270 (2011).
Cydylo, M. A., Davis, A. T. & Kavanagh, K. Fatty liver promotes fibrosis in monkeys consuming high fructose. Obesity (Silver Spring). 25(2), 290–293 (2017).
Fuentes-Hernandez, S. et al. Chronic administration of diethylnitrosamine to induce hepatocarcinogenesis and to evaluate its synergistic effect with other hepatotoxins in mice. Toxicol. Appl. Pharmacol. 378, 114611 (2019).
Strasser, F. Diagnostic criteria of cachexia and their assessment: decreased muscle strength and fatigue. Curr. Opin. Clin. Nutr. Metab. Care. 11(4), 417–421 (2008).
Hutton, J. L. et al. Dietary patterns in patients with advanced cancer: implications for anorexia-cachexia therapy. Am. J. Clin. Nutr. 84(5), 1163–1170 (2006).
Bosaeus, I., Daneryd, P., Svanberg, E. & Lundholm, K. Dietary intake and resting energy expenditure in relation to weight loss in unselected cancer patients. Int. J. Cancer. 93(3), 380–383 (2001).
Ambade, A., Satishchandran, A., Gyongyosi, B., Lowe, P. & Szabo, G. Adult mouse model of early hepatocellular carcinoma promoted by alcoholic liver disease. World J. Gastroenterol. 22(16), 4091–4108 (2016).
Parola, M. & Pinzani, M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 65, 37–55 (2019).
Satoh, K. Biochemical analysis of the initial carcinogenic changes that induce preneoplastic and neoplastic cell populations during chemical hepatocarcinogenesis in rats. Anal. Biochem.. 643, 114542. https://doi.org/10.1016/j.ab.2021.114542 (2022).
Flister, K. F. Long-term exposure to high-sucrose diet down-regulates hepatic endoplasmic reticulum-stress adaptive pathways and potentiates de novo lipogenesis in weaned male mice. J. Nutri. Biochem.. 62, 155–166. https://doi.org/10.1016/j.jnutbio.2018.09.007 (2018).
da Silva, B. S. et al. High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci. 267, 118944. https://doi.org/10.1016/j.lfs.2020.118944 (2021).
Tolba, R., Kraus, T., Liedtke, C., Schwarz, M. & Weiskirchen, R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Lab. Anim. 49(1 Suppl), 59–69 (2015).
Sánchez-Rodríguez, R. et al. Ptgr1 expression is regulated by NRF2 in rat hepatocarcinogenesis and promotes cell proliferation and resistance to oxidative stress. Free Radic. Biol. Med. 102, 87–99 (2017).
Guerrero-Escalera, D. et al. Comparative subcellular localization of NRF2 and KEAP1 during the hepatocellular carcinoma development in vivo. Biochim. Biophys. Acta Mol. Cell. Res. 1869(5), 119222. https://doi.org/10.1016/j.bbamcr.2022.119222 (2022).
Zhang, M. et al. Nrf2 is a potential prognostic marker and promotes proliferation and invasion in human hepatocellular carcinoma. BMC Cancer. 15, 531. https://doi.org/10.1186/s12885-015-1541-1 (2015).
Acknowledgements
Authors are deeply grateful to Maria Antonieta López-López, Benjamín Chávez-Álvarez, Alberto Rojo-Garcia, and Jorge Fernandez-Hernandez for their technical assistance at CINVESTAV-IPN, also to Ricardo Nava Monroy and Jose Luis Cruz at INMEGEN.
Funding
Isaac Aguirre Maldonado is a student from the Science Doctorate Program of the Department of Pharmacology at CINVESTAV and received fellowship number 1077133 from CONACYT. This study was supported from Grants to J.I.P.C. No. CF-2019-85552, CONACYT-Mexico, and from Instituto Nacional de Medicina Genómica, Grant No. 403.
Author information
Authors and Affiliations
Contributions
I.A.-M.: conceptualization, investigation, methodology, validation, writing—original draft. E.E.H.-L.: methodology, validation. F.L-Z: review and editing. J.C.R.-N: review and editing. A.L.-H.: methodology, validation. J.A.-R.: writing—review and editing. L.d.P.-Y.: writing—review and editing. R.G.-R: writing—review and editing. H.M.: writing—review and editing. A.A.-A.: writing—review and editing. J.M.N.-D: methodology. J.C.: conceptualization, investigation, resources, writing—review and editing. J.I.P.-C.: conceptualization, investigation, resources, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Aguirre-Maldonado, I., Herrera-López, E.E., López-Zenteno, F. et al. Intriguing hepatoprotective effects of sucrose on hepatocellular carcinoma pathogenesis. Sci Rep 14, 23689 (2024). https://doi.org/10.1038/s41598-024-74991-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-74991-4