Abstract
This is the first study aimed to detect morphological abnormalities in vivo in the skin capillaries of amyotrophic lateral sclerosis patients (ALS). Videocapillaroscopy assessed subungueal capillaries in 28 ALS patients (cases) and 35 controls (p = 0.42). The mean age was 61.46 and 61.23 years, respectively (p > 0.99). No statistically significant differences were observed between the groups regarding dominant hand, arterial hypertension, dyslipidemia, diabetes mellitus, active smoker, and former smoker variables. 78.57% of cases had spinal onset and 21.43% bulbar. The median disease duration (time between the onset of symptoms and the date of videocapillarscopy) was 29.71 months. Dilated capillaries were detected in 17.8% of cases and 11.43% of controls (p = 0.49). The median of capillary diameter in cases was 10.15 µm and 8.72 µm in controls (p = 0.011). 35.71% of cases and 2.86% of controls had severe capillary tortuosities (p < 0.001). Ramified capillaries were observed in 46.43% of cases and 11.43% of controls (p < 0.002). Micro-hemorrhages were only observed in 10.71% of cases. No significant correlations were observed between disease duration and dilated capillaries, tortuosity, ramified capillaries, and micro-hemorrhages. The present in vivo study shows abnormalities in the skin capillaries of ALS patients that do not depend on disease duration.
Similar content being viewed by others
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that affects upper and lower motor neurons, producing focal onset motor deficits. The course of the disease is rapidly progressive, leading to death, usually due to respiratory failure, in 3–5 years from the onset of symptoms1. A common pathological alteration is the mis-localization and abnormal formation of phosphorylated transactive response DNA binding protein of 43 kDa (TDP-43) deposits in neurons, threads, and oligodendrocytes2,3.
About 5–16% of patients have a family history of ALS, and they are classified as familial ALS (fALS), while the remaining patients are categorized as sporadic ALS (sALS)4.
In addition to motor neurons, blood vessels are also compromised in sALS and transgenic animal models bearing SOD1 mutations, which cause a small percentage of fALS. The blood vessel abnormalities in the nervous system involve thigh junctions, cell interactions, matrix metalloproteinases, transport systems, free radicals, and cytokines5. About 54% of pericytes, as revealed with platelet-derived growth factor receptor-β immunohistochemistry, are lost in the spinal cord in ALS6. In addition, immunoreactivity to vascular endothelial growth factor receptors VEGFR-1 and, to a lesser extent, VEGFR-2, is increased in blood vessels of ALS spinal cords7. Moreover, deposits of abnormal TDP-43 occur in blood vessels of the spinal cord and frontal cortex area 8 in sALS8. Electron microscopic examination of the spinal cord in SOD1 transgenic mice discloses disorganized mitochondrial cristae and degenerating mitochondria in endothelial cells and neuropil, swollen astrocyte foot processes, swollen and degenerating capillary endothelial cells and astrocytes, and extensive extracellular edema9. The blood vessel anomalies in SOD1 transgenic mice appear before motor neuron degeneration and inflammatory responses, indicating that vascular changes contribute to disease initiation in murine models10. Increased perivascular hemoglobin, hemosiderin deposits, plasma-derived immunoglobulin G, thrombin, fibrin, and blood vessel leakage are found in the spinal cord in ALS11. These data point to cumulative factors contributing to the blood–brain barrier (BBB) and blood-spinal cord barrier (BSCB) dysfunction in ALS12,13. Increased QAlb in the CSF is a marker of poor prognosis in a subset of sALS patients14.
Other studies have also shown altered blood vessels in ALS patients, including duplicated basement membranes, deposition of abnormal proteins, decreased collagen IV, fragmented collagen fibers, increased laminin IV, increased expression of VEGF, and altered pericytes15,16,17,18,19.
In vivo capillary imaging of spinal blood flow shows a progressive decrease in capillary diameter, capillary density, and red blood cell speed in the anterior gray matter from the pre-symptomatic stage of ALS model mice; moreover, local spinal glucose utilization is transiently increased at pre-symptomatic stages, only to decrease progressively at advanced stages of the disease in transgenic mice20.
Optical coherence tomography (OCT) has recently identified retinal abnormalities with increased thickening of the outer wall of the retinal vessels compared to controls21,22. Another study carried out on a tissue-engineered skin model derived from skin biopsies from patients with ALS demonstrated, among other alterations, the presence of TDP-43 positive cytoplasmic aggregates with indirect immunofluorescence on a standard microscope as well as with a confocal microscope23. These data suggest that vasculopathy in ALS is a systemic disorder not limited to the spinal cord and frontotemporal cortex.
The capillaroscopy is a non-invasive technique that visualizes the capillaries of the nail bed using a light source and an optical magnifying system. In recent years, the method has been carried out using new equipment called videocapillaroscopy with an optical probe that allows output to a computer and offers the possibility of saving the information digitally24. Various patterns of capillaroscopy indicative of different diseases have been established through the classification of Maricq et al.25, modified by Cutolo et al.26.
This methodology could provide valuable insights into the vascular characteristics of ALS-affected capillaries, offering a more accurate and real-time understanding of capillary modifications in ALS patients. It also holds significant value as it allows the observation of capillary dynamics without the potential modifications introduced during biopsy procedures or in the processing of skin biopsy samples.
Although numerous studies have explored vascular changes in the skin of ALS patients, it is noteworthy that the skin capillaries of ALS patients have never been evaluated in vivo. For this reason, our primary objective was to detect morphological abnormalities in vivo in the skin capillaries of ALS patients using this non-invasive technique, videocapillaroscopy.
Results
Twenty-eight ALS patients, 64.29% males and 35.71% females, and 35 healthy controls, 54.29% males and 45.71% females, were enrolled in the study. No statistically significant differences were observed between the groups (p = 0.42). The mean age of ALS patients was 61.46 (9.85) years, and for controls, it was 61.23 years (10.45). No statistically significant differences were observed between the groups (p > 0.99). All controls and 92.8% of ALS patients were right-handed, while 7.14% were left-handed, showing no statistically significant differences between the groups (p = 0.19).
Regarding the comorbidities, 35.71% of ALS patients and 37.14% of healthy controls had arterial hypertension and dyslipidemia, with no statistically significant differences between the groups (p = 0.91). The prevalence of diabetes mellitus was 3.57% among ALS patients and 11.43% among healthy controls; no statistically significant differences were observed between the groups (p = 0.37). Additionally, 3.57% of ALS patients and 20.00% of healthy controls were active smokers, and 46.43% of ALS patients and 34.29% of healthy controls were former smokers. No statistically significant differences were observed between the groups (p = 0.066 and p = 0.33, respectively).
Regarding the distribution of the ALS phenotype, 78.57% of patients exhibited a spinal onset, while 21.43% had a bulbar onset. Furthermore, 14.29% of ALS patients carried a C9orf72 hexanucleotide expansion. The median disease duration was 29.71 months [19.34, 48.38], and the ALS Functional Rate Scale (ALSFRS) slope median was 0.41 [0.26, 0.78] (Table 1).
Representative images of normal capillaries in healthy controls and altered capillaries in ALS patients are shown in Fig. 1.
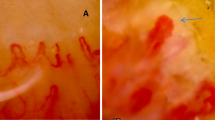
Capillaroscopic findings in healthy controls and ALS patients. (A) and (B): Characteristics of normal capillaroscopy of the nail bed without micro-hemorrhages, ramified capillaries, dilated capillaries, or tortuosities. We can identify a thin arterial afferent branch and a thicker venous efferent branch with a fork or U-shaped morphology without tortuosities. (C): Tortuosities with a dilated capillary. This image corresponds to a 47-year-old man with severe involvement of both upper and lower motor neurons, and rapid progression. (D): Micro-hemorrhages. This image corresponds to the same patients as image (C). (E): Tortuosity. This image corresponds to 70-year-old women with a spinal phenotype and slow progression. (F): ‘Ball of wool’ capillary resulting from severe damage of capillary structure; This image corresponds to 73-year-old women with spinal ALS with predominant involvement of upper motor neurons. (G) and (H): Ramified capillaries from a patient bearing an expansion of C9orf72.
Concerning capillary morphology, 17.86% of ALS patients and 11.43% of controls had dilated capillaries (p = 0.49). The median capillary diameter in ALS patients was 10.15 µm [8.5, 12.06], significantly larger than the 8.72 µm [8.00, 10.00] observed in healthy controls (p = 0.011) (Fig. 2).
In addition, 46.43% of ALS patients and 51.43% of healthy controls exhibited mild capillary tortuosities; 17.86% of ALS patients and 28.57% of healthy controls had moderate capillary tortuosities, while 35.71% of ALS patients and 2.86% of healthy controls presented severe capillary tortuosities. These differences were statistically significant (p < 0.001). Ramified capillaries were observed in 46.43% of ALS patients and 11.43% of healthy controls, with statistically significant differences (p < 0.002). The sub-papillary venous plexus was visible in all subjects, and no avascular zones or thrombosis were identified in any participant. Additionally, there were no significant differences in capillary density between ALS patients and controls (p = 0.76). Notably, micro-hemorrhages were observed in 10.71% of ALS patients and none of the healthy controls (Table 2; Fig. 3).
Stacked bar plots of capillaroscopic parameters. (A). Dilated capillary: ALS patients tend to have larger capillary diameters compared to controls. (B). Tortuosity: the majority of ALS patients have moderate or severe capillary tortuosity, whereas mild or no tortuosity occurs in controls. (C). Ramified capillary: ALS patients have more branches compared to controls. D: Micro-hemorrhages occur in a subgroup of ALS patients.
Finally, we analyzed potential correlations between disease duration and capillaroscopic abnormalities, including dilated capillaries, tortuosity, ramified capillaries, and micro-hemorrhages. As indicated in Material and Methods, disease duration is here considered the time between the beginning of symptoms and the time of videocapillaroscopy No significant differences were observed (Table 3 and Fig. 4).
Discussion
This study in vivo of patients with ALS utilizing video-capillaroscopy has produced compelling findings that shed new light on the nature of the disease. While capillaroscopy has been widely employed in diagnosing and monitoring various rheumatological disorders such as systemic lupus erythematosus27, scleroderma28, Raynaud’s phenomenon26,28, and dermatomyositis29, its application in ALS has been unexplored until now. It is recognized that various rheumatological diseases may show specific capillaroscopic patterns25,26. However, it is relevant to note that the current study findings do not align with any known patterns established for these diseases.
This study observed significant differences between ALS patients and the healthy control group. These differences encompassed moderate and severe tortuosity of capillaries, more ramified capillaries characterized by an increased number of branches, larger capillary diameter, and the presence of micro-hemorrhages. In contrast, no differences were detected in dilated capillary, capillary density, or avascular areas.
Our cohort has no statistically significant differences regarding age, gender, or cardiovascular comorbidities.
The study’s results have significant implications for two reasons. Firstly, they support that vascular alterations in ALS are not confined to the central nervous system. Rather, vascular pathology also affects skin capillaries, possibly through a different pathway than systemic sclerosis. These observations expand our understanding of ALS as a complex condition with effects extending beyond neuronal degeneration. Secondly, the specific findings identified in the study are of immense importance. Micro-hemorrhages indicate capillary damage, while the observation of increased ramified capillaries suggests increased angiogenesis26,30.
These observations align with microscopical studies in skin blood vessels showing duplicated basement membranes, deposition of abnormal proteins, decreased collagen IV, fragmented collagen fibers, increased laminin IV, increased expression of VEGF, and altered pericytes15,16,17,18,19.
The vascular etiology of ALS is not a novel concept. Previous research on SOD1 mutant mice demonstrated disruption of the blood-spinal cord barrier, resulting in micro-hemorrhages, reduced microcirculation, and hypoperfusion even before motor neuron degeneration and inflammatory responses occurred. These findings suggest that vascular changes contribute to disease initiation, at least in murine models10.
Moreover, other studies have shown significant increases in vascular endothelial growth factor (VEGF) levels in ALS patients’ cerebrospinal fluid (CSF) and serum. Elevated VEGF levels have been positively correlated with disease duration and inversely correlated with disease progression rate31. Importantly, increased immunoreactivity to VEGF receptors VEGFR-1 and, to a lesser extent, VEGFR-2 occurs in blood vessels of the ALS spinal cord7. VEGF promotes angiogenesis and is an essential neurotrophic factor for motor neurons32.
We hypothesized that VEGF upregulation may indicate an activation of compensatory responses in ALS31 to preserve and prolong motor neuron survival through neurotrophic mechanisms and to increase vascularity through angiogenic processes. The angiogenic activity may increase vascularization, leading to alterations in patient skin, including those observed with capillaroscopy in our study.
These distinct alterations were not linked to disease duration, thus suggesting that capillary alterations may be present individually at any time of the disease progression.
Whether these changes appear at early stages of the disease in a subpopulation of ALS cases cannot be excluded with the present data.
In conclusion, we found several abnormalities in the skin capillaries of ALS patients associated with angiogenic phenomena. Importantly, these alterations are not associated with the duration of the disease. Further studies involving larger patient cohorts are needed to evaluate whether these abnormalities could have prognostic implications for individuals with ALS.
Materials and methods
Ethics statement
The study was conducted by the Declaration of Helsinki and approved by the Ethics Committee of Bellvitge University Hospital (protocol code: PR396/20, date of approval: 03/12/2020). The protocol was updated and reapproved by the same Ethics Committee on 08/02/2024. The protocol includes the informed consent of the patients.
Inclusion of patients
ALS patients were examined at the motor neuron diseases multidisciplinary unit of the Bellvitge University Hospital. All patients met the El Escorial criteria for a definite and probable category33,34. Patients with other motor neuron diseases were excluded. All patients underwent a routine first visit in outpatient clinics, including a neurological examination, cranial and cervical magnetic resonance imaging (MRI), and subsequent electroneurography, electromyography, blood analysis, lumbar puncture, and transcranial magnetic stimulation in some cases. ALS patients were evaluated clinically according to the primary signs at onset (spinal, bulbar, and respiratory). Spinal-onset ALS was considered when the weakness started in the lower or upper limbs, and the clinical examination showed atrophy, weakness, fasciculations, and hyperreflexia. In bulbar-onset ALS, the disease begins with dysarthria, dysphagia, and, sometimes, tongue fasciculations; disease progression affects limbs and is accompanied by hyperreflexia. Respiratory-onset ALS is characterized by orthopnea or dyspnea, sometimes accompanied by mild spinal or bulbar signs. All the diagnoses were confirmed by electromyography and the monitoring of their evolution. The variables of age, gender, arterial hypertension, hyperlipidemia, diabetes mellitus, active smoking status, and former smoking status were collected for all the patients.
Inclusion of healthy controls
The healthy control group was recruited from employees at Bellvitge University Hospital.
Control participants were carefully selected to ensure the absence of neurological diseases and were verified to be free from systemic conditions known to influence capillaroscopy evaluations. The variables of age, gender, arterial hypertension, hyperlipidemia, diabetes mellitus, active smoking status, and former smoking status were collected for all healthy controls.
Exclusion criteria
Individuals with rheumatic diseases, Raynaud’s phenomenon, and digital ulcers were systematically excluded from the study. Furthermore, we excluded two ALS patients and one healthy control due to suboptimal visualization techniques. The resulting conclusive dataset comprised 28 ALS cases and 35 healthy controls, as detailed in Table 1.
Videocapillaroscopy evaluation
We employed videocapillaroscopy equipment to conduct our assessments, examining both hands in all participants. A drop of immersion oil was applied to the nail bed. The captured images were systematically saved on a computer for subsequent analysis. The evaluation encompassed various parameters, including capillary density, avascular zones, capillary diameter, morphology (tortuosity and ramified capillaries), micro-hemorrhages, thrombosis, and visibility of the venous plexus.
Videocapillaroscopy analysis
A neurologist initially reviewed the images, followed by assessments by two rheumatologists. This sequential and collaborative approach ensured a thorough and diverse evaluation of the recorded data, enhancing the robustness and reliability of our findings.
Definition of capillaroscopic parameters
-
Morphology of the capillaries: The typical capillary comprises a thin arterial afferent branch and a thicker venous efferent branch arranged in a fork or U-shape. Capillary thickening, capillary tortuosity, and ramified capillaries were also assessed24,25.
-
Capillary tortuosity classification: We defined four categories using a qualitative classification: without tortuosities, when the capillaries have a hairpin shape; mild tortuosities, when the limbs bend but do not cross; moderate tortuosities, when the limbs cross once or twice; and severe tortuosities, when the capillaries have a significant distortion and twisting leading to a disrupted capillary pattern35.
-
Capillary diameter: Capillary diameter was determined by measuring three capillaries on the fourth finger of the non-dominant hand; subsequently, the mean was calculated.
-
Ramified capillaries are characterized by branching, bushy, or coiled capillaries, often originating from a single normal-sized capillary36. Our study classified it as ramified if we observed more than two ramified capillaries in more than two fingers.
-
Dilated capillaries are defined as those with a caliber ranging from > 20 µm to < 50 µm, considered non-specific dimension abnormalities36.
-
Sub-papillary venous plexus: located in the papillary portion of the dermis and assessed depending on its ease of visualization; grade 0: no visualization; grade 4: optimal visualization. The assessment of this parameter is subjective and relies on the examiner’s experience.
-
Subungueal micro-hemorrhages: Grade 1: fewer than two bleeds per finger; Grade 2: more than two punctate micro-hemorrhages per finger; Grade 3: extensive and confluent hemorrhagic areas. Micro-hemorrhages must appear in more than two fingers in each hand. All other micro-hemorrhages are categorized as likely traumatic and excluded.
-
Capillary density: In adults and under normal conditions, capillaries are usually ≥ 7/mm2. The classification of avascular zones ranges from 0 to 3. Grade 0 means no avascular zones; grade 1 means one or two avascular zones; grade 2 means more than two avascular zones; and grade 3 means confluent avascular areas24,25,26,37.
Clinical variables
We calculated the diagnostic delay as the diagnosis date minus the start age, the start age as the start date minus the date of birth, and disease duration as the videocapillaroscopy evaluation date minus the start date.
The ALSFRS-r Slope was calculated on the date of the videocapillaroscopy evaluation using the following formula:
ALSFRS-r Slope = (ALSFRS-r before disease onset—ALSFRS-r at the time of videocapillaroscopy evaluation) / disease duration (in months).
Genetic analysis
DNA extraction was performed in the Maxwell® 16 automatic DNA extraction system from leukocytes present in peripheral blood, obtained with EDTA-K3 tubes, and kept at (2–8) ºC for a maximum of one week until the DNA was obtained.
A fragment analysis by capillary electrophoresis was made after PCR amplification of the polymorphic GGGGCC region in the c9orf72 gene, using primers (1 uM) 5’-FAM- CAAGGAGGGAAACAACCGCAGCC-3’ and 5’- GCAGGCACCGCAACCGCAG-3’, and HotStart G2 GoTaq polymerase (Promega), with betaine 1 M and DMSO 5%; the PCR program consisted of 40 cycles of 97ºC-30 s, 68ºC-30 s, 72ºC-60 s, with a final extension at 72 º for 5 min.
Besides, a confirmatory RP-PCR amplification was performed using primers 5’-FAM-CGGGCGCAGGCACCGCAACC-3’ (1,6 uM), 5’-TACGCATCCCAGTTTGAGACG-3’ (1,6 uM) and 5’-TACGCATCCCAGTTTGAGACGGGGGCCGGGGCCGGGGCCGGGG-3’ (0,8uM), and HotStart G2 GoTaq polymerase (Promega), with DMSO 5%, deaza-GTP (0,04 mM) and 1 × SuperFi GC Enhancer (ThermoFisher); the PCR consisted of an 8 cycle-touchdown program (with 2 ºC decrement per cycle, from 70 ºC to 56 ºC) followed by 40 cycles of 95ºC-30 s, 56ºC-30 s, 72ºC-3 min, with a final extension at 72 º for 5 min.
GGGGCC hexanucleotide repeat length of > 30 was used as the standard threshold to distinguish between neutral and pathogenic expansions38.
Statistical analysis
The number of cases and percentages are presented as categorical variables; continuous variables are shown as mean and standard deviation (SD) or median with the first and last quantile [Q1, Q3], depending on whether the data distribution was normal.
The Wilcoxon rank sum test was used for continuous variables to compare the demographic and clinical profiles of the recruited sample groups. Pearson’s chi-squared test or Fisher’s exact test for categorical variables. Box plots and stacked-bar plots were also used.
The association of disease duration (months since diagnosis) with capillaroscopy parameters was assessed in ALS patients. Scatter plots and Spearman correlation tests were performed for numerical non-normal capillaroscopy parameters; box plots and Wilcoxon tests were used for the categorical parameters.
All analyses were done with the statistical package R version 4.2.2 for Windows.
Accepting an alpha risk of 0.05 in a two-tailed test with 35 subjects in the control group and 28 subjects in the ALS group, the statistical power to detect a statistically significant difference was 89% for ramified capillaries (from 0.1143 in the control group to 0.4643 in the ALS group) and 93% for severe tortuosity (from 0.0286 to 0.3571).
Data availability
Data supporting the findings of this study are available from the corresponding author upon request.
References
Brown, R. H. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Eng. J. Med. https://doi.org/10.1056/NEJMra1603471 (2017).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science https://doi.org/10.1126/science.1134108 (2006).
Kwong, L. K., Neumann, M., Sampathu, D. M., Lee, V. M. Y. & Trojanowski, J. Q. TDP-43 proteinopathy: The neuropathology underlying major forms of sporadic and familial frontotemporal lobar degeneration and motor neuron disease. Acta Neuropathol. 114 (1), 63–70. https://doi.org/10.1007/s00401-007-0226-5 (2007).
Byrne, S. et al. Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: A population-based case-control cohort study of familial and sporadic amyotrophic lateral sclerosis. Ann. Neurol. 74 (5), 699–708. https://doi.org/10.1002/ana.23969 (2013).
Garbuzova-Davis, S. et al. Amyotrophic lateral sclerosis: A neurovascular disease. Brain Res. 1398, 113–125. https://doi.org/10.1016/j.brainres.2011.04.049 (2011).
Winkler, E. A., Sengillo, J. D., Bell, R. D., Wang, J. & Zlokovic, B. V. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J. Cerebral Blood Flow Metab. 32 (10), 1841–1852. https://doi.org/10.1038/jcbfm.2012.113 (2012).
Spliet, W. G. M. et al. Immunohistochemical localization of vascular endothelial growth factor receptors-1, -2 and -3 in human spinal cord: Altered expression in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 30 (4), 351–359. https://doi.org/10.1111/j.1365-2990.2003.00543.x (2004).
Ferrer, I., Andrés-Benito, P., Carmona, M., Assialioui, A. & Povedano, M. TDP-43 Vasculopathy in the Spinal Cord in Sporadic Amyotrophic Lateral Sclerosis (sALS) and Frontal Cortex in sALS/FTLD-TDP. J. Neuropathol. Exp. Neurol. 80 (3), 229–239. https://doi.org/10.1093/jnen/nlaa162 (2021).
Garbuzova-Davis, S. et al. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 1157 (1), 126–137. https://doi.org/10.1016/j.brainres.2007.04.044 (2007).
Zhong, Z. et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 11 (4), 420–422. https://doi.org/10.1038/nn2073 (2008).
Winkler, E. A. et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 125 (1), 111–120. https://doi.org/10.1007/s00401-012-1039-8 (2013).
Evans, M. C., Couch, Y., Sibson, N. & Turner, M. R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 53, 34–41. https://doi.org/10.1016/j.mcn.2012.10.008 (2013).
Garbuzova-Davis, S. et al. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 1469, 114–128. https://doi.org/10.1016/j.brainres.2012.05.056 (2012).
Assialioui, A., Domínguez, R., Ferrer, I., Andrés-Benito, P. & M.,. Povedano, ‘Elevated Cerebrospinal Fluid Proteins and Albumin Determine a Poor Prognosis for Spinal Amyotrophic Lateral Sclerosis’. Int. J. Mol. Sci. https://doi.org/10.3390/ijms231911063 (2022).
Kolde, G., Bachus, R. & Ludolph, A. C. Skin involvement in amyotrophic lateral sclerosis. Lancet 347 (9010), 1226–1227. https://doi.org/10.1016/S0140-6736(96)90737-0 (1996).
Ono, S. et al. Decreased type IV collagen of skin and serum in patients with amyotrophic lateral sclerosis. Neurology 51 (1), 114–120. https://doi.org/10.1212/WNL.51.1.114 (1998).
Ono, S., Shimizu, N., Imai, T., Mihori, A. & Nagao, K. Increased cystatin C immunoreactivity in the skin in amyotrophic lateral sclerosis. Acta Neurol. Scand. 102 (1), 47–52. https://doi.org/10.1034/j.1600-0404.2000.102001047.x (2000).
Suzuki, M. et al. Immunohistochemical studies of vascular endothelial growth factor in skin of patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 285 (1–2), 125–129. https://doi.org/10.1016/j.jns.2009.06.021 (2009).
Coatti, G. C., Cavaçana, N. & Zatz, M. The Role of Pericytes in Amyotrophic Lateral Sclerosis’. In Advances in Experimental Medicine and Biology (ed. Coatti, G. C.) (Springer, 2019).
Miyazaki, K. et al. Early and progressive impairment of spinal blood flow-glucose metabolism coupling in motor neuron degeneration of ALS model mice. J. Cerebral Blood Flow Metab. 32 (3), 456–467. https://doi.org/10.1038/jcbfm.2011.155 (2012).
Abdelhak, A. et al. In vivo assessment of retinal vessel pathology in amyotrophic lateral sclerosis. J. Neurol. 265 (4), 949–953. https://doi.org/10.1007/s00415-018-8787-x (2018).
Cerveró, A., Casado, A. & Riancho, J. Retinal changes in amyotrophic lateral sclerosis: Looking at the disease through a new window. J. Neurol. https://doi.org/10.1007/s00415-019-09654-w (2019).
Paré, B. et al. Early detection of structural abnormalities and cytoplasmic accumulation of TDP-43 in tissue-engineered skins derived from ALS patients. Acta Neuropathol. Commun. 3, 5. https://doi.org/10.1186/s40478-014-0181-z (2015).
Juanola, X., Sirvent, E. & Reina, D. Capilaroscopia en las unidades de reumatología. Usos y aplicaciones. Revista Española de Reumatología 31 (9), 514–520 (2004).
Maricq, H. R. & Carwile LeRoy, E. Patterns of finger capillary abnormalities in connective tissue disease by “wide-field” microscopy. Arthritis Rheum. https://doi.org/10.1002/art.1780160506 (1973).
Cutolo, M., Grassi, W. & Cerinic, M. M. Raynaud’s Phenomenon and the Role of Capillaroscopy. Arthritis Rheum. 48 (11), 3023–3030. https://doi.org/10.1002/art.11310 (2003).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis 1 (5), 293–299. https://doi.org/10.1080/146608200300079536 (2000).
De Carvalho, M. & Swash, M. Awaji diagnostic algorithm increases sensitivity of El Escorial criteria for ALS diagnosis. Amyotr. Lateral Scler. 10 (1), 53–57. https://doi.org/10.1080/17482960802521126 (2009).
Smith, V. et al. An EULAR study group pilot study on reliability of simple capillaroscopic definitions to describe capillary morphology in rheumatic diseases. Rheumatology (United Kingdom) 55 (5), 883–890. https://doi.org/10.1093/rheumatology/kev441 (2016).
Smith, V. et al. Nailfold capillaroscopy. Best Pract. Res. Clin. Rheumatol. https://doi.org/10.1016/j.berh.2023.101849 (2023).
Piette, Y. et al. Standardised interpretation of capillaroscopy in autoimmune idiopathic inflammatory myopathies: A structured review on behalf of the EULAR study group on microcirculation in Rheumatic Diseases. Autoimmun. Rev. https://doi.org/10.1016/j.autrev.2022.103087 (2022).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-Linked FTD and ALS. Neuron 72 (2), 245–256. https://doi.org/10.1016/j.neuron.2011.09.011 (2011).
Cutolo, M. et al. Nailfold capillaroscopy in systemic lupus erythematosus: A systematic review and critical appraisal. Autoimmun. Rev. https://doi.org/10.1016/j.autrev.2017.11.025 (2018).
Smith, V. et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. https://doi.org/10.1016/j.autrev.2020.102458 (2020).
Bertolazzi, C., Cutolo, M., Smith, V. & Gutierrez, M. State of the art on nailfold capillaroscopy in dermatomyositis and polymyositis. Seminars Arthr. Rheumat. https://doi.org/10.1016/j.semarthrit.2017.06.001 (2017).
Cutolo, M., Pizzorni, C., Secchi, M. E. & Sulli, A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 22 (6), 1093–1108. https://doi.org/10.1016/j.berh.2008.09.001 (2008).
Gao, L., Zhou, S., Cai, H., Gong, Z. & Zang, D. VEGF levels in CSF and serum in mild ALS patients. J. Neurol. Sci. 346 (1), 216–220. https://doi.org/10.1016/j.jns.2014.08.031 (2014).
Calvo, P., Hernández, R., De La Cruz, R. & Pastor, A. Role of vascular endothelial growth factor as a critical neurotrophic factor for the survival and physiology of motoneurons. Neural Regen Res. https://doi.org/10.4103/1673-5374.363194 (2023).
Acknowledgments
We wish to thank Tom Yohannan for editorial help. The authors thank Cristian Tebé and Pau Satorra for their help with the statistical analysis.
Funding
The project leading to these results received funding from “Fundació Catalana d’ELA Miquel Valls”, “Retos todos unidos contra la ELA” and “Proyecto DGeneracion conexiones con sentido” to MP. We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Author information
Authors and Affiliations
Contributions
A.A., R.D., and M.P. clinically selected ALS cases and controls; C.M.P. and X.J. carried out skin capillaroscopy; A.A., C.M.P., X.J. and V.T. analysed the skin capillaroscopy; A.A. performed the statistical analysis of the observations; N.S. and J.P. revised the statistical analysis; A.A. designed the study and wrote the manuscript; M.P and I.F. designed the study, revised the results and the final version of the manuscript; all authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Assialioui, A., Marco-Pascual, C., Torrente-Segarra, V. et al. Microvascular abnormalities in skin capillaries of individuals with amyotrophic lateral sclerosis. Sci Rep 14, 24648 (2024). https://doi.org/10.1038/s41598-024-75899-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-75899-9