Abstract
Background
Video-assisted thoracic surgery decortication for phase 3 thoracic empyema is widely accepted, but its optimal timing has not been established. We aim to investigate and assess this timing, in terms of overall survival, for chronic empyema.
Methods
Two hundred four patients with pneumonia-caused phase 3 empyema were treated with video-assisted thoracic surgery decortication over 10-years at Changhua Christian Hospital. The 90-day post-operative survival status was analyzed, and we compared the survivor group versus the non-survivor group. A receiver operating characteristic curve was used to identify the optimal decortication timing.
Results
A comparison between survivors and non-survivors showed statistical differences among age (p=0.004), presence of cardiovascular disease (p=0.018), presence of end-stage renal disease (p=0.002), duration to surgery (p=0.013), length of intensive care unit stay (p=0.010), and overall length of hospital stay (p=0.015). ROC curve analysis determined the cut-off for video-assisted thoracic surgery decortication, based on optimal 90-day post-operative survival, to be 7.5 days after hospitalization; mortality increases threefold thereafter (14.2% vs 44.6%, p<0.001). Multivariate analysis revealed that age, end-stage renal disease, pleural effusion pH≦7.2 and duration to surgery >7.5 days negatively impacted 90-day post-operative survival.
Conclusions
Patients receiving decortication surgery within 7.5 days of hospital admission had better overall survival.
Similar content being viewed by others
Introduction
Thoracic empyema, also known as pyothorax or empyema thoracis, is a collection of purulent fluid in the pleural space1. The most common cause of thoracic empyema in developed countries is the infection of parapneumonic effusion as a complication of community-acquired pneumonia2. Annually, more than 1.5 million adults with pneumonia are hospitalized in the United States3. Of these hospitalized patients, 20-40% will develop parapneumonic effusion and 10% of these parapneumonic effusions will progress to an empyema4,5. About 15% of these empyema patients will expire and 30% will require surgical debridement of the pleural space6,7. Interestingly, there is a rising trend in thoracic empyema8,9,10. A Canadian population-based cohort study found a decrease in the proportion of patients younger than 50 years old and an increase in patients aged 50–70 years old and no change in those older than 70 years old10. The proportion of patients with higher scores on the Charlson Comorbidity Index is also rising10. With an aging population, pneumonia-caused empyema is of medical concern.
The development of empyema is a dynamic three-step process in which treatment failure can escalate to the subsequent phase11. During phase 1 (acute exudative phase), increased permeability of pleural membranes due to pleural inflammation causes an influx of inflammatory cells and protein-rich exudate. Progression into phase 2 (fibrinopurulent phase) consists of an increased procoagulant activity in the pleural space, promoting fibrin deposition and septum formation. At phase 3 (chronic organizing phase) fibroblast proliferation generates a fibrous pleural peel that encases the lung11. This leads to narrowing of intercostal space and secondary atelectasis that produces a unilateral loss of lung volume, resulting in restrictive ventilatory syndrome with ventilation-perfusion mismatch12. The whole process takes about 4–6 weeks and involves progressive changes in the pleural cavity, the appearance of the effusion and the need for surgical treatment12.
Clinically, both the 2015 European Association for Cardiothoracic Surgery expert consensus statement and the 2017 American Association for Thoracic Surgery guidelines recommend surgical approaches to patients with phase 2 and 3 empyema1,13. However, there is heterogeneity in present treatment for phase 2 empyema. Treating with intrapleural fibrinolytic agents versus surgical intervention is a topic of hot debate, and current practice is not unanimous14,15. On the other hand, surgical decortication is the consensus for phase 3 empyema treatment. Many studies have reported on the effectiveness and safety of video-assisted thoracic surgery (VATS) for decortication, but its optimal intervention timing has not yet been established in guidelines.
The purpose of this study is to quantitatively demonstrate the association between surgical intervention timing and mortality for pneumonia-caused phase 3 thoracic empyema, focusing on the optimal decortication timing for best surgical outcome. We present this article in accordance with the STROBE reporting checklist.
Results
Patient characteristics
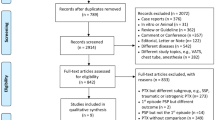
A total of 204 adult patients who underwent VATS decortication for phase 3 thoracic empyema were included in our study according to Fig. 1. Their characteristics are presented in Table 1. The mean age was 66.46 years old. A majority of patients (79.9%) were male, and the laterality of the affected pleural cavity was comparable (52% right, 47% left). One hundred thirty-two patients (64.7%) had hypertension, ninety-two patients (44.9%) had diabetes, sixty-nine patients (33.8%) had cardiovascular disease, forty-six patients (22.5%) had chronic pulmonary obstructive disease and thirty patients (14.7%) had end-stage renal disease.
On average, patients had leukocytosis with a mean white blood cell count of 14.27 × 103/µL and a mean hemoglobin level of 9.88 g/dL. The pleural effusion characteristics of pH ≤ 7.2, glucose level ≤ 40 mg/dL and lactate dehydrogenase (LDH) ≥ 1000 IU/L are indicative of an advanced phase of empyema and are predictors of a complicated clinical course. Of all our patients, approximately half had a pleural effusion pH ≤ 7.2 and a pleural effusion glucose level ≤ 40 mg/dL. Over 60% of the cases had LDH ≥ 1000 IU/L. There were 10 cases (4.9%) of pleural tuberculosis, evident by the presence of mycobacterium tuberculosis complex in pleural effusion analysis. Duration to surgery (DTS) is defined as the duration from admission day to operation day. Overall mean DTS was 13.36 days, and the mean operation time was 117.22 min. The average length of stay at the intensive care unit (ICU) and average overall hospital course were 12.83 days and 51.80 days, respectively.
A comparison of characteristics between the survivor group and the non-survivor group is shown in Table 1. There are statistical differences among age, presence of cardiovascular disease, presence of end-stage renal disease, duration to surgery, length of ICU stay and overall hospital stay. There is no statistical difference in gender, affected chest laterality, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), lab data, pleural effusion analysis and operative time.
Optimal VATS decortication timing and overall survival
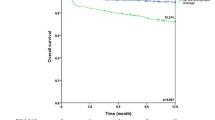
ROC curve analysis (Fig. 2) indicated that the cut-off, based on optimal survival 90 days after VATS decortication, was 7.5 days after hospital admission, with a sensitivity of 0.543 and a specificity of 0.196 (area under the curve was 0.698, 95% confidence interval was 0.605–0.790; p < 0.001). Table 2 compared the characteristics of patients who were treated within 7.5 days of hospital admission and patients treated thereafter. Significant differences were observed in age, end-stage renal disease, hemoglobin level, pleural pH, re-operation, post-operative ICU admission, post-operative ICU duration, length of ICU stay, length of hospital stay, as well as mortality within 90 days. Figure 3 shows the overall survival graph of patients who underwent VATS decortication within and after 7.5 days of hospital admission.
Univariate and multivariate analysis
Univariate and multivariate analyses of independent predictors of survival outcome for phase 3 empyema post VATS decortication are shown in Table 3. Univariate logistic regression analysis revealed that age (p = 0.005), cardiovascular disease (p = 0.009), end-stage renal disease (p = 0.001), pleural effusion pH ≦ 7.2 (p = 0.033) and duration to surgery > 7.5 days (p < 0.001) were factors that had a negative impact on 90-day survival post VATS decortication. Multivariate analysis revealed that age (p = 0.015), end-stage renal disease (p = 0.014), pleural effusion pH ≦ 7.2 (p = 0.014) and duration to surgery > 7.5 days (p = 0.015) negatively impacted 90-day survival post VATS decortication.
Cause of death
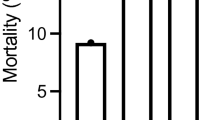
Overall mortality rate of phase 3 empyema in our study group is 22.5%. Figure 4 compared expired patients who underwent VATS decortication ≤ 7.5 days versus > 7.5 days, revealing that septic shock as cause of death was 6.10% and 12.50%, respectively. Earlier intervention group also has less respiratory failure leading to death (4.70% vs. 16.10%). Cancer-caused death was similar in both groups (6.80% vs. 7.10%).
Discussion
In this study, we retrospectively reviewed 204 pneumonia-caused phase 3 thoracic empyema cases involving VATS decortication to determine the optimal timing for VATS decortication. Although surgical decortication is recommended for phase 2 and phase 3 empyema, timing considerations have not been adequately addressed. Our analysis showed that phase 3 empyema patients have better outcomes when decortication is performed within 7.5 days of hospitalization. Knowing the optimal surgical intervention timing is of utter importance for thoracic surgeons to provide better prognosis for phase 3 empyema.
Empyema progression follows a dynamic triphasic development: acute exudation, fibrinopurulence and chronic organization12. During phase 1, pleural inflammation results in the output of sterile pleural fluid with low viscosity, a normal glucose level and a normal pH value. Transition into phase 2 is marked by increases in turbidity, white blood cell count, and LDH level. Both glucose level and pleural fluid pH decrease while fibrin deposits on pleural surfaces due to a reduced tissue type plasminogen activator level. This phase is considered as noncomplex pleural disease and a prospective study done by Migliore demonstrated the possibility to perform uniport VATS decortication under sedation with single-trocar technique16. Uniport VATS has several advantages over traditional three-port VATS and thoracotomy, such as less pain, decreased physiological trauma, and better cosmesis17. Transition into phase 3 typically occurs 4–6 weeks after the development of pleural effusion. The pleural fibrin deposits thicken and begin to organize12. If left untreated, the thick peel exerts a restrictive effect on the lung parenchyma, encasing and immobilizing the lung during respiration18. Goals for the treatment of thoracic empyema include infection control via removal of the purulent material with obliteration and sterilization of the pleural space, re-expansion of the lung, and the elimination of the underlying disease process19.
Empyema patients are rarely asymptomatic, and most patients have varying symptoms depending on the underlying disease process, the extent of the pleural involvement, and the immunologic state of the patient. Patients typically complain of fever, pleuritic chest pain, dyspnea and purulent sputum. Both the 2015 European Association for Cardiothoracic Surgery expert consensus statement and the 2017 American Association for Thoracic Surgery guidelines recommend surgical approaches to patients with phase 2 and 3 empyema, but they did not mention optimal surgical timing1,13. Previous studies on optimal timing of surgery for empyema by Chung et al. and Matsudaira et al. both used disease duration to surgery as their reported timing19,20. In the study by Chung et al., patients in the chronic organization phase constituted only a small fraction of the study group20. Matsudaira et al. reported VATS timing for acute pyothorax but no specific phase21. For phase-specific studies, such as the study by Luciani et al. on uniportal VATS in the treatment of stage II pleural empyema, “surgery at days after admission” was used to standardize treatment timing22. Similarly, for our particular group and focus, we choose a more specific and objective index – duration to surgery since admission day.
Of the 204 patients with phase 3 empyema that had VATS decortication surgery at our institution, the survivor group had less cardiovascular disease and end-stage renal disease. They also received VATS decortication much earlier than the non-survivor group. It is evident from Table 2; Fig. 3 that undergoing VATS decortication more than 7.5 days after admission not only has poor overall survival but also a threefold increase in mortality rate from 14.2 to 44.6%. The reported mortality rate in the literature is 10-20%23. According to Stefani et al., the probability of thoracotomy increases according to the waiting time for surgery24. As empyema evolves into phase 3, there are usually multiple loculations and fibrothorax with diffuse lung entrapment. Pleural adhesion was considered one of the main limitations to using the VATS approach in phase 3 patients because of difficult access to the thoracic cavity25. Our study had no VATS-to-thoracotomy conversions. There were also no re-operations for complications such as air leakage and wound infection. However, there is a higher rate of secondary VATS decortication (14.3%) and a higher rate of post-operative ICU admission (67.9%) in patients that had their first VATS decortication performed within 7.5 days of admission compared to patients that had surgery done afterwards. This may be attributed to the development of fibrothorax and the fact that loculations were not completely eradicated during the earlier approach, so secondary decortication to enhance recovery would be required. Patients that were approached later had a more severe form of fibrothorax with less loculation of fluid, so secondary decortication effects were of minimal help. This extrapolation is reflected on our analysis. Although there is a higher rate of secondary VATS decortication and a higher rate of post-operative ICU admission in patients that had their first VATS decortication within 7.5 days of hospital admission, this group’s overall length of ICU admission and length of hospital stay are much shorter than the patients that had VATS decortication done more than 7.5 days after admission day.
Several factors were associated with poor outcome after VATS decortication. In the present study, univariate analysis shows that age, cardiovascular disease, end-stage renal disease, pleural pH < 7.2 and duration to surgery > 7.5 days are all significant predictors of non-survival within 90 days of VATS decortication. In multivariable analysis, age, end-stage renal disease, pleural pH < 7.2 and duration to surgery > 7.5 days were associated with mortality. Our findings are in concordance with previous studies. Mikkola et al. found that older age was strongly correlated with perioperative mortality in surgical treatment of pleural empyema26. Analysis from the Society of Thoracic Surgeons General Thoracic Surgery Database revealed that preoperative dialysis and poor renal function are associated with poor outcome after decortication27. Moreover, Towe et al. demonstrate that delays in operative intervention for empyema, either VATS or thoracotomy, were associated with operative mortality in the first 5 days for each day delayed27. This suggests that earlier intervention significantly affects outcomes and highlights the need to determine the optimal decortication timing. Patients who received surgical treatment after our defined “golden period” of 7.5 days had longer stays in the intensive care unit and longer hospital stays. Chronic pleural empyema led to passive atelectasis of the underlying compressed lung. Respiratory mechanics and ventilation become compromised due to the restrictive ventilatory effect, and due to lesser oxygen penetration into the alveoli, the perfusion and gas exchange in the lungs are also decreased18. Hence, delayed effective treatment hinders recovery. Some of the compromised lung may not achieve functional recovery post operation28.
We encountered several limitations in this study. First, since the study is a retrospective single-center analysis, selection bias is inevitable and could affect data analysis. We also did not consider the antimicrobial agents that patients received before visiting our institution and during hospitalization. Third, this is a single-institution study and the extrapolated conclusion may not be applicable to all cases. The pathogens and prescribed antibiotics may vary slightly among different demographics, thus a larger-scale multi-center study is needed to confirm our results.
To the best of our knowledge, this is the first study that focuses on optimal VATS decortication timing for chronic thoracic empyema. While it may be instinctive to thoracic surgeons that prompt surgical therapy improves empyema outcomes, there is scarce evidence in the literature regarding quantitative analysis of this surgical timing. Of the reviewed literature to date, our study included the largest case number, 204 patients, and spans the longest study period, one decade. We provide a specific and objective timeframe (7.5 days since admission day) for thoracic surgeons to perform VATS decortication in phase 3 empyema patients in order to achieve a higher rate of overall survival.
Methods
Ethical declaration
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee at Changhua Christian Hospital, Changhua, Taiwan (CCH IRB No. 221001). Informed consent was waived by the Ethics Committee of Changhua Christian Hospital, Changhua, Taiwan.
Patient population and selection
Between May 2011 and December 2021, 262 patients with phase 3 thoracic empyema underwent decortication at Changhua Christian Hospital in Changhua City, Taiwan. A retrospective review of their medical records was performed. Exclusion criteria include open thoracotomy decortication (2 cases), patients younger than 18 years old (3 cases) and non-pneumonia-caused thoracic empyema (53 cases). The remaining 204 cases constitute our study group. The study group was further divided based on survival status at 90 days post VATS decortication: 158 patients lived (survivor group) and 46 patients expired (non-survivor group). Non-survivors are patients who passed away during hospitalization or within the post-operative 90-day period. Figure 1 shows the study design flow chart. Data were collected and compared between the two groups. Results are summarized in Table 1. This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board and ethics committee of Changhua Christian Hospital (CCH IRB No. 221001), which waived the need for patient consent.
VATS decortication
Under general anesthesia with double-lumen endotracheal intubation, the patient was placed in true lateral decubitus position with the affected side positioned up. The number of the ports and their positions depended on the location of the empyema. Thoracoscopic curettage was done under single lung ventilation. Fluid, loculations and septa were removed under endoscopic vision via a suction device and endoscopic forceps. Best-effort decortication was done to remove visceral and parietal pleural peels, freeing the entire lung from apex to diaphragm, and to open the fissures. Hyperthermic pleural irrigation with povidone-iodine and several liters of normal saline was performed. The affected lung was re-expanded and checked for air leakage from possible parenchymal injury during decortication. Two Fr. 28 chest tubes were placed: a straight one placed near the apex and another curved one placed at the base of the thoracic cavity. Post operation, chest tubes were connected to an Emerson suction pump at negative pressure of 15cmH2O for one day to ensure the residual space was obliterated by lung expansion. The timing of chest tube removal was based on clinical condition and the following objective parameters: the inflammatory reaction had subsided, the patient remained afebrile for 48 h, a chest x-ray showed satisfactory lung re-expansion, the daily drainage amount was less than 200 cc, and the drainage had turned serous in color.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation whereas categorical variables are presented as percentages. Independent t tests and chi-square tests were used to evaluate differences between continuous variables and categorical variables, respectively. A receiver operating characteristic (ROC) curve was constructed, and a cutoff value was calculated to evaluate the optimal timing of surgical intervention. The clinical outcomes of overall survival (OS) were calculated by Kaplan-Meier survival curves. Univariate and multivariate analyses were performed to examine the associations of each predictor with survival outcome. Odds ratios and 95% confidence intervals were calculated. All statistical analyses were performed using the statistical package SPSS for Windows (version 23; SPSS Inc, Chicago, IL). Statistical analysis with p < 0.05 was considered statistically significant.
Data availability
Data can be provided upon reasonable request to our corresponding author.
References
Shen, K. R. et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J. Thorac. Cardiovasc. Surg.153, e129–e146. https://doi.org/10.1016/j.jtcvs.2017.01.030 (2017).
Light, R. W. Parapneumonic effusion and empyema. In: Rhyner S, Winter N, Koleth K, editors. Pleural Disease. 5th edition. Lippincott Williams and Wilkins; :179–210 (2007).
Ramirez, J. A. et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin. Infect. Dis.65, 1806–1812. https://doi.org/10.1093/cid/cix647 (2017).
Light, R. W. et al. Parapneumonic effusions. Am. J. Med.69, 507–512 (1980).
Light, R. W. Parapneumonic effusions and empyema. Proc. Am. Thorac. Soc.3, 75–80 (2006).
Maskell, N. A. et al. The bacteriology of pleural infection by genetic and standard methods and its mortality significance. Am. J. Respir Crit. Care Med.174, 817–823 (2006).
Ahmed, R. A., Marrie, T. J. & Huang, J. Q. Thoracic empyema in patients with community-acquired pneumonia. Am. J. Med.119, 877–883 (2006).
Finley, C., Clifton, J., Fitzgerald, J. M. & Yee, J. Empyema: an increasing concern in Canada. Can. Respir J.15, 85–89. https://doi.org/10.1155/2008/975312 (2008).
Grijalva, C. G., Zhu, Y., Nuorti, J. P. & Griffin, M. R. Emergence of parapneumonic empyema in the USA. Thorax. 66, 663–668. https://doi.org/10.1136/thx.2010.156406 (2011).
Nayak, R., Brogly, S. B., Lajkosz, K., Lougheed, M. D. & Petsikas, D. Two decades of thoracic empyema in Ontario, Canada. Chest. 157, 1114–1116. https://doi.org/10.1016/j.chest.2019.11.040 (2020).
Kapp, C. M. & Feller-Kopman, D. Nonmalignant pleural effusions. In: (eds Michael, A., Grippi et al.) Fishman’s Pulmonary Diseases and Disorders. 6th ed. McGraw Hill; https://accessmedicine.mhmedical.com/content.aspx?bookid=3242§ionid=270517391. (2023).
Lo Cicero, J. III. & Feins, R. H. Parapneumonic effusion, empyema, and fibrothorax. In Shields’ General Thoracic Surgery. 8th ed 1513–1534 (Wolters Kluwer, Philadelphia, 2019). Chapter 60.
Scarci, M. et al. EACTS expert consensus statement for surgical management of pleural empyema. Eur. J. Cardiothorac. Surg.48, 642–653. https://doi.org/10.1093/ejcts/ezv272 (2015).
Sorino, C., Mondoni, M., Lococo, F., Marchetti, G. & Feller-Kopman, D. Optimizing the management of complicated pleural effusion: from intrapleural agents to surgery. Respir Med.191, 106706. https://doi.org/10.1016/j.rmed.2021.106706 (2022).
Christensen, T. D. et al. Intrapleural fibrinolysis and DNase versus video-assisted thoracic surgery (VATS) for the treatment of pleural empyema (FIVERVATS): protocol for a randomised, controlled trial - surgery as first-line treatment. BMJ Open.12, e054236. https://doi.org/10.1136/bmjopen-2021-054236 (2022).
Migliore, M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J. Thorac. Cardiovasc. Surg.126 (5), 1618–1623. https://doi.org/10.1016/s0022-5223(03)00592-0 (2003).
Xia, Z. et al. Uniportal versus triportal video-assisted thoracic surgery in the treatment of tuberculous empyema. J. Int. Med. Res.51 (5), 3000605231169901. https://doi.org/10.1177/03000605231169901 (2023).
Abraham, S. V. & Chikkahonnaiah, P. Change in pulmonary function following decortication for chronic pleural empyema. Turk. Thorac. J.21, 27–31. https://doi.org/10.5152/TurkThoracJ.2019.180146 (2020).
Cheng, Y. F., Cheng, C. Y., Huang, C. L., Hung, W. H. & Wang, B. Y. Pleural peels tissue culture plus pleural fluid culture help to improve culture rate for empyema. J. Clin. Med.11, 1882. https://doi.org/10.3390/jcm11071882 (2022).
Chung, J. H. et al. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann. Thorac. Surg.97, 224–229. https://doi.org/10.1016/j.athoracsur.2013.08.039 (2014).
Matsudaira, H. et al. Optimal timing of video-assisted thoracic surgery for acute pyothorax: a retrospective study. Gen. Thorac. Cardiovasc. Surg.69, 1476–1481. https://doi.org/10.1007/s11748-021-01649-7 (2021).
Luciani, C. et al. The uniportal VATS in the treatment of stage II pleural empyema: a safe and effective approach for adults and elderly patients-a single-center experience and literature review. World J. Emerg. Surg.17, 46. https://doi.org/10.1186/s13017-022-00438-8 (2022).
Kanai, E. & Matsutani, N. Management of empyema: a comprehensive review. Curr. Chall. Thorac. Surg.2, 38. https://doi.org/10.21037/ccts.2020.03.02 (2020).
Stefani, A. et al. Preoperative predictors of successful surgical treatment in the management of parapneumonic empyema. Ann. Thorac. Surg.96, 1812–1819. https://doi.org/10.1016/j.athoracsur.2013.06.013 (2013).
Semenkovich, T. R., Olsen, M. A., Puri, V., Meyers, B. F. & Kozower, B. D. Current state of empyema management. Ann. Thorac. Surg.105, 1589–1596. https://doi.org/10.1016/j.athoracsur.2018.02.027 (2018).
Mikkola, R., Kelahaara, J., Heikkinen, J., Lahtinen, J. & Biancari, F. Poor late survival after surgical treatment of pleural empyema. World J. Surg.34, 266–271. https://doi.org/10.1007/s00268-009-0324-8 (2010).
Towe, C. W. et al. Morbidity and 30-day mortality after decortication for parapneumonic empyema and pleural effusion among patients in the Society of thoracic surgeons’ General thoracic surgery database. J. Thorac. Cardiovasc. Surg.157, 1288–1297e4. https://doi.org/10.1016/j.jtcvs.2018.10.157 (2019).
Choi, S. S., Kim, D. J., Kim, K. D. & Chung, K. Y. Change in pulmonary function following empyemectomy and decortication in tuberculous and non-tuberculous chronic empyema thoracis. Yonsei Med. J.45, 643–648. https://doi.org/10.3349/ymj.2004.45.4.643 (2004).
Acknowledgements
The authors thank their patients for entrusting them with their care. They also thank all medical staffs for their assistance in providing excellent care to the patients.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: CML, YLC, BYW; (II) Administrative support: YFC; (III) Provision of study materials or patients: YFC, CYC, CLH, WHH, BYW; (IV) Collection and assembly of data: CML, YLC, YFC, CYC, CLH, WHH, BYW; (V) Data analysis and interpretation CML, YLC, YFC, BYW; (VI) Manuscript writing: all authors; (VII) Final approval of manuscript: All author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, CM., Chen, YL., Cheng, YF. et al. Optimal timing for video assisted thoracic surgery decortication for improved survival in chronic empyema. Sci Rep 14, 24548 (2024). https://doi.org/10.1038/s41598-024-75569-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75569-w