Abstract
Accessory nerve (CNXI) has been known to be the primary conduit for motor control of the trapezius, while the supplementary cervical nerves (C3 and C4) are responsible for processing sensory information from muscle. However, the lack of substantial direct evidence has led to these conclusions being regarded as mere speculation. This study used immunostaining (using antibodies against neurofilament 200 for all axons, choline acetyltransferase for cholinergic axons, tyrosine hydroxylase for sympathetic axons, and alpha 3 sodium potassium ATPase for proprioceptive afferent axons) of human samples to verify the functional contributions of nerves. Study highlights the pivotal role of C3 and C4 in regulating precise movements of trapezius, contributing to motor control, proprioceptive feedback, and sympathetic modulation. CNXI is composed primarily of somatic efferent fibers, with significant numbers of sympathetic or sensory fibers. Furthermore, C3-4 have both cholinergic and non-cholinergic axons, suggesting their involvement in proprioceptive feedback and somatic efferent functions. Although less common, mechanosensors such as nociceptive sensor and sympathetic fibers are also supplied by these cervical nerves. The study demonstrated that these nerves contain motor fibers and significant proprioceptive and sympathetic axons, challenging the long-held notion that CNXI are motor and upper spinal nerves are sensory.
Similar content being viewed by others
Introduction
The accessory nerve, the 11th cranial nerve (CNXI), transmits nerve signals to the trapezius muscle in conjunction with the upper cervical spinal nerves1,2. The CNXI is considered to be primarily responsible for motor functions, while the supplementary cervical nerves are considered to be responsible for sensory information3,4. However, recent studies have indicated that the accessory nerves can also transmit sensory or afferent pain signals5,6,7,8. Conversely, there is an electrophysiological study that raises the possibility of a motor contribution of the cervical nerves to the trapezius muscle9.
To understand muscle movement, elucidating the peripheral pathways that transmit sensory feedback from mechanoreceptors or proprioceptors is imperative10,11,12,13. Anatomically, mixed nerves provide pathways for both afferent and efferent axons to the same muscle, as demonstrated by the mandibular nerve, which contains efferent axons for the chewing muscles and afferent axons for tactile perception and proprioception. Traditionally, the term “motor nerve” has been defined as a bundle of efferent axons that trigger the release of acetylcholine from the motor endplate, resulting in muscle contraction. The term “muscle nerve” or “nerve to muscle” is used to indicate a nerve that morphologically innervates a muscle, regardless of its component, however, this term and “motor nerve” are used interchangeably. However, Mioton et al.14 proposed that most motor nerves consist of both efferent and afferent axons that facilitate proprioception or mechanoreception for muscle movement control. If pure “motor nerves” exist, complementary sensory nerves must be involved in this process. For instance, the sensory branches of the trigeminal nerve may transmit sensory information from mechanoreceptors on the facial skin to facial expression muscles. Reports on nervous anastomoses between the facial and trigeminal nerves suggest potential routes for afferent axons along the motor nerve to facial muscles13. Furthermore, craniospinal connections between the cervical spinal nerve and cervical branch of the facial nerve also suggest the possibility of shared sensory feedback to the platysma15.
The trapezius muscle, one of the largest extrinsic back muscles, is responsible for complex movements of the upper limb, shoulder, and neck. It plays an additional role in maintaining posture by providing sensory feedback to the upper body. Given the variability in clinical symptoms based on the distribution and quantity of axonal differences in the involved nerves, previous anatomical and clinical studies have reported co-innervation of the trapezius by the cranial and spinal nerves1,3,16,17,18. However, detailed anatomical information on the functional contribution of these components to the trapezius muscle is scarce. Additionally, to date, no studies have categorized these components using axonal profiling. Gavid et al19. conducted an electric stimulation study indirectly indicating that the C2, C3, and C4 nerves are distributed in the trapezius, along with the CNXI, suggesting alternative or complementary roles. Nevertheless, to gain a more profound understanding of the functional relationship between the nerves and the trapezius muscle, an anatomical study is necessary to directly identify the various components of the nerves, including the motor, sensory, and sympathetic axons.
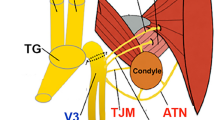
Thus, this study aimed to elucidate the functional roles (motor, proprioceptive, and sympathetic) of the CNXI, C3, and C4 nerves in the regulation of trapezius movement using immunofluorescence analysis. We developed an axonal profiling technique using immunofluorescence to analyze the motor and sensory axon bundles in human samples. Anti-neurofilament 200 (NF200) antibodies were used to stain nerve axons20,21, and anti-choline acetyltransferase (ChAT) antibodies were used to tag motor axons11,20. Anti-tyrosine hydroxylase (TH) and anti-ATPase Na+/K+-transporting subunit alpha 3 (ATP1A3) were also used to label sympathetic axons22 and proprioceptive afferent axons23 (Fig. 1).
Results
Fascicles of the nerves
Masson’s trichrome staining and immunohistochemistry revealed that nerve fascicles were deposited within the epineurium of each nerve. The mean number of fascicles observed per nerve was as follows: 2.67 ± 1.63 fascicles in CNXI, 3.33 ± 1.52 fascicles in C3, and 2.0 ± 0 fascicles in C4 (Fig. 2). Herein, the number of axons exhibiting positive immunofluorescence staining for each antibody was calculated as the number per fascicle, except that in Fig. 7. The majority of axons in the CNXI, C3, and C4 nerves were cholinergic fibers (NF200+/ChAT+): 51.77% in CNXI, 55.12% in C3, and 46.52% in C4. In all the three nerves, no somatotopic trend was identified in the axonal arrangement of the two functional categories. Even when considering the same nerve type, in some cases, the nerve exhibited clear compartmentalization of cholinergic and non-cholinergic innervation, whereas in others, the nerve displayed an atypical mixture of these two types of innervations.
Functional modality of the axons
To determine the functional modality (cholinergic motor, non-cholinergic sympathetic, and non-cholinergic proprioceptive or non-proprioceptive sensory fibers) of the nerve, a quantitative analysis of its composition was performed based on the number of axons expressing NF200, ChAT, TH, ATP1A3, and MBP (Figs. 3 and 4). Cholinergic and non-cholinergic axons were counted based on NF200 and ChAT expression (Figs. 3A and 4B and C). Figure 3B; Table 1 show detailed counts of somatic (TH-) and non-cholinergic axons in the three nerves. Their proportions are displayed in Fig. 4C; Table 2. Each cadaver exhibited a higher number of cholinergic axons in C3 compared to C4. Visualization of myelination with toluidine blue (Fig. 3D) indicated the formation of a cluster of small unmyelinated axons, which were indicative of sympathetic fibers. Myelinated fibers (cholinergic motor or non-cholinergic sensory) are typically larger than unmyelinated fibers. Unmyelinated fibers that were not identified as sympathetic axons and were solely located among myelinated fibers were rarely observed.
(A) Immunofluorescence staining for the accessory (CNXI), C3, and C4 nerves. The sample has been stained with antibodies against NF200 (green) and ChAT (red). (B) The bar graph illustrates the comparison of total axons (NF200+) and cholinergic axons (ChAT+/NF200+) per fascicle for each nerve. (C) A comparison of the detailed proportion of cholinergic (NF200+/ChAT+) and non-cholinergic axons (NF200+/ChAT-) per nerve fascicle in the three nerves. (D) The visualization of myelination of the axons via toluidine blue staining. Myelinated axons (arrows) represent motor or sensory fibers. Some unmyelinated axons (arrowheads) represent sensory fibers (e.g., afferent c-fiber). A cluster of small unmyelinated axons (dashed line) represents sympathetic fibers. (E) Bar graph comparing the somatic and visceral-sympathetic proportions of the three nerves. NF200, neurofilament 200; ChAT, choline acetyltransferase.
Somatic efferent and afferent axons of the C4 nerve. (A) All axons were stained with anti-NF200 antibody (green). (B) The cholinergic axons were stained with an anti-ChAT antibody (red). (C) Merged immunofluorescence staining images of NF200 and ChAT. The dashed lines indicate non-cholinergic axons in C and D. (D) Merged immunofluorescence staining images of MBP (red) and ATP1A3 (green). The co-expression of ATP1A3 with MBP suggests that they are myelinated proprioceptive sensory fibers. The large nonsympathetic fiber that lacks ATP1A3 expression (MBP+/ATP1A3-) appears to be involved in mechanosensation rather than in proprioception. NF200, neurofilament 200; ChAT, choline acetyltransferase; MBP, myelin basic protein; ATP1A3, alpha 3 sodium potassium ATPase.
Table3 presents the number of non-cholinergic axons expressing TH and ATP1A3 and their proportions in each nerve. As anticipated, non-cholinergic axons (NF200+/ChAT-) exhibited a clear morphological dichotomy. The small non-cholinergic axons closely matched the TH + axons, indicating that they have morphologic characteristics indicative of sympathetic nerves (Fig. 5A). These sympathetic nerves relay fibers to muscles after synapsing at the cervical sympathetic ganglia. In contrast, large noncholinergic axons were predominantly positive for ATP1A3 and MBP, indicating that they are myelinated proprioceptive sensory fibers (Fig. 4D).
The motor, proprioceptive, and sympathetic axons of the accessory (CNXI), C3, and C4 nerves. (A) The images show immunofluorescence staining for NF200, ChAT, ATP1A3, and TH in the three nerves. The percentages of axons with ChAT (cholinergic), ATP1A3 (proprioceptive), and TH (sympathetic) in total axons (NF200) and the number of axons per fascicle are presented. (B) Graph illustrating the proportions of motor, proprioceptive, others, and sympathetic axons of the three nerves per fascicle. Detailed percentages are presented in Table 3. NF200, neurofilament 200; ChAT, choline acetyltransferase; ATP1A3, alpha 3 sodium potassium ATPase; CNXI, 11th cranial nerve; TH, tyrosine hydroxylase.
In summary, sympathetic fibers constituted 34.96%, 30.91%, and 33.35% of all axons in CNXI, C3, and C4, respectively, whereas proprioceptive sensory fibers accounted for 6.87%, 9.64%, and 10.24% of all axons in these regions, respectively (Fig. 5B; Table 2).
Large nonsympathetic fibers without ATP1A3 expression (MBP+/ATP1A3-) appear to be involved in low-threshold mechanosensation such as nociception instead of proprioception. Notably, a branch from C3 had an anomalous course in all individuals (Fig. 6). This branch did not join CNXI but directly entered the upper part of the trapezius. The branch clearly showed both cholinergic (38.01% of all axons) and proprioceptive sensory axons (61.99% of all axons), indicating that the cervical spinal nerve can independently innervate the trapezius as a motor nerve with its own proprioception.
Contribution of the nerves in controlling and monitoring the trapezius
Regarding the overall axonal composition of the three nerves, approximately half of the motor efferent, proprioceptive, and sympathetic axons innervating the muscle are supplied by the accessory nerve (Fig. 7). The two spinal nerves supplied the remaining half of the axons. C3 delivered a greater proportion of motor (34.5% vs. 16.4%) and proprioceptive (30.1% vs. 18.3%) axons than did C4, whereas C4 was associated with a greater proportion of sympathetic axons (28.4% vs. 22.1%) than was C3.
Functional contribution of the accessory (CNXI), C3, and C4 nerves to the trapezius muscle (all fascicles). C3 and C4 contribute axons to the remaining half of the relevant neural pathway. C3 provides a significantly higher proportion of motor axons (34.5% vs. 16.4%) and proprioceptive axons (30.1% vs. 18.3%) than does C4. Conversely, C4 supplies a greater proportion of sympathetic axons (28.4% vs. 22.1%) than does C3. CNXI, 11th cranial nerve.
Discussion
All specimens in the present study showed convergence of C3 and C4 to CNXI before entering the trapezius, with subsequent distribution of the merged nerve to the trapezius. Our comprehensive examination at multiple scales, from macroscopic observation at the nerve level to detailed histological examination at the fascicle, axon, and myelin levels, revealed that this convergence is not merely the union of two spinal nerves with CNXI but also involves a functional rearrangement to modulate motor function.
This study highlights the pivotal role of C3 and C4 in regulating the precise movements of the trapezius, which contributes to motor control, proprioceptive feedback, and sympathetic modulation. The cholinergic population (NF200+/ChAT+) appears to influence motor function by acting on the motor endplate, whereas the non-cholinergic population (NF200+/ChAT-) contains axons with sympathetic (MBP-/TH+), proprioceptive (MBP+/ATP1A3+), and mechanosensation (MBP+/ATP1A3-) properties. The CNXI is primarily composed of somatic efferent fibers with a considerable number of sympathetic or sensory fibers. C3 and C4 also exhibit a clear presence of both cholinergic and non-cholinergic axons, indicating their involvement in both proprioceptive feedback and somatic efferent fibers in the muscle. Although less common, nociceptive sensory and sympathetic fibers are supplied by these cervical nerves.
Our findings align with those of previous studies showing that motor nerves, previously believed to consist solely of efferent axons, also contain afferent fibers that convey proprioceptive or mechanical sensations24. For example, although the extracranial facial nerve is considered to be a purely somatic efferent nerve that innervates facial expression muscles, Tereshenko et al.13 reported that only 68% of the temporal, 61% of the zygomatic, 53% of the buccal, 62% of the mandibular, and 43% of the cervical branches of the facial nerve were efferent axons.
Neuroanatomy
The CNXI comprises two components: the spinal component, which innervates the trapezius and sternocleidomastoid (SCM) muscles, and the cranial component, which innervates the laryngeal musculature. The spinal component is composed of axons from lower motor neurons in the upper spinal cord segments, consistent with C3 and C4. The spinal CNXI connects to the vagus nerve within the jugular foramen via the internal branches. The superior ganglion of the vagus nerve, which is formed by sensory neurons, provides general somatic afferent signals, and the internal branches between the two nerves may transmit somatic sensations from the ganglion to CNXI15. Although Gesslbauer et al.11 indicated that the accessory nerve is primarily composed of efferent axons with few proprioceptive components, our study demonstrated that this nerve contains a notable proportion of noncholinergic axons, exceeding 40%. In fact, CNXI and its accompanying cervical nerves provide both motor and sensory components, albeit in varying compositions Our findings indicate that somatic efferent fibers are transmitted via the CNXI, C3, and C4 nerves from the lower motor neurons within the spinal cord, whereas the somatic afferent fibers are conveyed via CNXI from the superior vagal ganglion or via C3 or C4 from the dorsal root ganglion.
Developmental and evolutionary consideration
The presence of a considerable number of C3 cholinergic fibers distributed independently in the descending (upper) part of the trapezius muscle is noteworthy and indicates that the descending part of the trapezius is subject to independent regional motor control by spinal nerves. Bocca et al.25 observed an absence of accessory nerve innervation in the descending part of the trapezius in 16% of their cases, which supports the possibility of an alternative spinal contribution, as suggested in the present study. The trapezius muscle originates from the mesoderm of the occipital lateral plate, whereas the muscles innervated by the trigeminal, facial, and vagus nerves (special visceral efferent fibers) originate from the pharyngeal apparatus26. The occipital origins of these muscles may be related to the additional motor distribution of the cervical spinal nerves. Furthermore, the identification of the trapezius as a single mesenchymal condensation with the SCM in 11 cm-long human specimens may be related to a similar dual innervation pattern of the SCM27.
Phylogenetically, the spinal accessory nerve represents an evolutionary adaptation that integrates features of both head and trunk musculature, reflecting the dual metameric organization of the vertebrate body. Early vertebrates, such as agnathans (lampreys), lack a distinct spinal accessory nerve but have structures suggestive of their evolutionary ancestors. In jawed vertebrates (gnathostomes), however, it became distinct from the vagus nerve and began to innervate the cucullaris muscle, which evolved into the sternocleidomastoid and trapezius muscles in mammals and shares many cytoarchitectural features with somatic spinal nerves.
Molecular aspect and methodological consideration
Previous studies have shown that small non-cholinergic axons clustered in the motor nerve are positive for TH, a marker of the sympathetic nervous system13. Given that muscle sympathetic nerve activity controls blood pressure and blood flow to support the metabolic needs of skeletal muscles, sympathetic control of the trapezius, as a large shoulder muscle, must be metabolically appropriate for its activities, such as upper limb movement or prolonged postural maintenance28. Sympathetic innervation appears to be mainly carried out by the cervical spinal nerves, whereas the accessory nerve has few sympathetic fibers. Previously, sympathetic fibers were also found to comprise 15–25% of the facial nerve and were thought to maintain the unconscious muscle tone of the facial musculature13; however, it remains unclear whether a similar sympathetic role exists for the trapezius.
In this study, ATP1A3 was used as a proprioceptive axon marker. Markers for proprioceptive or mechanoreceptive sensory fibers have been identified in rodents, with most studies conducted in this species. Wei et al.29 found that rats express higher levels of Hgf and inhibin beta A in cutaneous nerves, which transmit tactile or nociceptive sensations, than in motor nerves. The histological localization of the muscle spindle, a small intramuscular proprioceptive organ, is challenging. To address this challenge, Bornstein et al.23 used mice expressing TdTomato to label muscle spindles. Transcriptomic analysis revealed high expression of ATP1A3, DYPSL3, HSPA12A, VAT1L, PLP1, and TUBB3 in the nerves surrounding the spindle. ATP1A3expression was robust, as confirmed by immunostaining. Maintaining transcriptome stability and protein antigenicity in humans is challenging because of the difficulty in obtaining cadavers with short postmortem intervals (PMI), optimizing tissue preprocessing, and the localization of tiny intramuscular sensory organs. The absence of further molecular evidence in human tissues precludes the ability to determine whether intramuscular afferent fibers mediate mechanoreception or nociception from the trapezius. Muscle nociceptors can be sensitized and activated by strong mechanical stimuli such as trauma or mechanical overload. They are also activated by inflammatory mediators and ATP, resulting in the release of neuropeptides, such as substance P and CGRP, which cause blood vessel dilation and local edema30. Nevertheless, the afferent fibers of these cervical nerves, owing to their intramuscular location, are likely to mediate proprioception from the muscle spindle, regardless of any accompanying mechanosensation. Accordingly, herein, the type of MBP+/ATP1A3- is rare in CNXI (6.40% of afferent axons), C3 (4.33%), and C4 (9.88%), suggesting that afferent fibers within the trapezius are involved in proprioception rather than mechanosensation such as nociception.
Clinical implication
We show that the cervical nerve contributes not only to sensory function but also to motor function, which means that iatrogenic lesion of the cervical nerve to the trapezius muscle, e.g. during neck dissection, would result in trapezius muscle dysfunction. In addition, one of the most crucial considerations in the decision to use a donor nerve is the extent to which its composition affects functional recovery following nerve grafting. Donor nerve axons serve as guides for axonal regeneration after Wallerian degeneration. Some studies have indicated that the prevalence of efferent axons or density of sympathetic fibers in donor nerves may influence outcomes after grafting13. Our findings suggest broader applications for the accessory nerve in nerve grafting. For instance, it could be used to restore the suprascapular nerve or to repair the spinal accessory nerve with another donor31,32. Such insights can guide clinicians in selecting optimal donor nerves, potentially leading to better functional outcomes. The factors that influence functional recovery after nerve graft, including somatotopic arrangement, proportion of afferent axons to efferent axons, amount of total axonal area, or compositional purity, remain unclear. The number of axons within a nerve can be considered as a proxy for the number of neuronal components involved in that nerve. Thus, in this study, the predominance of axonal modality was assessed based on the total number of axons, rather than their total area, which is related to the speed of the signal. Furthermore, no somatotopic trend was observed in the arrangement of axons in the perineurium. This indicates that the mixed character of these axons may be influenced by the composition of the ventral and dorsal rami during spinal nerve formation.
Limitations
This anatomical and histological study has some limitations. Morphological differences in peripheral nerves may arise depending on genetic diversity or advanced age, which can lead to variations in axonal regeneration efficiency and redistribution capacity33. Consequently, the relative composition of the motor and sensory nerve axons may fluctuate depending on the age of the cadavers. Additionally, the generalization of absolute axonal values regarding motor and sensory ratios is challenging because of the restricted sample size. Consequently, further clinical validation with a larger sample size is imperative to clarify this issue. While we used several well-established markers, a more nuanced identification could provide a more detailed axonal profile. Especially for a more robust identification of somatic efferent fibers, the TH-/ChAT + axons should be further validated using a nitric oxide synthase (NOS) marker, which is expressed in parasympathetic fibers.
In our study, we used cadavers that had been fixed and stored for 72 h or longer before applying the IHC protocol. Reissig et al. reported a time-dependent reduction in antibody-specific signals, noting that the quality for most antibodies, including NF and ChAT, remained acceptable at 48 h postmortem but significantly deteriorated by 72 h34. Despite this, the NF and ChAT expression signals in our samples remained sufficiently detectable for analysis. Our approach of counting expressed axons, rather than measuring expression signal density, allowed us to effectively use these signals despite potential postmortem changes35.
Conclusion
This study successfully identified the functional components of the nerves supplying the trapezius (CNXI, C3, and C4) using immunostaining. Specifically, all these nerves contain motor fibers and considerable amounts of proprioceptive and sympathetic axons. The notions that CNXI is exclusively a motor nerve and that the upper spinal nerves merely supplement the sensory components for proprioceptive monitoring are questionable. Generally, the functional nature of a nerve is more complex than that previously considered, and its peripheral or central arrangement requires further elucidation. This complexity should be considered when determining the actual functions of these nerves.
Materials and methods
Specimen harvest
All experiments were conducted with the approval of the Yonsei University Medical College Surgical and Anatomy Education Center (Approval number: YSAEC 24 − 004). We utilized nine fixed cadavers (five males and four females; mean age at death, 83.4 years) whose donors had consented to donate their bodies for research purposes. The authors underscore their adherence to all pertinent local and international ethical guidelines and laws pertaining to the use of human cadaver donors in anatomical research. The trapezius nerves of the cadavers were meticulously separated from the surrounding skin and connective tissue. All specimens were harvested from cadavers free of trauma, congenital anomalies, or neurological diseases affecting the trapezius. For immunofluorescence staining, the CNXI, C3, and C4 nerve sections were isolated, fixed in 10% formalin, and embedded in paraffin.
Immunofluorescence labeling
Histological examination was conducted on six cadaveric nerve samples. Double immunofluorescence and immunofluorescence staining techniques were used to enhance the visibility of the nerve axonal elements. Masson’s trichrome staining was used to evaluate the structural characteristics of nerve bundles. The nerve specimens were prepared by cutting them into transverse sections with thicknesses of 5 μm.
To distinguish between sensory and motor axons, antigen retrieval was performed at 95 °C for 30 min. Subsequently, a mixture of anti-NF200 (mouse, diluted 1:400; Sigma-Aldrich, Inc., St. Louis, MO, USA) and anti-ChAT (goat, diluted 1:50; Sigma-Aldrich Inc., St. Louis, MO, USA) was applied evenly to the tissue and incubated overnight at 4 °C. Alexa Fluor 488 donkey anti-mouse IgG (1:500; Thermo Fisher Scientific Inc., Waltham, MA, USA) and Alexa Fluor 555 donkey anti-goat IgG (1:400; Thermo Fisher Scientific Inc., Waltham, MA, USA) were employed as secondary antibodies, and their visualization was conducted for a period of 2 h at room temperature. To identify proprioceptive axons, double immunofluorescence staining was performed with myelin basic protein (MBP) and ATP1A3. The antigen was retrieved at 95 °C for 30 min. Subsequently, a mixture of anti-MBP and anti-ATP1A3 antibodies was applied to the tissue in an even distribution and incubated at 4 °C overnight. Alexa Fluor 594 goat anti-rabbit IgG (1:500; Thermo Fisher Scientific Inc., Waltham, MA, USA) and Alexa Fluor 488 donkey anti-mouse IgG (1:500; Thermo Fisher Scientific Inc., Waltham, MA, USA) were employed as secondary antibodies, and their visualization was conducted for a period of 2 h at room temperature. To distinguish the sympathetic nerves, immunofluorescence staining for tyrosine hydroxylase (TH) was performed. The antigen was retrieved at 95 °C for 30 min. Subsequently, a mixture of anti-TH (rabbit, diluted 1:100; Chemicon®, JP) was applied evenly to the tissue and incubated at 4 °C overnight. Alexa Fluor 488 donkey anti-rabbit IgG (1:500; Thermo Fisher Scientific Inc., Waltham, MA, USA) was used as the secondary antibody, and visualization was conducted for a period of 2 h at room temperature.
Data analysis
Motor nerve bundles exhibited positive staining for both NF200 and ChAT, whereas sensory nerve bundles exhibited positive staining for NF200 only. Proprioceptive axons were positive for both MBP and ATP1A3, and sympathetic nerve bundles were positive for TH. Axonal composition proportions were calculated by measuring the area of each axon relative to the total nerve area, and the results are expressed as percentages. The analyses were conducted using ImageJ version 1.54d, a software program developed by the National Institutes of Health (NIH) in the United States. The statistical significance of the presence of positively stained fibers for each antibody marker was determined using R software (version 4.2.1, MacOS Sonoma 14.4.1). As the Shapiro–Wilk test demonstrated that the data collected did not follow a normal distribution, the Wilcoxon signed-rank test was employed to assess statistically significant differences. Statistical significance was set at p < 0.05.
Toluidine blue staining
The specimens were fixed for 12 h in a solution of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). They were then washed in 0.1 M phosphate buffer. Post-fixation was performed with 1% osmium tetroxide in 0.1 M phosphate buffer for 2 h. Dehydration was performed using an ascending ethanol series (50, 60, 70, 80, 90, 95, 100, and 100%) for 10 min each. Finally, the specimens were infiltrated with propylene oxide for 10 min. The specimens were embedded with a Poly/Bed 812 kit (Polysciences) and polymerized in an electron microscope oven (TD-700, DOSAKA, Japan) at 65 ℃ for 12 h. The block was equipped with a diamond knife in an ultra-microtome (UC7, Leica Microsystems Ltd., Vienna, Austria) and cut into 200-nm semi-thin sections, which were then stained with toluidine blue for observation under an optical microscope.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Change history
29 November 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-78826-0
Abbreviations
- CNXI:
-
Accessory nerve
- NF200:
-
Neurofilament
- ChAT:
-
Choline acetyltransferase
- TH:
-
Tyrosine hydroxylase
- ATP1A3:
-
ATPase Na+/K+-transporting subunit alpha 3
- MBP:
-
Myelin basic protein
- SCM:
-
Sternocleidomastoid
- PMI:
-
Postmortem intervals
References
Hovorka, M. S. & Uray, N. J. Microscopic clusters of sensory neurons in C1 spinal nerve roots and in the C1 level of the spinal accessory nerve in adult humans. Anat. Rec. 296, 1588–1593. https://doi.org/10.1002/ar.22757 (2013).
Watkinson, J. & Gleeson, M. Neck: The accessory nerve. In Gray’s Anatomy: The Anatomical Basis of Clinical Practice. 41st ed. 467–468 (Elsevier Limited P, London, 2016).
Soo, K. C. et al. Innervation of the trapezius muscle by the intra-operative measurement of motor action potentials. Head neck. 15, 216–221. https://doi.org/10.1002/hed.2880150308 (1993).
Benninger, B. The accessory nerve (CN XI) in Nerves and Nerve Injuries (eds. Tubbs, R. S., Rizk, E. B., Shoja, M. M., Loukas, M., Barbaro, N., & Spinner, R. J.). 399–415 (Elsevier, Academic Press, 2015).
Bremner-Smith, A. T., Unwin, A. J. & Williams, W. W. Sensory pathways in the spinal accessory nerve. J. Bone Joint Surg. Br. 81, 226–228. https://doi.org/10.1302/0301-620x.81b2.9027 (1999).
Tubbs, R. S. et al. Histologic confirmation of neuronal cell bodies along the spinal accessory nerve. Br. J. Neurosurg. 28, 746–749. https://doi.org/10.3109/02688697.2014.920485 (2014).
Restrepo, C. E., Tubbs, R. S. & Spinner, R. J. Expanding what is known of the anatomy of the spinal accessory nerve. Clin. Anat. 28, 467–471. https://doi.org/10.1002/ca.22492 (2015).
Overland, J., Hodge, J. C., Breik, O. & Krishnan, S. Surgical anatomy of the spinal accessory nerve: review of the literature and case report of a rare anatomical variant. J. Laryngol Otol. 130, 969–972. https://doi.org/10.1017/S0022215116008148 (2016).
Brînzeu, A. & Sindou, M. Functional anatomy of the accessory nerve studied through intraoperative electrophysiological mapping. J. Neurosurg. 126, 913–921. https://doi.org/10.3171/2015.11 (2017).
Wen, Q. et al. Proprioceptive coupling within motor neurons drives C. Elegans forward locomotion. Neuron. 76, 750–761. https://doi.org/10.1016/j.neuron.2012.08.039 (2012).
Gesslbauer, B. et al. Axonal components of nerves innervating the human arm. Ann. Neurol. 82, 396–408. https://doi.org/10.1002/ana.25018 (2017).
Rodrigues, A. C. Z. et al. The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability. Acta Physiol. (Oxf). 225, e13195. https://doi.org/10.1111/apha.13195 (2019).
Tereshenko, V. et al. Axonal mapping of the motor cranial nerves. Front. Neuroanat. 17, 1198042. https://doi.org/10.3389/fnana.2023.1198042 (2023).
Mioton, L. M., Dumanian, G. A., De la Garza, M. & Ko, J. H. Histologic analysis of sensory and motor axons in branches of the human brachial plexus. Plast. Reconstr. Surg. 144, 1359–1368. https://doi.org/10.1097/PRS.0000000000006278 (2019).
Johal, J. et al. The accessory nerve: a comprehensive review of its anatomy, development, variations, landmarks and clinical considerations. Anat. Rec (Hoboken). 302, 620–629. https://doi.org/10.1002/ar.23823 (2019).
Karuman, P. M. & Soo, K. C. Motor innervation of the trapezius muscle: a histochemical study. Head neck. 18, 254–258. https://doi.org/10.1002/1097-0347 (1996).
Nori, S. et al. Utilization of intraoperative electroneurography to understand the innervation of the trapezius muscle. Muscle Nerve. 20, 279–285. https://doi.org/10.1002/(SICI)1097-4598(199703)20:3%3C;279::AID-MUS3%3E;3.0.CO;2-8 (1997).
Tubbs, R. S. et al. Study of the cervical plexus innervation of the trapezius muscle. J. Neurosurg. Spine. 14, 626–629. https://doi.org/10.3171/2011.1.SPINE10717 (2011).
Gavid, M. et al. Topographical and functional anatomy of trapezius muscle innervation by spinal accessory nerve and C2 to C4 nerves of cervical plexus. Surg. Radiol. Anat. 38, 917–922. https://doi.org/10.1007/s00276-016-1658-1 (2016).
Yuan, Q. et al. Assessment of the rate of spinal motor axon regeneration by choline acetyltransferase immunohistochemistry following sciatic nerve crush injury in mice. J. Neurosurg. 120, 502–508. https://doi.org/10.3171/2013.8.JNS121648 (2014).
Cho, T. H., Won, S. Y. & Yang, H. M. Delineation and histological examination of the intramuscular innervation of the platysma: application to botulinum neurotoxin injection. Clin. Anat. 36, 277–284. https://doi.org/10.1002/ca.23984 (2023).
Burgi, K. et al. Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: simultaneous evaluation in different tissues of hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R264–R271. https://doi.org/10.1152/ajpregu.00687.2009 (2011).
Bornstein, B. et al. Molecular characterization of the intact mouse muscle spindle using a multi-omics approach. Elife. 12, e81843. https://doi.org/10.7554/eLife.81843 (2023).
Cobo, J. L. et al. Searching for proprioceptors in human facial muscles. Neurosci. Lett. 640, 1–5. https://doi.org/10.1016/j.neulet.2017.01.016 (2017).
Bocca, E., Pignataro, O., Oldini, C. & Cappa, C. Functional neck dissection: an evaluation and review of 843 cases. Laryngoscope. 94, 942–945. https://doi.org/10.1288/00005537-198407000-00015 (1984).
Pu, Q., Huang, R. & Brand-Saberi, B. Development of the shoulder girdle musculature. Dev. Dyn. 245, 342–350. https://doi.org/10.1002/dvdy.24378 (2016).
Cho, K. H. et al. Fetal development of the human trapezius and sternocleidomastoid muscles. Anat. Cell. Biol. 53, 405–410. https://doi.org/10.5115/acb.20.202 (2020).
Katayama, K. & Saito, M. Muscle sympathetic nerve activity during exercise. J. Physiol. Sci. 69, 589–598. https://doi.org/10.1007/s12576-019-00669-6 (2019).
Wei, S. et al. Differences in the structure and protein expression of femoral nerve branches in rats. Front. Neuroanat. 14, 16. https://doi.org/10.3389/fnana.2020.00016 (2020).
Mense, S. Muscle pain: mechanisms and clinical significance. Dtsch. Arztebl Int. 105, 214–219. https://doi.org/10.3238/artzebl.2008.0214 (2008).
Mayer, J. et al. Reconstruction of the spinal accessory nerve with selective fascicular nerve transfer of the upper trunk. J. Neurosurg. Spine. 31, 133–138. https://doi.org/10.3171/2018.12.SPINE18498 (2019).
Terzis, J. K. & Barmpitsioti, A. Axillary nerve reconstruction in 176 posttraumatic plexopathy patients. Plast. Reconstr. Surg. 125, 233–247. https://doi.org/10.1097/PRS.0b013e3181c496e4 (2010).
Verdú, E., Ceballos, D., Vilches, J. J. & Navarro, X. Influence of aging on peripheral nerve function and regeneration. J. Peripher Nerv. Syst. 5, 191–208. https://doi.org/10.1046/j.1529-8027.2000.00026.x (2000).
Reissig, L. F. et al. Spinal cord from body donors is suitable for multicolor immunofluorescence. Histochem. Cell. Biol. 159, 23–45. https://doi.org/10.1007/s00418-022-02154-5 (2023).
Tereshenko, V. et al. Axonal mapping of motor and sensory components within the ulnar nerve and its branches. J. Neurosurg. 139, 1396–1404. https://doi.org/10.3171/2023.2.JNS23180 (2023).
Acknowledgements
The authors sincerely thank those who donated their bodies to science for anatomical research. The results of such research support the advancement of medical knowledge, which can improve patient care. Therefore, the donors and their families deserve the highest gratitude. We also wish to thank Jun Ho Kim, Jong Ho Bang, and Tae-Jun Ha (staff members of the Surgical Anatomy Education Centre at Yonsei University College of Medicine) for their technical support.
Funding
This work was supported by the National Research Foundation Korea grant funded by the Korean government (No. RS-2023-00246638) (to H-M.Y).
Author information
Authors and Affiliations
Contributions
MK: Formal analysis, Investigation, Visualization, Software, Writing – original draft. I-S.Y: Data curation, Visualization, Writing – original draft. T-H.C: Visualization, Methodology, Writing – review and editing. J-E.H: Visualization, Methodology.SHK: Conceptualization, Data curation, Supervision, Validation, Writing – review and editing. H-M.Y: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review and editing. All authors read and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics statement
The body donors previously provided the informed consent to participate in the body donation program of the Surgical Anatomy Education Centre at Yonsei University College of Medicine (Approval number: YSAEC 24 − 004). The study was approved by the Institutional Review Board of Yonsei University College of Medicine (Yonsei IRB Approval number: 4-2024-0041) and conducted in accordance with the principles of the Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article Miri Kim and In-Seung Yeo were omitted as equally contributing authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, M., Yeo, IS., Cho, TH. et al. Peripheral cranio-spinal nerve communication for trapezius muscle control using axonal profiling through immunostaining. Sci Rep 14, 25266 (2024). https://doi.org/10.1038/s41598-024-76645-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76645-x