Abstract
Climate change will lead to changes in the suitable ranges of certain species and accelerate the decline of global biodiversity, with alpine plants are particularly sensitive to these changes. Fritillaria przewalskii, a primary source for the traditional Chinese medicine Fritillariae Cirrhosae Bulbus, thrives in alpine meadows at altitudes of 2,800 to 4,400 m. In recent years, the rapid decline of wild plant populations, driven by rising market demand and global warming, has led to its classification as a rare and endangered species. This study utilized 83 distribution records of F. przewalskii and 39 climatic variables to assess its distribution range across different historical periods using an optimized MaxEnt model and analyzed the environmental factors affecting its distribution. The MaxEnt model was optimized with feature combination (FC) = LQ and regularization multiplier β = 1.5, based on the values of AUCdiff, OR10, and ΔAICc. The results indicate that elevation (Elev), solar radiation (Srad5, Srad10), precipitation of warmest quarter (Bio18), and temperature seasonality (Bio4) are key factors influencing its distribution. Ecological niche modeling revealed that this species is currently found mainly in southern Gansu, northwestern Sichuan, eastern Qinghai, and eastern Tibet. In addition, projections from three high-resolution GCM models (BCC-CSM1.1, MIROC, and BCC-CSM2-MR) suggest that the suitable habitat for F. przewalskii will shrink by the 2050s and 2090s, with its distribution shifting toward higher elevations and latitudes. In conclusion, this study aims to predict trends in the adaptive distribution of F. przewalskii under climate change, identify threatened and priority conservation areas, and provide a scientific foundation for the conservation of its germplasm resources.
Similar content being viewed by others
Introduction
Global warming is one of the most pressing environmental challenges the world currently faces1. According to the IPCC Fifth Assessment Report, global temperatures have risen by nearly 1 ℃ over the past century, with the rate of increase significantly accelerating, particularly over the last three decades2. The IPCC’s Sixth Assessment further projects that, by the end of this century, global average temperatures will increase by an additional 1.0 to 5.7 ℃, potentially driving plant species to migrate towards higher latitudes3. Climate change may alter future species distribution patterns, exacerbate biodiversity loss, deplete germplasm resources, and even accelerate the rate of species extinction4. Understanding the relationship between species distribution and environmental factors remains a crucial scientific issue in contemporary ecology5. Therefore, research on the distribution pattern and change of species’ suitable areas under climate change, and clarification of the characteristics of the future geographic distribution pattern of species and the trend of change, are of vital importance to the conservation of species and the sustainable utilization of resources.
In exploring the impact of climate change on the geographical distribution of plants, species distribution models (SDMs) have become a key tool for predicting suitable habitats. SDMs utilize ecological principles, niche theory, and specific algorithms to infer a plant’s niche characteristics from its known geographic distribution and environmental variables, thereby predicting suitable habitat areas6,7. Various predictive models have been developed based on different algorithmic approaches and objectives, including the Maximum Entropy Model (MaxEnt), CLIMEX, BIOCLIM, Artificial Neural Networks (ANN), Ecological Niche Factor Analysis (ENFA), and the Genetic Algorithm for Ruleset Prediction (GARP)8. The MaxEnt model proposed by Phillips in 2004 has excellent predictive performance. It is a density estimation and species distribution prediction model based on maximum entropy theory, which has the advantages of short running time, simple operation, small sample size, high predictive accuracy, and superior stability9. This model has been extensively applied in identifying conservation areas for endangered and rare species, analyzing the growth environments of medicinal plants, and predicting the potential distribution of invasive species10,11,12. Hu et al. utilized this model to predict the suitable area distribution of the medicinal plant Gastrodia elata under future climate scenarios13. Wang et al. employed the model to identify the main environmental factors influencing the growth of Codonopsis pilosula14. Yang et al. utilized the model to assess the environmental variables affecting the potential distribution and suitable habitat areas of Astragalus membranaceus var. mongholicus, receiving positive feedback for its applicability in guiding species cultivation15.
F. przewalskii is one of the source plants for the traditional Chinese medicine Fritillariae Cirrhosae Bulbus, commonly known as “Min Bei”, which is used in its dried bulb form to clear heat, moisturize the lungs, stop coughing, and resolve phlegm16. Its wild resources are distributed in an extremely narrow area, primarily in alpine grasslands at altitudes of 2,800 to 4,400 m. These regions are characterized by fragile ecological conditions, including high altitude, cold climates, large diurnal temperature fluctuations, strong ultraviolet radiation, and a short frost-free period, which together contribute to the slow natural regeneration rate of wild populations of F. przewalskii17. It has been listed as a national level III protected plant in the “Catalogue of China’s Rare and Endangered Plants”18. In recent years, increasing population growth and heightened awareness of healthcare have brought more attention to its medicinal and economic value. The domestic and international market demand for its raw materials has grown annually, and wild resources can no longer meet market needs. Long-term overharvesting and habitat degradation have led to a sharp decrease in the reserves of wild resources, exacerbating the risk of extinction. However, there are no reports on the prediction of suitable habitats in its current or potential distribution areas under future climate change scenarios.
This study employed the MaxEnt model, based on 83 existing geographical distribution points of F. przewalskii, to primarily investigate the effects of environmental factors such as temperature, precipitation, elevation, solar radiation, and soil on its potential distribution. The results offer valuable insights for the strategic planning of F. przewalskii conservation areas and hold significant implications for the research, development, and conservation of its germplasm resources, as well as understanding its spatial distribution patterns. Figure 1 depicts a schematic representation of the proposed methodology.
Materials and methods
Source and processing of species distribution point data
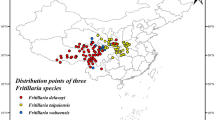
This study integrated a comprehensive literature review and field survey data with records from the Chinese Virtual Herbarium (CVH, http://www.cvh.ac.cn/) and the Global Biodiversity Information Facility (GBIF, https://www.gbif.org). The distribution of F. przewalskii was initially determined based on these records, and the precise longitude and latitude coordinates for 92 sites were obtained via Google Maps (https://ditu.google.cn/)19. To reduce modeling errors, we removed specimen points with obviously incorrect geographic information, especially the loss of precision caused by clustering effects, duplicate distribution points were removed using the ENMTools of the R package, and only one observation was retained in a 5 km x 5 km grid cell, resulting in 83 species distribution records of F. przewalskii data (Fig. 2).
Environmental variable data collection and screening
Environmental variables related to habitat and niche distribution are critical, as those used in SDMs may directly or indirectly influence the model’s accuracy and performance20,21. The 19 bioclimatic layers, solar radiation, and elevation were procured from the WorldClim database (https://www.worldclim.org), characterized by a 2.5 arc-min spatial resolution and spanning 1970 to 2000. The soil data were retrieved from the Harmonized World Soil Database (HWSD, http://www.fao.org/soils-portal/)22. Two periods were selected for past climate data: LIG (last interglacial period) (130 ka BP), and LGM (last glacial maximum) (21 ka BP) downloaded at a spatial resolution of 30″. For the LGM period, we used paleoclimatic layers simulated by the Model for Interdisciplinary Research on Climate Earth System Model (MIROC-ESM)23; and the Community Climate System Model Version Version 4 (CCSM4)24. To simulate the future distribution of F. przewalskii, we employed Representative Concentration Pathways (RCPs) and Shared Socio-economic Pathways (SSPs) emission scenarios25. The outputs of simulated precipitation and temperature from three high-resolution General Circulation Models (MIROC5, BCC-CSM2-MR, and BCC-CSM1.1) were utilized in this study. The climate change model data for MIROC5 and BCC-CSM1.1 were derived from the Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report (AR5). Four Representative Concentration Pathways (RCP2.6, RCP4.5, RCP6.0, and RCP8.5) were modeled to represent low, medium, and high greenhouse gas concentration scenarios (Table 1). In addition, four Shared Socioeconomic Pathways (SSP126, SSP245, SSP370, and SSP585) were selected under the BCC-CSM2-MR model to reflect varying trajectories of socio-economic, environmental, and policy impacts on global greenhouse gas emissions26. In this study, the prediction of suitable areas assumed that topography and soil factors would remain relatively stable over the next 100 years27.
To mitigate multicollinearity among environmental variables, which could affect model output and lead to overfitting, this study applied Spearman correlation and variance inflation factor (VIF) analysis using the ENMeval package in R28 (Fig. 3). A VIF value below 10 indicates no multicollinearity, values between 10 and 100 suggest moderate multicollinearity, and values exceeding 100 indicate severe multicollinearity. Environmental variables with Spearman correlation coefficients below 0.7 and VIFs less than 10 were selected for distribution modeling29,30. Ultimately, 10 environmental variables were retained (Table 2).
Optimization and operation of MaxEnt
In predicting the potential geographical distribution of species, while most studies use default parameters in modeling, some research has demonstrated that the relative complexity of models built with default settings can result in overfitting, making the outcomes difficult to interpret31. Therefore, we used the R package ENMeval to adjust the regularization multiplier (RM) and feature combination (FC), analyzing model complexity across different parameter combinations to select those with lower complexity for model optimization29,32. In this study, the RM parameter was set between 1 and 4, with an interval of 1, resulting in four RM configurations. For the FC parameter, the MaxEnt model provides five feature types: linear (L), quadratic (Q), hinge (H), product (P), and threshold (T). The model’s fit and complexity were evaluated using the Akaike Information Criterion Correction (AICc), the difference between training and testing AUC (AUC DIFF), and the 10% training omission rate (OR10) to assess model overfitting. The parameter combination with the smallest AICc increase was selected as the optimal configuration for model construction33. Based on the lambdas file, RM was set to 1, and FC was set to LQHP, representing the best combination for the MaxEnt model in this study (Table S1).
Species distribution records and filtered data for 10 environmental variables were imported into MaxEnt software to run linear, quadratic, hinge, and product features. By randomly selecting 75% of the observation records as the training set and the remaining 25% as the test set, and conducting 100 iterations, the MaxEnt prediction model was constructed34. The final model output was in Logistic format, with the file type saved in ASC format35.
Model performance evaluation
To evaluate the overall performance of the MaxEnt model’s simulation results, we used the Area Under the Curve (AUC) of the Receiver Operating Characteristic (ROC) curve to assess the model’s discriminative ability. However, research by Ahmadi et al. suggests that AUC alone is insufficient for evaluating potential species distribution models. Therefore, we also calculated the True Skill Statistic (TSS) to further verify the model’s accuracy36,37.
The AUC evaluation ranges from 0 to 1. AUC < 0.6 means the prediction results are not up to standard; 0.6 to 0.7 indicates lower performance; 0.7 to 0.8 indicates medium performance; 0.8 to 0.9 indicates good performance; 0.9 to 1.0 represents excellent performance38,39. TSS, an intuitive threshold-related measure, ranges from − 1 to 1, with 1 representing perfect model performance and 0 or below indicating poor performance40. A TSS value > 0.75 signifies that the model’s predictive performance is outstanding41.
Centroid displacement in suitable distribution areas
This study aimed to investigate the distribution and migration trends of F. przewalskii over time and under different scenarios by calculating centroid shifts in the species’ range using SDMtoolbox, developed in Python42. Habitat distributions were determined based on a threshold of 0.5 by importing MaxEnt’s output ASC files into ArcGIS v.10.6 and converting them into binary format using SDMtoolbox v.2.4. Using the “Centroid Changes (Lines)” function, we summarized the centroid of suitable habitats for F. przewalskii in each period and analyzed the latitudinal and longitudinal changes to evaluate the species’ migration paths and distances within suitable areas.
Results
Evaluation of model prediction accuracy
The model with optimized parameters was used for the MaxEnt simulation of F. przewalskii, employing ROC to verify the model’s accuracy, and both AUC and TSS were calculated. Under current climatic conditions, the model achieved an AUC of 0.984 (standard deviation 0.004) and a TSS of 0.83 (Fig. 4 and Fig. S1). In both historical and future climate scenarios, the AUC exceeded 0.95, and the TSS was above 0.8 (Table S2). These results indicate that the MaxEnt model constructed in this study exhibits high predictive accuracy for the potential distribution of F. przewalskii, effectively revealing the relationship between the species’ potential distribution and environmental factors.
The importance of environment variables
In this study, 10 environmental variables were used for MaxEnt model construction, including 5 climatic factors, 1 topographical factor, 2 soil factors, and 2 solar radiation factors. The response of F. przewalskii suitable habitat distribution to climatic variables was primarily evaluated through percent contribution and Jackknife tests (Fig. 5). The top five environmental variables by percent contribution were elevation (Elev, 45.5%), solar radiation in May (Srad5, 17%), solar radiation in October (Srad10, 13.5%), precipitation of warmest quarter (Bio18, 9.8%), and temperature seasonality (Bio4, 8.5%), together accounting for 94.3%. The environmental variables with the top five importance percentages were elevation (Elev, 59.7%), mean temperature of driest quarter (Bio9, 8.3%), temperature seasonality (Bio4, 7.8%), solar radiation in October (Srad10, 6.4%), and solar radiation in May (Srad5, 5.7%), together representing 87.9% of the total (Table 3). The response curves generated by the MaxEnt model reflect the impact of different environmental factors on the distribution probability of F. przewalskii, especially the relationship between key environmental factors and the species’ occurrence probability. The distribution probability exceeding 0.5 is considered indicative of a potentially suitable environment for the species, which aids in determining thresholds for critical environmental variables (Fig. 6). The graph shows that temperature seasonality (Bio4) is associated with suitable distribution conditions between 700 and 830, with the highest distribution probability at 750. The threshold for precipitation of warmest quarter (Bio18) is 250–370 mm, peaking at 310 mm. Suitable elevation for growth ranges from 2600 to 4100 m. The threshold for solar radiation in May (Srad5) is 18,000–19,800 kJm−2day−1, with the highest distribution probability at 19,000 kJm−2day−1. The species occurs in areas where topsoil organic carbon exceeds 2, peaking at 4.9, after which the probability of distribution gradually decreases. Topsoil pH (H2O) suitable for the growth of F. przewalskii ranges from 5.8 to 7.6, peaking at 7.5.
Predicting the distribution of suitable areas in the glacial period versus the current period
The MaxEnt model’s prediction results (.asc file) were imported into ArcGIS 10.6 for reclassification and habitat suitability zoning. Using this approach, suitability distribution maps for F. przewalskii during the LIG, LGM (CCSM, MIROC), and the contemporary period were created (Fig. 7). In addition, using the natural breaks method, the potential distribution of F. przewalskii was divided into four categories: unsuitable, marginally suitable, moderately suitable, and highly suitable areas (Table 4). In the current period, F. przewalskii is primarily distributed in Sichuan, Tibet, Gansu, and Qinghai in China, with smaller distributions in Ningxia, Shanxi, and Shaanxi, covering a total area of 71.90 × 104 km2. The high suitability areas are located in southern Gansu, central Sichuan, and eastern Qinghai, covering an area of 5.26 × 104 km2. The medium suitability areas mostly surround the highly suitable regions, particularly in southern Qinghai and the Gansu-Sichuan border, covering 32.64 × 104 km2. Low suitability areas are found in eastern Qinghai, southern Tibet, northeastern Sichuan, and regions spanning from southern to central Gansu, as well as sporadic regions between Qinghai and Shaanxi, with a total area of 34.00 × 104 km2. During the LIG, suitable growth regions for F. przewalskii expanded to central Qinghai, southeastern Gansu, southern Shaanxi, and the border between Sichuan and Shaanxi, with medium and high suitability areas densely distributed, particularly in southeastern Gansu and northern and central Sichuan. In the LGM, both the MIROC and CCSM models produced consistent results, with the overall suitable areas expanding into central Sichuan, southern Ningxia, and southeastern Gansu, while medium and high suitability zones were primarily distributed in eastern Tibet, central Sichuan, and southern Gansu.
Forecast of Future suitable distribution and fluctuation analysis
By comparing changes in suitable habitat areas under different climate scenarios, trends of expansion, stability, or contraction in the species’ distribution and corresponding geographical range were revealed (Fig. 8, Fig. S2, Fig. S3, and Table 5). Under three future GCMs, F. przewalskii exhibits various distribution patterns. The BCC-CSM2-MR model of the Shared Socioeconomic Pathways projects suitable areas to expand mainly into central and southern Sichuan and the Sichuan-Yunnan border, while parts of western Sichuan and central Gansu contract (Fig. 8). In the analysis using RCP scenarios with the MIROC5 and BCC-CSM1.1 models, suitable habitats for F. przewalskii are projected to expand toward southeastern Gansu, the intersection of Qinghai and Gansu, and the Tibet-Sichuan border (Fig. S2, Fig. S3). Meanwhile, suitable areas in central Sichuan and northern Tibet are anticipated to shrink, with parts of Qinghai also exhibiting a shrinking trend towards the southeast (Fig. S2, Fig. S3). Regarding changes in suitable area size, most future climate scenarios show an overall increasing trend in suitable regions. However, under the SSP5-8.5 extreme scenario, a decrease occurs, reflecting that the area of expansion exceeds the area of contraction (Fig. 8). For the 2050s, the areas of expansion under the SSP126, SSP245, and SSP370 scenarios show a gradually decreasing trend, but by the 2090s, the areas of expansion under these scenarios increase (Fig. 8). The statistical results from the MIROC and BCC-CSM1.1 models indicate that the suitable area increases only under the 2050s-RCP2.6 emission scenario, while it decreases under all other scenarios (Fig. S2, Fig. S3).
Spatial changes of F. przewalskii in China under emission scenarios of the 2050s and 2090s. White, Gray, Red and Blue areas represent not suitable, unchanged suitable, expansion suitable, and contraction suitable areas, respectively. (a–c), the 2050s; (d–f), the 2090s; (a, d), future climate scenario SSP126; (b, e), future climate scenario SSP370; (c, f), future climate scenario SSP585.
Changes in centroid migration in suitable areas over time
To better understand the migration dynamics of F. przewalskii, centroids were used to represent the centers of potential suitable areas across different periods. By comparing the positions and migration distances under various climate scenarios, the changing routes and trends of future suitable areas for F. przewalskii are intuitively illustrated (Fig. 9). Based on the analysis of different historical periods and three high-resolution GCMs, the centroids of F. przewalskii are consistently located in either the Guoluo Tibetan Autonomous Prefecture or Huangnan Tibetan Autonomous Prefecture in Qinghai Province (Fig. 9a). During the LIG, the distribution centroid was located in Dari County, while in the LGM (MIROC), the centroid shifted northeast to Maqu County, covering a migration distance of 94.98 km (Fig. 9b). In comparison, the current centroid of F. przewalskii has moved southwest from its LGM location, with a migration distance of 72.31 km (Fig. 9b). Future predictions from various models and emission scenarios indicate that, despite differing migration directions, there is an overall trend of movement toward higher latitudes in the southeast. The migration distance increases with higher carbon emissions, but the overall migration range remains relatively small, primarily stabilizing within eastern Qinghai between 33° 08′ N to 34° 28′ N and 100° 13′ E to 101° 09′ E.
BCC-CSM2-MR model migration routes of F. przewalskii suitable habitat centres under current and future climate scenarios. (a) Arrows indicate migratory routes and direction of the suitable habitat distribution center under current and future climate scenarios. (b) The bar chart represents the core distribution shift distance for F. przewalskii under different scenarios/years. Among them, the meaning of the letters were (A) LIG, (B) LGM, (C) Current, (D) SSP1-2.6–2050 s, (E) SSP2-4.5–2050 s, (F) SSP3-7.0–2050 s, (G) SSP5-8.5–2050 s, (H) SSP1-2.6–2090 s, (I) SSP2-4.5–2090 s, (J) SSP3-7.0–2050 s, (K) SSP5-8.5–2090 s.
Discussion
Rationalization of the model
Species distribution models play a crucial role in studying ecological issues under the interaction between species and the environment, especially against the backdrop of global environmental changes43. These models are pivotal for predicting species’ potential distribution shifts across historical periods and into the future, with prediction accuracy heavily influenced by model selection44. Among the various SDMs, the MaxEnt model is widely acclaimed for its robustness and precision. In this study, we optimized the MaxEnt model using the ENMeval package to mitigate overfitting and sampling bias45. The optimized MaxEnt model achieved AUC and TSS scores greater than 0.95 and 0.80, respectively, for glacial periods (LIG, LGM), the current, and future scenarios (2050s, 2090s) (Table S2), highlighting its high accuracy and strong discriminatory power46. Under current climate conditions, predicted suitable habitats for F. przewalskii are mainly distributed in Gansu, Qinghai, and Sichuan, closely aligning with its documented range in the Flora of China.
Adaptive distribution of species in response to climate change
Environmental factors, particularly temperature and precipitation, are vital to plant physiology and phenological traits and are key determinants of growth, regeneration, and distribution in alpine plants47. Zu et al. identified elevation, radiation, and temperature as the primary factors influencing alpine plant distribution patterns48. In this study, we used MaxEnt model outputs, including PI, PC, and Jackknife tests, to analyze the environmental variables affecting the distribution of F. przewalskii. The results revealed that elevation (Elev), solar radiation (Srad5, Srad10), temperature seasonality (Bio4), and precipitation of the warmest quarter (Bio18) are the main factors influencing the distribution of F. przewalskii, with elevation showing the highest PI and PC. Among various climatic variables, annual mean temperature (Bio1) and temperature seasonality (Bio4) were the dominant factors influencing the spatial distribution of species during the glacial periods. The LGM was characterized by cold and dry conditions, with global temperatures 4.6 to 8.3ºC lower than today49. The CCSM and MIROC models for the LGM indicate that compared to LIG, the suitable habitats for LIG were relatively limited. Both models show that core distribution areas were located in the Sichuan Basin, southern Gansu, and eastern Qinghai. During the LGM (CCSM) period, the distribution area of F. przewalskii increased. This expansion phenomenon during the LGM can also be found in other alpine plant groups, such as Gymnadenia orchidis50 and Rhodiola rosea51. In this study, the largest area of distribution was observed during the LGM, followed by the LIG, and finally the present period (Table 4). The LIG is considered one of the warmest interglacials in the past 800,000 years, with geological evidence suggesting significant warming compared to present conditions52. Contrary to expectations, the suitable habitat range of F. przewalskii during the LIG did not shrink but rather expanded significantly, exhibiting a shift towards higher altitudes in the northwest. This pattern of expansion during the LIG is consistent with other alpine cold-tolerant plant species, such as Hansenia weberbaueriana, as demonstrated by Liu et al. in their study of its niche distribution in the Tibetan Plateau53.
Current climate analysis reveals that the primary factors influencing species distribution are elevation, Srad10, Srad5, Bio18, and Bio4, in that order. Since the Industrial Revolution, climate change has continually reshaped the distribution of flora and fauna, particularly affecting the altitudinal distribution of mountain species48. A notable feature of mountain climates is that even slight changes in elevation can lead to significant climate variation. Furthermore, global warming has accelerated glacier melt and permafrost thaw, leading to more complex changes in mountain precipitation patterns beyond those caused by temperature increases alone48. Understanding regional shifts in precipitation is vital for predicting potential changes in vegetation and climate in alpine regions54. Solar radiation intensity impacts plant growth and physiological processes, influencing plant phenology and, consequently, species distribution patterns55. F. przewalskii thrives in moist environments with sufficient water, primarily inhabiting the harsh alpine meadows and grasslands at elevations of 3,000–5,000 m in the western Tibetan Plateau56. Its growth period requires adequate solar radiation, which also influences temperature fluctuations55. Due to constraints imposed by elevation, precipitation, and temperature, F. przewalskii has limited ability to expand into lower-altitude, lower-latitude areas, restricting its current distribution to Gansu, Sichuan, Qinghai, Tibet, and neighboring regions. Studies indicate that while adult Fritillaria plants need strong light, juvenile plants require a certain level of humidity for growth, aligning with model predictions57.
Impacts of future climate change on distribution patterns and conservation measures
Under future climate change scenarios, our results suggest that the suitable distribution areas for the F. przewalskii will tend to shrink in the future (Table 5). This phenomenon has also been observed in other rare and endangered species, such as Nardostachys chinensis and Liriodendron chinense58,59. High-emission RCP scenarios predict temperature increases of 1.3 °C by 2050 and 2.2 °C by 207060. This will lead to a general reduction in the suitable range for F. przewalskii and exacerbate habitat fragmentation. However, under the BCC-CSM2-MR model for SSP1-2.6, SSP2-4.5, SSP3-7.0, and SSP5-8.5 scenarios, projections for the 2050s and 2090s, except for the extreme SSP5-8.5 scenario, suggest an expansion in suitable habitat. The divergence in predictions between CMIP6 and CMIP5 is mainly due to improvements in the physical components of the climate models. Studies indicate that CMIP6 provides better representation of extreme climate events in China, particularly extreme precipitation61. Simulation results from three high-resolution GCMs (BCC-CSM1.1, BCC-CSM2-MR and MIROC5) show that the centers of the suitable areas are predicted to tend to displacement toward high latitudes and elevations, under the emissions scenarios for the 2050s and 2070s (Fig. 9). Due to the influence of climate change, the rate of glacier melting and permafrost thawing are gradually increasing, and the migration rate of species altitudinal distribution is also gradually enhanced, therefore, climate change will lead to a significant change in the distribution of biological species, especially on the composition of mountain forest ecosystems of the biological community has been affected to varying degrees62. Lenoir et al. selected 171 plant species in an alpine forest area in the Alps, and compared species changes along the altitude in the time period 1986–2005, and found that a large number of plants migrated to higher altitudes63. Zu et al. found that most species have migrated upward along the altitude since the mid-1980s by studying the altitudinal changes of seed plants in Gongga Mountain64. Climate warming is the main factor influencing the altitudinal migration of species. This is because altitude is associated with many factors that directly affect the phenology of organisms, especially with climatic factors, such as seasonal variations in mean annual temperature and temperature, which may directly influence key processes that determine the dynamics of species distributions64. Secondly, disturbance also has an important effect on changes in the altitude distribution of species. Natural disturbances (e.g., extreme climate, insect pests, fires, volcanic eruptions, etc.) or anthropogenic disturbances (e.g., deforestation, grazing, farming, tourism, etc.) can significantly affect the change of species’ altitudinal distribution65.
F. przewalskii has long been harvested extensively for its high medicinal value, significantly reducing its natural population61. With the potential for increased impacts from ongoing climate change and human activities, the species may face extinction without prompt conservation efforts, making such measures critical. Niche studies suggest that under future climate conditions, most of the current suitable areas will remain conducive to the species’ growth. Therefore, it is recommended to establish core conservation areas in the high-altitude regions of the Tibetan Plateau, Gansu, and Qinghai, restrict development in these regions, and implement measures to partially protect the species’ wild populations. Additionally, collecting mature seeds from various populations and introducing them to other populations through cross-seeding could alleviate habitat fragmentation, promote genetic exchange, and enhance the genetic diversity of wild populations.
Conclusions
This study, using the optimized MaxEnt model, integrated five climatic variables, one topographical variable, two soil variables, and two radiation variables to predict the potentially suitable habitats of F. przewalskii across different periods. The AUC and TSS values from the model outputs for each period demonstrate that the model accurately simulates the distribution range of F. przewalskii. Among the environmental factors, elevation (Elev), solar radiation in October (Srad10), solar radiation in May (Srad5), temperature seasonality (Bio4), and precipitation of warmest quarter (Bio18) significantly influence the species’ distribution. From the ice age to the future, F. przewalskii has exhibited relative stability in its geographical range, primarily concentrated in southern Gansu, western Sichuan, eastern Qinghai, and eastern Tibet. Future projections from three GCM models suggest a reduction in suitable habitats, with the distribution center shifting to higher altitudes and latitudes. These findings enhance understanding of the potential distribution patterns of F. przewalskii and provide a scientific basis for defining ecological core areas and conserving genetic resources.
Data availability
The data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bayer, A. D. et al. Diverging land-use projections cause large variability in their impacts on ecosystems and related indicators for ecosystem services. Earth Syst. Dynam. 12, 327–351. https://doi.org/10.5194/esd-12-327-2021 (2021).
Tahir, N. et al. 20 years nitrogen dynamics study by using APSIM nitrogen model simulation for sustainable management in Jilin China. Sci. Rep. 11, 17505. https://doi.org/10.1038/s41598-021-96386-5 (2021).
Wang, L. et al. Revealing the long-term trend of the global-scale Ginkgo biloba distribution and the impact of future climate change based on the ensemble modeling. Biodivers. Conserv. 32, 2077–2100. https://doi.org/10.1007/s10531-023-02593-z (2023).
Pounds, J. A. et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 439, 161–167. https://doi.org/10.1038/nature04246 (2006).
Lawler, J. J. et al. Projected climate-induced faunal change in the Western Hemisphere. Ecology. 90, 588–597. https://doi.org/10.1890/08-0823.1 (2009).
Tariq, M. et al. Phytosociological and niche distribution study of Paris polyphylla Smith, an important medicinal herb of Indian himalayan region. Trop. Ecol. 62, 163–173. https://doi.org/10.1007/s42965-020-00125-2 (2021).
Kearney, M. R., Wintle, B. A. & Porter, W. P. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213. https://doi.org/10.1111/j.1755-263X.2010.00097.x (2010).
Wiens, J. A., Stralberg, D., Jongsomjit, D., Howell, C. A. & Snyder, M. A. Niches, models, and climate change: Assessing the assumptions and uncertainties. Pnas. 106, 19729–19736. https://doi.org/10.1073/pnas.0901639106 (2009).
Phillips, S. J., Anderson, R. P. & Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026 (2006).
Yang, J. et al. Potential geographic distribution of relict plant Pteroceltis tatarinowii in China under climate change scenarios. PloS One. 17, e0266133. https://doi.org/10.1371/journal.pone.0266133 (2022).
Wang, Y. E., Zhang, L. I., Du, Z., Pei, J. & Huang, L. Chemical diversity and prediction of potential cultivation areas of Cistanche herbs. Sci. Rep. 9, 19737. https://doi.org/10.1038/s41598-019-56379-x (2019).
Zhang, H., Song, J., Zhao, H., Li, M. & Han, W. Predicting the distribution of the invasive species Leptocybe invasa: Combining MaxEnt and geodetector models. Insects. 12, 92. https://doi.org/10.3390/insects12020092 (2021).
Hu, J. et al. Impact of climate change on the geographical distribution and niche dynamics of Gastrodia elata. PeerJ. 11, e15741. https://doi.org/10.7717/peerj.15741 (2023).
Wang, Z. et al. Study on environmental factors affecting the quality of codonopsis radix based on MaxEnt model and all-in-one functional factor. Sci. Rep. 13, 20726. https://doi.org/10.1038/s41598-023-46546-6 (2023).
Yang, M. et al. Ecological niche modeling of Astragalus membranaceus var. Mongholicus medicinal plants in Inner Mongolia. China Sci. Rep. 10, 12482. https://doi.org/10.1038/s41598-020-69391-3 (2020).
Chinese Pharmacopoeia. Commission Pharmacopoeia of the people’s Republic of China. China Med. Sci. Technol. Press. 36–38. (2020).
Ma, R., Chen, Y., Guo, F., Wu, R. & Jiao, X. Research on Reproductive allocation of Biomass and nutrients of Fritillaria przewalskii Maxim. Acta Agrestia Sinica. 27, 1291–1300. https://doi.org/10.11733/j.issn.1007-0435.2019.05.023 (2019).
Fu, L. List of rare and Endangered Plants in China Science, (1987).
Dou, P., Dong, Y., Chen, L. & Yang, H. Q. Modeling the potential distribution of different types of Dendrocalamus sinicus, the strongest woody bamboo in the world, with MaxEnt model. PeerJ. 10, e13847. (2022). https://doi.org/10.7717/peerj.13847
Heikkinen, R. K. et al. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog Phys. Geog. 30, 751–777. https://doi.org/10.1177/0309133306071957 (2006).
Wang, R. et al. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri (Kuwayama), in China using the MaxEnt model. PeerJ. 7, e7323. https://doi.org/10.7717/peerj.7323 (2019).
FAO/IIASA/ISRIC/ISSCAS/JRC Harmonized World Soil Database (Version 1.2) (FAO, Rome, Italy and IIASA, Laxenburg, 2012).
Watanabe, S. et al. MIROC-ESM 2010: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model. Dev. 4, 845–872. https://doi.org/10.5194/gmd-4-845-2011 (2011).
Gent, P. R. et al. The community climate system model version 4. J. Clim. 24, 4973–4991. https://doi.org/10.1175/2011JCLI4083.1 (2011).
Alizadeh, M. R., Adamowski, J. & Inam, A. Integrated assessment of localized SSP–RCP narratives for climate change adaptation in coupled human-water systems. Sci. Total Environ. 823, 153660. https://doi.org/10.1016/j.scitotenv.2022.153660 (2022).
Riahi, K. et al. The Shared Socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Global Environ. Chang. 42, 153–168. https://doi.org/10.1016/j.gloenvcha.2016.05.009 (2017).
Piwowarczyk, R. & Kolanowska, M. Effect of global warming on the potential distribution of a holoparasitic plant (Phelypaea tournefortii): Both climate and host distribution matter. Sci. Rep-UK. 13, 10741. https://doi.org/10.1038/s41598-023-37897-1 (2023).
Muscarella, R. et al. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 5, 1198–1205. https://doi.org/10.1111/2041-210X.12261 (2014).
Ranjitkar, S., Xu, J., Shrestha, K. K. & Kindt, R. Ensemble forecast of climate suitability for the Trans-Himalayan Nyctaginaceae species. Ecol. Model. 282, 18–24. https://doi.org/10.1016/j.ecolmodel.2014.03.003 (2014).
Zhan, P. et al. Assessment of suitable cultivation region for Panax notoginseng under different climatic conditions using MaxEnt model and high-performance liquid chromatography in China. Ind. Crop Prod. 176, 114416. https://doi.org/10.1016/j.indcrop.2021.114416 (2022).
Radosavljevic, A. & Anderson, R. P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 41, 629–643. https://doi.org/10.1111/jbi.12227 (2014).
Yang, M. et al. Potential global geographical distribution of Lolium temulentum L. under climate change. Front. Plant. Sci. 13, 1024635. https://doi.org/10.3389/fpls.2022.1024635 (2022).
Zhao, Y., Deng, X., Xiang, W., Chen, L. & Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inf. 64, 101393. https://doi.org/10.1016/j.ecoinf.2021.101393 (2021).
Kong, F. et al. Assessing the impact of climate change on the distribution of Osmanthus fragrans using Maxent. Environ. Sci. Pollutr. 28, 34655–34663. https://doi.org/10.1007/s11356-021-13121-3 (2021).
Hosni, E. M. et al. Evaluation of climate change impacts on the global distribution of the calliphorid fly Chrysomya albiceps using GIS. Diversity, 14, 578 .
Ahmadi, M., Hemami, M. R., Kaboli, M. & Shabani, F. MaxEnt brings comparable results when the input data are being completed; Model parameterization of four species distribution models. Ecol. Evol. 13, e9827. https://doi.org/10.1002/ece3.9827 (2023).
Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob Ecol. Biogeogr. 21, 498–507. https://doi.org/10.1111/j.1466-8238.2011.00683.x (2012).
Swets, J. A. Measuring the accuracy of diagnostic systems. Science. 240 (4857), 1285-93. (1988). https://doi.org/10.1126/science.3287615. PMID: 3287615.
Zhao, M., Duan, Q., Shen, X. & Zhang, S. Climate Change influences the Population density and suitable area of Hippotiscus Dorsalis (Hemiptera: Pentatomidae) in China. Insects. 14, 135. https://doi.org/10.3390/insects14020135 (2023).
Liang, W. et al. Predicting the potential invasion of kudzu bug, Megacopta cribraria (Heteroptera: Plataspidae), in North and South America and determining its climatic preference. Biol. Invasions. 20, 2899–2913. https://doi.org/10.1007/s10530-018-1743-y (2018).
Georgopoulou, E., Djursvoll, P. & Simaiakis, S. M. Predicting species richness and distribution ranges of centipedes at the northern edge of Europe. Acta Oecol. 74, 1–10. https://doi.org/10.1016/j.actao.2016.03.006 (2016).
Brown, J. L., Bennett, J. R. & French, C. M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ. 5, e4095. https://doi.org/10.7717/peerj.4095 (2017).
Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. https://doi.org/10.1111/j.1461-0248.2011.01736.x (2012).
Lee, M. & Min, K. A comparative study of the performance for predicting biodegradability classification: The quantitative structure–activity relationship model vs the graph convolutional network. ACS Omega. 7, 3649–3655. https://doi.org/10.1021/acsomega.1c06274 (2022).
Xu, Y. et al. Predicting the current and future distributions of Pennisetum alopecuroides (L.) in China under climate change based on the MaxEnt model. PloS One. 18, e0281254. https://doi.org/10.1371/journal.pone.0281254 (2023).
Li, S., Wang, Z., Zhu, Z., Tao, Y. & Xiang, J. Predicting the potential suitable distribution area of Emeia pseudosauteri in Zhejiang Province based on the MaxEnt model. Sci. Rep. 13, 1806. https://doi.org/10.1038/s41598-023-29009-w (2023).
Verslues, P. E. et al. Burning questions for a warming and changing world: 15 unknowns in plant abiotic stress. Plant. Cell. 35, 67–108. https://doi.org/10.1093/plcell/koac263 (2023).
Zu, K. & Wang, Z. Research progress on the elevational distribution of mountain species in response to climate change. Sheng Wu Duo Yang Xing. 30, 21451. https://doi.org/10.17520/biods.2021451 (2022).
Holden, P. B., Edwards, N. R., Oliver, K. I. C., Lenton, T. M. & Wilkinson, R. D. A probabilistic calibration of climate sensitivity and terrestrial carbon change in GENIE-1. Clim. Dyn. 35, 785–806. https://doi.org/10.1007/s00382-009-0630-8 (2010).
Li, M. et al. Prediction of historical, current, and future configuration of tibetan Medicinal Herb Gymnadenia orchidis based on the optimized MaxEnt in the Qinghai–Tibet Plateau. Plants. 13, 645. https://doi.org/10.3390/plants13050645 (2024).
You, J. et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 8, 5879. https://doi.org/10.1038/s41598-018-24360-9 (2018).
Wang, Y. V. et al. Higher sea surface temperature in the Indian Ocean during the last interglacial weakened the south Asian monsoon. Proc. Natl. Acad. Sci. 119 (e2107720119). https://doi.org/10.1073/pnas.2107720119 (2022).
Liu, M. L., He, Y. L., López-Pujol, J., Jia, Y. & Li, Z. H. Complex population evolutionary history of four cold-tolerant Notopterygium herb species in the Qinghai-Tibetan Plateau and adjacent areas. Heredity. 123, 242–263. https://doi.org/10.1038/s41437-019-0186-2 (2019).
McCullough, I. M. et al. High and dry: High elevations disproportionately exposed to regional climate change in Mediterranean-climate landscapes. Landsc. Ecol. 31, 1063–1075. https://doi.org/10.1007/s10980-015-0318-x (2016).
Liu, P. et al. Numerical investigation of the temperature field effect on the mechanical responses of conventional and cool pavements. Materials. 15, 6813. https://doi.org/10.3390/ma15196813 (2022).
Ma, R., Xu, S., Chen, Y., Guo, F. & Wu, R. Allometric relationships between leaf and bulb traits of Fritillaria przewalskii Maxim. Grown at different altitudes. PLoS One. 15, e0239427. https://doi.org/10.1371/journal.pone.0239427 (2020).
Song, Y. C. et al. Resource investigation on Fritillariae Cirrhosae Bulbus on Tibetan Plateau and its adjacent regions. Mod. Chin. Med. 23, 611–618 (2021).
Li, J. et al. Simulating the effects of climate change across the geographical distribution of two medicinal plants in the genus Nardostachys. PeerJ. 7, e6730. https://doi.org/10.7717/peerj.6730 (2019).
Zhu, Y. Y. & Xu, X. T. Effects of climate change on the distribution of wild population of Metasequoia glyptostroboides, an endangered and endemic species in China. Chin. J. Ecol. 38, 1629. (2019).
Ford, J. D., Vanderbilt, W. & Berrang-Ford, L. Authorship in IPCC AR5 and its implications for content: Climate change and indigenous populations in WGII. Clim. Change. 113, 201–213. https://doi.org/10.1007/s10584-011-0350-z (2012).
Zhu, H., Jiang, Z. & Li, L. Projection of climate extremes in China, an incremental exercise from CMIP5 to CMIP6. Sci. Bull. 66, 2528–2537. https://doi.org/10.1016/j.scib.2021.07.026 (2021).
Gobiet, A. et al. 21st century climate change in the European Alps—A review. Sci. Total Environ. 493, 1138–1151. https://doi.org/10.1016/j.scitotenv.2013.07.050 (2014).
Lenoir, J., Gégout, J. C., Marquet, P. A., de Ruffray, P. & Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 320, 1768–1771. https://doi.org/10.1126/science.1156831 (2008).
Zu, K. et al. Upward shift and elevational range contractions of subtropical mountain plants in response to climate change. Sci. Total Environ. 783, 146896. https://doi.org/10.1016/j.scitotenv.2021.146896 (2021).
Guo, F., Lenoir, J. & Bonebrake, T. C. Land-use change interacts with climate to determine elevational species redistribution. Nat. Commun.91315. https://doi.org/10.1038/s41467-018-03786-9 (2018).
Funding
This study was financially supported by A Study on The Mechanism of Growth Age on Seed Quality and Production Performance of Astragalus membranaceus (22JR5RA846); 2021 Provincial Modern Silk Road Cold and Arid Agriculture Chinese Herb Industry Development Project (2130122); Education Department of Gansu Province, Innovation Fund Project for Higher Education Institutions, 2021 A-001. Science and Technology Department of Gansu Province, Key R&D Program, Research on Monitoring Powdery Mildew of Astragalus membranaceus Based on Unmanned Aerial Vehicle and Machine Learning Technology (21YF5NA070).
Author information
Authors and Affiliations
Contributions
YC and FXG conceived and coordinated the study. PBD and LYW analyzed the data and performed bioinformatics analyses. YC, FXG, PBD, LYW, LJW, DYQ and GB provided some materials and analytical tools. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, P., Wang, L., Wang, L. et al. Investigating the distributional response of the rare and endangered plant Fritillaria przewalskii to climate change based on optimized MaxEnt model. Sci Rep 15, 35939 (2025). https://doi.org/10.1038/s41598-024-75872-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-75872-6