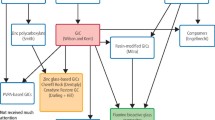
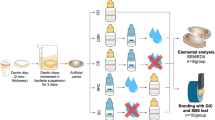
Abstract
Glass ionomer cement (GIC) is one of the most widely used restorative materials for temporary fillings and reconstructions in dentistry, but it has relatively poor mechanical properties that make its use limited, especially in places subject to high pressure. Thus, to extend the applicability of GIC, samples based on SiO2, P2O5, Al2O3, CaF2, and NaF were prepared with the addition of calcium oxide CaO extracted from natural sources (oyster shells) in different ratios of 0, 5, 10, 15, 20, and 25% wt. The suggested glass samples were evaluated, and their physical and mechanical properties were compared. XRD, SEM, and FTIR were performed on the samples. 24 specimens were prepared for each test in order to assess the mechanical properties as per the specific requirements. The tests included measuring bending strength, elastic modulus, adjusted direct tensile strength, absorption, water solubility, and diffusion coefficients after the specimens were stored in distilled water for 60 days. All calculations were carried out in accordance with standard procedures. The findings indicated a slight improvement in the bending resistance of the recommended GIC. Glass modified with 20% by weight of calcium oxide was the best among the ratios in terms of the results obtained and compared to the traditional commercial type. The malleable strength of the sample was 54.121 MPa, while the flexural modulus increased, the tensile strength reached 10.154 MPa, and the solubility was 25.87 µg/mm3 after storage for 60 days. These indicate that the developed material is suitable for use as a dental restoration material when compared to international commercial cement specifications.
Similar content being viewed by others
Introduction
Dental health is a major indicator of public health, well-being, and freedom from disability, with approximately two billion of the world’s population suffering from permanent tooth decay. Furthermore, about 530 million children suffer from dental caries of milk teeth (milk or baby teeth), and this is one of the most common non-communicable diseases and causes of public health problems1. Thus, the development of new and better restorative materials is inevitable. Glass ionomer cement (GIC) is an important restorative dental material. It was proposed by Wilson and Kent in 1969 and first applied in 19722. It is widely used in dental applications, repairing cracks in the teeth, as an adhesive for crowns and bridges, as well as for the manufacture of dental linings and bases. Generally, GIC consists of two parts powder and liquid. The powder is an aluminosilicate that contains fluoride and the liquid is polyacrylic acid3. Its working principle is based on the interaction between glass powder (fluorosilicate glass) capable of ion filtration and the liquid represented by weak polymeric acids. Despite the many advantages, GIC has weak mechanical properties and high sensitivity to moisture in the preparation stage that made it use in dental restoration. Thus, with the presence of friction and chewing force, the compressibility and insufficient bending resistance of GIC will lead to corrosion4. Therefore, the ongoing development of GIC in recent years aims to enhance its poor mechanical properties. Optimizing the powder/liquid (p/L) ratio, particle size and distribution has resulted in better physical properties, and some studies have shown that high-viscosity materials have superior physical properties compared to conventional ones5. Researchers also concentrated on identifying materials that could enhance the physical and chemical characteristics of innovative glass compositions6,7.
Nevertheless, the role of environmental factors in recent advancements cannot be overlooked, and there is a growing need to incorporate natural formulations in materials research. As a result, both fully and partially natural sources are being tested for use in dental applications. Oyster shells, a readily available natural waste with low cost, are composed mainly of calcium carbonate (98%) with very minimal impurities5, and they have been utilized in various medical applications6,7, including dental restorative materials. Studies have indicated that incorporating oyster shell powder into dental fillings can improve their mechanical and physical characteristics in composites, due to the shells’ toughness and durability8,9,10. Moreover, oyster shells are utilized in the production of hydroxyapatite, which is crucial for the development of bones and teeth11,12, as well as in various applications in orthopedic, maxillofacial, and reconstructive surgery13.
The purpose of this study is to evaluate the effects of a new glass ionomer cement (GIC) that includes recycled exhaust materials from our local environment, at a cost-effective price, and with high-quality physical and mechanical properties. In this study, oyster shells were treated and converted into calcium oxide, which was then combined with a specific glass composition, replacing one of its components, sodium fluoride, with calcium oxide. Therefore, when calcium oxide is added to GIC, it is anticipated that the vitreous network will be strengthened. This is evidenced by an increase in the glass transition temperature, a decrease in molar volume, and a reduction in dissolution rate. This phenomenon occurs when NaF bonds are replaced with more covalent CaO bonds. Additionally, the replacement of NaF with CaO results in alterations to the physicochemical and thermal properties of the GIC.
Materials and methods
In this study oyster shells were selected, processed, and converted into calcium oxide, then this was incorporated into a glass mixture containing SiO2, P2O5, Al2O3, CaF2, and NaF, in various weight percentages of 0, 5, 10, 15, 20, and 25% wt. Therefore, this work adjusted the ratio of CaO in the mixed powder and reduced the amount of sodium fluoride while maintaining the other components constant. The resulting glass powder underwent comprehensive physio-chemical analysis to confirm its compatibility with GIC. Subsequently, the GIC based on each ratio were prepared and evaluated its physical and mechanical properties, and then conducted a comparative analysis with existing research findings. Oyster shells with dimensions ranging from 3 to 5 cm were collected and purified by repeatedly washing them with water and alcohol. After that, the oyster shells were dried in an oven at 80 °C for 24 h. Then, the dried oyster shells were crushed with a pestle and sieved with a sieve of less than 36 μm to obtain calcium carbonate. An electric furnace (MV MIHM-VOGT P6/B) converts CaCO3 to calcium oxide by heating it to 1200 °C for 2 h at a rate of 10 °C/min. Then, the sample was cooled, grounded again, and sieved using a 500-mesh sieve to obtain calcium oxide (CaO) particles with a size less than 25 μm12,14. 70 g for each ratio of GIC powder was created for each suggested proportion of glass divided from SiO2, P2O5, AL2O3, CaF2, and NaF according to Table 1. SiO2 was manufactured by Sigma Aldrich with a purity of 99% and a particle size range of 0.5–10 nm. P2O5, Al2O3, and NaF are manufactured by Merck (Germany) with a purity > 99.9% and 99% and a particle size within 1–200 nm. CaF2 was purchased from UNI-CHEM, C08676-41 (South Carolina, USA), with a purity > 99% and a particle size of 1–200 nm. In this work, all the compositions have a fixed value except NaF, which is changed as the concentration of CaO is also changed because the substances NaF and CaO are isoelectronic (they have the same number of valence electrons). The bond distance is nearly equal (about 235 p.m.). In addition, reducing sodium fluoride and replacing it with calcium oxide increases the viscosity of the GIC, which leads to an increase in setting working time (Table 1).
The powder was mixed with a ball mill (RETSCH PM 100 Germany) for 2 h until the components were homogeneous. Then, using the fusion method, the resulting mixture was heated in an electric furnace (MIHM-VOGT P6/B Germany) at a temperature range of about 1200 °C, starting (5 °C min intervals, 50–500 °C, 10 °C min intervals, and 500–1200 °C) for 2 h when reaching 1200 °C to obtain the required glass. The resulting glass was grounded in a ball mill for an hour before passing it through a sieve with a mesh opening of about 25 m. The produced powder was used for subsequent diagnostic tests. It was then mixed well with the liquid and molded according to mechanical and physical tests.
Glass powder (control) was also used within the international specifications (Cave, Germany, Lot 2033134, ISO 9917-1:2007) with good mechanical and physical properties to compare with the prepared GIC compositions. In this work, polyacrylic acid (Cave, Germany, Lot 2033134) was used as the liquid for mixing with glass.
Physio-chemical characterization of glasses
A Cu-Kα beam equal to 1.5405 A° (Philips PW 1700 series diffractometer, Leiden, Netherlands) was used to measure the XRD patterns and was scanned between 2θ (10–70°) with a step size of 0.02° in continuous mode and a counting time of 0.35 s per step. The Scherer equation was used to quantify the crystalline volume (D) for the prepared glass-models.
Where λ represents the incident X-ray wavelength (0.154060 nm), β is the Scherer constant between 0.85 and 0.99 which depends on the particle morphology, β = 0.89 for spherical crystals with cubic symmetry, θ represents the diffraction angle, and W is the full width at half maximum (FWHM in radians). Scanning electron microscopy (SEM) (VEGA TESCAN - Czech) was used to evaluate the microstructure of the surfaces of the prepared GIC samples and to characterize the type and size of the particles.
Fourier-transform infrared spectroscopy (FTIR) (FTIR; Nicolet Magna-IR 550 spectrometer, Madison, Wisconsin) was used to analyze the functional groups of the as-prepared glass models. FTIR spectra were acquired over the 500–4000 cm−1 region using the KBr pellet technique. Energy dispersive X-ray spectroscopy (EDX) associated with SEM was employed for semi-quantitative verification of the chemical compositions of the GIC samples. The specific surface area of the glass powder was calculated by Brunauer-Emmett-Teller (BET) analysis. The BET method has been used with Chembet-3000 quantachrome. The mean diameter (dBET) obtained by applying the BET method as follows:
whereas As is the specific surface area (m2/g) and ρ is the theoretical density of the GIC sample (g/cm3). Malvern Zetasizer version 7.03 (serial number: MAL1032006) was used to analyze the particle size. The volume distribution report was calculated by intensity and the statistical report volume by severity.
Setting and working time
The setting characteristics of the viscous cement should allow sufficient time to mix the substance, and its application in the restorations, tooth preparation, and gluing recovery. During the setting process, the mixture changes from a liquid to a solid within minutes. If the cement sets too quickly, the viscosity increases very fast, and it will be impossible to fully install the casting. On the other hand, if the setup time is very long, and the chair time will be unduly prolonged15. The international standard test for working times and setting times is the piercing by the needle method. Working time at 23 °C is measured using an indentation of mass 28 g and diameter 2 mm. The working time is the time from the beginning of mixing until the needle fails to make a complete indentation on the surface of the cement. Setup time is measured using an indentation of 400 g and a diameter of 1 mm. Setting time is the time from the beginning from mixing until the needle fails to make a complete circular indentation on the cement surface. Adjustment times of GICs can also be measured using an oscillating oscilloscope. It allows continuous monitoring of the setting and gives a record of the hardness16.
Flexural strength and modulus
The prepared GIC samples’ strength was evaluated by bending test because it was considered the most suitable test for this type of material and one of the most important conditions to be met in dental applications15,17. 24 specimens in bar shape of the six prepared glasses (4 specimens for each weight ratio) with dimensions of 2 × 2 × 25 mm according to the ISO 997-1 2007 standard. After completing the formation, these were stored in distilled water for 24 h at 37 °C and then dried before the measurement was carried out. The bending strength (FS) was measured by using a universal testing machine (ZwickRoell BT1-FR2.5TN, Germany) at a crosshead speed of 0.5 mm/min. FS in MPa was calculated by using the following Eqs16,18,19.
where F is the maximum load exerted on the sample at the point of fracture (N), L is the distance between two supports (20 mm), b is width (mm), and d is the thickness (mm).
Flexural modulus E (MPa) was also determined as follows10,20:
where p is the deflection corresponding to the load F (mm). The flexural modulus was determined from the slope of the initial part of the stress-strain curve.
Diametral tensile strength (DTS)
24 cylindrical specimens of the six prepared glasses (4 specimens for each weight ratio) with dimensions 6 mm diameter x 3 mm height according to ANSI/ADA specification No. 27 to measure the diametral tensile strength (DTS) using the following equation10:
where F’ is the load at fracture (N), D (mm) is the sample’s diameter, and L (mm) is the sample’s height.
Water sorption, solubility, and diffusion coefficients
Water sorption and solubility tests were determined according to the specification standard for composites (ISO 4049:2008). 18 specimen discs, approximately 15 ± 0.2 mm in diameter and 1 ± 0.1 mm in thickness, were fabricated (4 specimens for each experimental glass). At first, the thickness of the samples was measured accurately at three points using a micrometer. Also, their diameters were measured, and their volumes were then calculated in mm3. Then all specimens were placed in a desiccator at room temperature for 7 days. The mass of the specimens, labeled as M0 with an accuracy of ± 0.1 mg, was measured. They were then placed in a 250-ml bottle filled with distilled water and stored at 37 ± 1 °C for 60 days until equilibrium was reached. The specimens were placed in a Memmert Oven UN30 from Germany, and their weights were recorded daily after blotting dry to remove excess water. The difference in weights, with a mass change of less than ± 0.1 mg, was recorded as M1. The specimens were then weighed again after a week, and this new mass was labeled as M2. Then, the solubility (WS) and sorption (WA) were calculated according to a glass cement standard (Cave, Germany, Lot 2033134, ISO 9917-1:2007) using the following equations15,18,21:
The second Fick equation was used to calculate the diffusion coefficient, by using Stefan’s approximation as follows22,23:
where t (s) is time, and c represents concentration. The diffusion coefficient of the one-dimensional model of mass flow in solids is) mm/s), and the differential equation is expressed in the following Eq. (7):
This solution to Fick’s second law for long periods of propagation is represented as follows24:
The above equation8 is reduced by the initial stage of absorption when the value of Mt / M∞≤ 0.6
The diffusion coefficient of the liquid through the adsorption process is D (mm/s), Mt expresses absorption in time t (s), M∞ is sorption representing mass (g) when weight is constant, and L represents thickness. In case of Eq. 8, for the initial absorption stages (when Mt/M∞ < 0.6), the equation can be used since the absorption of Mt has been determined at different intervals of time until which the equilibrium state is reached. There is a straight line in the early stages of the graph of Mt / M∞ vs. t ½ and from which the slope is calculated.
S is represented by the following equation:
Data analysis
Statistical analysis of the data was carried out by using the SPSS Statistics 20 package and the differences in the results of the mechanical properties, water sorption, and water solubility were determined. Intra-differences in the parameters between the values were evaluated using the one-way analysis of variance (ANOVA) with several post hoc comparisons of the mean and least significant difference (LSD) performed at the probability level p-values < 0.05.
Results and discussion
Physio-chemical characterization of glasses
The results for XRD patterns of the prepared glass powders and for all samples are represented in (Figure S1). Amorphous structure was observed in the case of the prepared glass powder samples. The XRD pattern of the prepared GIC showed peaks at 2θ = 24° and 53° which can be associated with the presence of SiO2. Additional peaks were observed at 2θ = 31° and 2θ = 45° which indicates the presence of CaF2 and Al2O3, respectively, which similar to the results in the literature25. It also shows a characteristic orthogonal lattice peak centered in 2θ = 20° indicating its semi crystalline nature with a slight shift of this peak26.
The crystal size of the experimental glasses was determined (Table 2). The size of the crystals was in the range of 29.78 to 61.48 nm. The smallest belongs to the sample with the highest CaO content (25 wt%), while the largest is associated with the 5.0 wt% CaO containing sample (Table 2). The results indicate that this glass completely contains amorphous structure27.
SEM images of the microstructures of experimental glasses show homogeneous distribution of the glass and the absence of any intermolecular agglomeration, which helps the fluid distribution during formation of dental filling (Fig. 1). Furthermore, there are no agglomerates, which facilitates the homogenization process and the ease of penetration of the liquid to all the powder particles and this in turn increases the efficiency of mechanical properties. It can also be noted that the shape is irregular, which enhances the surface area, and this in turn leads to an increase in the adhesion of the liquid in the particles. The particle size of GIC samples was measured using the NIH Image program, with approximately 250 particles analyzed. The average particle size of the glass samples was in the range of 22.50–33.56 μm. The sample with 25% CaO had the highest particle size, while the sample with only 10% CaO had the smallest.
The FTIR spectra of the samples has a peak at 471 cm−1 which is associated with the bending vibration mode of SiO, and other peaks at 875 cm−1, and 1099 cm−1 which corresponds to the symmetric stretching vibration of Si–O–Si, the absorption band near 1060 cm−1 shows a gradual enhancement in its left shoulder with the CaO content in the glass increases28. The O–Al peak is located at 450 cm−1 (Figure S2). Thus, the presence of Si–O–Si and Al–O–Si bonds verified by FTIR analysis as well (Figure S2). These are the oxides that make up the GIC samples lattice, and they are vulnerable to acid attack needed to form dental compounds. Additionally, there are peaks at 530 and 620 cm−1 in all spectra which can be attributed to P–O29. The presence of phosphate in the glass structure is demonstrated with the peak at 555 cm−1 in the case of P−O bending vibrations of the PO4 structure. Furthermore, a high Ca2+ content causes band formation at 1432 cm−130. In conclusion, as the CaO content in the glass increased, the absorption peak bands around 1060 cm−1 shifted from a higher wave number range to a lower wave number range, and the intensity of the peak gradually decreased.
As for the EDX measurements verified the chemical and elemental composition of the prepared samples (Figure S3). It is possible to see the weight ratio of each element as well.
The density of the prepared GIC samples was determined and the BET surface area and particle size were also compared (Table S1). The density of the samples is in a range between 3.167 and 4.002 g/cm3, and the lowest value belongs to the sample which does not contain CaO, while the highest density belongs to the sample which prepared with 25% CaO. The GIC samples show an increase in CaO, with a density of 3.35 g/cm3, and a decrease in NaF, with a density of 2.56 g/cm3 compared with international specifications for commercial glass (Cave, Germany, Lot 2033134, ISO: 9917-1).
Setting and working time
By testing the time of work and preparation of the prepared glass, the preparation time (3–8 min) of the counter was recorded for the filling and its hardening, and this in turn facilitates the formation of the filling inside the tooth cavity before it is completely hardened29. The solidification process depends on several factors, including the ratio of powder to liquid, the type of liquid used, the molecular weight of the liquid, as well as the size of the powder particles. In this study, a powder to liquid ratio of 3:2 was used. The filling preparation and hardening time and the duration ranged between 3.86 and 6.29 s (Table S2). The best result was experienced in the case of 20% CaO sample, because this period the dentist considers (6.29 s) suitable for preparing the filling.
Mechanical and physical properties of tested GIC
The physical and mechanical properties of the tested GIC were determined. The prepared models were placed in distilled water for 24 h at 37 °C. The result for bending strength indicated that the FS values cover a range from 17.221 to 54.121 MPa, where the lowest value belongs to the sample with the highest CaO content while the highest value is associated with the sample containing 20% CaO (Table 3). The results were compared to a conventional glass cement (Cave, Germany, LT 2033134, ISO 9917-1: 2007) and it was found that the prepared samples have better properties. Incorporation of CaO enhanced external force compared to the control. The increase in the surface area caused by CaO and its good diffusion in the powder increased the bonding, which provided more flexibility. This is attributed to the smaller size of the nanoparticles which leads to a larger particle size distribution occupation of empty spaces between GIC particles resulting in higher mechanical properties.
The bending modulus results for all prepared samples and different groups were measured and it was found that the values are between 245.6 and 397.92 MPa. The largest value belonged to the sample with 20% CaO by weight, and the lowest value is associated with the sample which does not include CaO (Table 3).
The diametral tensile strength (DTS) is a crucial factor to consider, as a significant number of clinical failures are caused by tensile stress. Since it is challenging to directly measure the tensile strength of brittle materials such as Glass Ionomer Cements (GICs)31,32.
It was found that DTS covers a range from 2.541 to 10.154 MPa where the lowest one is the sample with 10% CaO while the highest one belongs to the sample with 20% CaO (Table 3).
The study found that the addition of CaO enhances the DTS of GIC, regardless of the amount added. Additionally, CaO reduces the plastic deformation of GIC, leading to an overall increase in strength. This is achieved through finely controlled micronization to achieve the optimal particle size and characteristics. Furthermore, CaO can fill any gaps in the matrix, enabling the cement to withstand higher loads. The homogeneous incorporation of CaO particles in the glass component further strengthens the material for lasting durability and high tolerance to occlusal load. The addition of 20 wt% of calcium oxide to the prepared glass powder showed promising results when compared to the mechanical properties of commercial glass (Cave, Germany, Lot 2033134, ISO: 9917-1). The measurement of resistance to bending is a crucial property of dental restoration material, and the results indicate that the addition of CaO significantly improves this property.
The sorption during 60 days of the GIC samples was also recorded (Fig. 2; Table 4). It can be noted the sorption is ranging between 25.87 and 42.17 µg/mm3. The 20% CaO sample had the lowest absorption value of 25.87 ± 2.08 µg/mm3 in 60 days, while the highest value, 42.17 ± 2.08 µg/mm3 was recorded for system with 10% CaO. In the first stage of the preparation time, the GIC is subject to hydrolysis, and this represents one of its weaknesses, due to its exposure to air and moisture. By comparing the mechanical properties of glass powder prepared with different weight ratios with commercial glass, the sample with 20% calcium oxide is better, which makes it a good material to be used for dental restoration. In addition, the increase in absorption and solubility can be linked to the characteristic of surface roughness.
The water solubility of samples was determined and the highest solubility at 60 days was 47.82 ± 5.06 µg/mm3 for the sample including 5% CaO, while the lowest value was 32.54 ± 2.02 µg/mm3 and associated with the system including 20% CaO (Fig. 2; Table 4).
For GIC solubility CaO concentrations 0 wt%. and 5% by weight. Occupying the higher range, the 15% and 20% groups showed a statistically significant decrease in solubility. This result can be attributed to the improved microstructure of the tested GIC by adding different ratios of CaO.
Statistical analysis of the mechanical properties, water solubility, and water sorption data was carried out, and the one-way analysis of variance (ANOVA) with several post hoc comparisons of the mean and least significant difference (LSD) performed (Tables 3 and 4). This was used to identify differences between the values at a probability level (p-value) < 0.05 or less. The p-values of the mechanical properties for all samples have been studied (Table 3) and were in a range from 0.000 to 0.013 for flexural strength, indicating high significance compared to the control. When recording the DTS data (Table 3), the results indicate that all ratios had p-values of 0.000, indicating a strong statistical comparison with the control. However, in the case of flexural modulus, all the ratios were highly significant, except for 5% (p = 0.175) and 25% (p = 0.275), indicating that no statistically significant difference was found between these ratios and the control. As for water sorption and water solubility (Table 4), the p = 0.000 for all ratios, which means these data have high statistical significance compared to the control and as for water solubility, the p values were 0.000 for all samples expect the one including 25% CaO (p = 0.038) but still have high significance because it is less than 0.05.
The diffusion coefficients were determined by using the slope of the Mt/M∞vs. t1/2 plot (Fig. 3).
The diffusion coefficient describes the rate of dispersion of the powder within the liquid. The diffusion coefficients are in a range between 3.9 and 6.2 mm/s, and the highest value is associated with the 25% CaO sample, while the smallest one belonged to the CaO free sample (Figure S4).
All in all, it can be seen that the sample with 20% CaO is suitable to be applied as dental restoration material, due to the good mechanical properties such as flexural and compressive strength which were compared with the international specifications of restoration materials (Cave, Germany, Lot 2033134 ISO 9917-1:2007). Thus, calcium oxide prepared from natural resources (oyster shells) proved to be excellent material to contribute to the development of dental restoration materials5,29 .
Conclusion
The aim of the study was to develop dental restorative materials using natural resources that are readily available and cost-effective. Oyster shells were proposed as a potential ingredient in tested GIC, along with SiO2, P2O5, Al2O3, CaF2, and NaF. Different weight ratios of calcium oxide derived from oyster shells (0%, 5%, 10%, 15%, 20% and 25%wt.) were added to the composition. The findings of the study suggest that agglomeration of the fine particles of the powder did not occur, leading to improved powder/liquid interaction and enhanced adhesion. The presence of CaO in the tested GIC increased both setting and working time. Furthermore, the addition of calcium oxide enhanced the physical and mechanical properties of the tested GIC. The prepared GIC with CaO exhibited lower water sorption values, making it a suitable material for dental restoration. The developed GIC composition with CaO 20%wt. showed significantly better properties compared to other samples tested in this study. Further clinical studies are recommended to assess the mechanical strength of the materials on a larger scale and evaluate their success rate in real-life applications.
Data availability
Data is provided within the manuscript and supplementary information files.
References
Primus, C., Gutmann, J. L., Tay, F. R. & Fuks, A. B. Calcium silicate and calcium aluminate cements for dentistry reviewed. J. Am. Ceram. Soc. 105, 1841–1863 (2022).
Moshaverinia, A. et al. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 4, 432–440 (2008).
Kim, H.-J. et al. Effects of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 11, 7016 (2021).
Makanjuola, J. O. et al. A new hydrolytic route to an experimental glass for use in bioactive glass-ionomer cement. J. Market. Res. 18, 2013–2024 (2022).
Prabhakar, M., Lee, D.-W., Kim, B.-S. & Song, J. I. Development and characterization of oyster shell powder filled polypropylene composite. Compos. Res. 27, 201–206 (2014).
Dittrich, B., Wartig, K.-A., Mülhaupt, R. & Schartel, B. Flame-retardancy properties of intumescent ammonium poly (phosphate) and mineral filler magnesium hydroxide in combination with graphene. Polymers 6, 2875–2895 (2014).
Al-Samaray, M., Al-Somaiday, H., Rafeeq, A. K. Effect of adding different concentrations of CaCO3-SiO2 nanoparticles on tear strength and hardness of maxillofacial silicone elastomers. Nano Biomed. Eng. 13 (2021).
Zhu, K. et al. Mechanical, antibacterial, biocompatible and microleakage evaluation of glass ionomer cement modified by nanohydroxyapatite/polyhexamethylene biguanide. Dental Mater. J. 41, 197–208 (2022).
Uresha, M., Pitawala, H. & Thushari, G. Development of a novel dental filling using hydroxyapatite derived from waste oyster shells. J. Technol. Value Addit. 1, 1–18 (2021).
AlBadr, R. M., Halfi, S. A. & Ziadan, K. M. The effectiveness of oyster filler on the physical and mechanical properties of novel dental restorative composite. AIP Conf. Proc. 1, 050001-1–050001-8 (2020).
Nisa, A., Sari, M. & Yusuf, Y. Fabrication and characterization of HA-oyster shell based on biopolymer-propolis as an agent of dental enamel remineralization material. Mater. Res. Express 9, 115401 (2022).
Aljaberi, K., AlBadr, R. M. & Ziadan, K. M. A new approach to prepare nano hydroxyapatite from oyster shells used for dental applications. J. Kufa-Phys. 14, 35–46 (2022).
Oktar, F. N., Unal, S., Gunduz, O., Nissan, B. B., Macha, I. J., Akyol, S., Duta, L., Ekren, N., Altan, E., Yetmez, M. Correction to: Marine‑derived bioceramics for orthopedic, reconstructive and dental surgery applications, 1, 57–81. (2023)
Thbayh, K. K., AlBadr, R. M., Ziadan, K. M. & Fiser, B. Expanding potential dental applications of glass ionomer cement by incorporating with nano-hydroxyapatite. Karbala Int. J. Modern Sci. 9, 10 (2023).
Najeeb, S. et al. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 17, 1134 (2016).
Awan, M. A Study Investigating the Mechanical Testing of a Novel Dental Restorative Material and Its Biocompatibility (University of Birmingham, 2010).
Núñez, D., Elgueta, E., Varaprasad, K. & Oyarzún, P. Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater. Lett. 230, 64–68 (2018).
Habib, H. B. Modifying playfair cipher algorithm by using Legendre symbol. Diyala J. Pure Sci. 15, 74–84 (2019).
Hilfi, S. A., AlBadr, R. M. & Mahdi, K. M. Z. Physical-mechanical properties of dental composites by addition oyster shell powder as fillers. Basrah J. Sci. 37, 103–112 (2019).
Singer, L., Bierbaum, G., Kehl, K. & Bourauel, C. Evaluation of the flexural strength, water sorption, and solubility of a glass ionomer dental cement modified using phytomedicine. Materials 13, 5352 (2020).
Al-Badr, R. M. Sorption kinetics of restorative dental composites immersed in different solutions. Basrah J. Sci. 34, 87–99 (2016).
Al Badr, R. M. & Hassan, H. A. Effect of immersion in different media on the mechanical properties of dental composite resins. Int. J. Appl. Dent. Sci 3, 81–88 (2017).
Saddam Abd al-Rahman al-Hilfi Preparing optical dental restoration overlays and studying the effect of adding biological materials on the physical and mechanical properties University of Basrah, College of Science, Department of Physics, Iraq (2019).
Moheet, I. A. et al. Novel nano-hydroxyapatite-silica–added glass ionomer cement for dental application: Evaluation of surface roughness and sol-sorption. Polym. Polym. Compos. 28, 299–308 (2020).
Al-Bader, R. M., Ziadan, K. M. & Al-Ajely, M. Water adsorption characteristics of new dental composites. Int. J. Med. Res. Health Sci. 4, 281–286 (2015).
Santos, C., Clarke, R., Braden, M., Guitian, F. & Davy, K. Water absorption characteristics of dental composites incorporating hydroxyapatite filler. Biomaterials 23, 1897–1904 (2002).
Djebaili, K., Mekhalif, Z., Boumaza, A., Djelloul, A. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J. Spectrosc. 2015 (2015).
Fan, Y., He, F., Li, Z. & Xie, J. Influence of the CaO content in glass on the sinterability and properties of a Glass/Al2O3 composite for LTCC applications. Ceram. Silikáty 66, 310–324 (2022).
Al-Bader, R. M., Ziadan, K. M. & Al-Ajely, M. Preparation and characterization of Calcium-Fluoroaluminosilicate glass fillers for dental composite. Basrah J. Sci. 33, 11–21 (2015).
Bhargav, P. B., Mohan, V. M., Sharma, A. & Rao, V. N. Investigations on electrical properties of (PVA: NaF) polymer electrolytes for electrochemical cell applications. Curr. Appl. Phys. 9, 165–171 (2009).
Bresciani, E. et al. Resistência à compressão e à tração diametral de cimentos de ionômero de vidro. J. Appl. Oral Sci. 12, 344–348 (2004).
Kunte, S. et al. Comparative evaluation of compressive strength and diametral tensile strength of conventional glass ionomer cement and a glass hybrid glass ionomer cement. Int. J. Clin. Pediatric Dent. 15, 398 (2022).
Acknowledgements
KKT express her gratitude to Basrah Teaching Hospital and to the University of Basrah, College of Science, Department of Physics for giving the opportunity to complete postgraduate studies.
Author information
Authors and Affiliations
Contributions
K.K.T. were carried out experiments and characterisation of the samples. K.K.T., D.K.T. wrote the main manuscript text and prepared figures. S.M.M. carried out additional analysis. R.M.A., K.M.Z. and B.F. supervised the work and carried out finetuning of the text. All authors participated in the formal analysis of data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Thbayh, K.K., AlBadr, R.M., Ziadan, K.M. et al. Fabrication and characterization of novel glass-ionomer cement prepared from oyster shells. Sci Rep 14, 24083 (2024). https://doi.org/10.1038/s41598-024-75040-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75040-w