Abstract
Underground coal gasification (UCG) is process of directly recovering energy as combustible gases such as hydrogen and carbon monoxide by combusting unmined coal resources in situ. During UCG process, the temperature in the gasification zone can exceed 1,300 °C, raising concerns about the potential melting of the steel pipe for oxidant injection. To control the temperature in the gasification zone, the use of water injection as an injection agent can be an option. Injecting water during UCG process serves two purposes: it decreases the temperature in the reaction zone by the endothermic effect of water, and it enhances the production of H2 by the reduction reaction of water. However, injecting excessive amounts of water may lead to a significant decrease in the temperature in the reaction zone, consequently cannot maintain the temperature range required for the UCG reaction. This study discusses the effects of water injection on the temperature of the gasification zone and the product gas by developing a chemical reaction model of UCG using COMSOL Multiphysics® software. The model was developed based on the temperature and produced gas results obtained from laboratory-scale artificial coal seam UCG experiments. Our findings reveal that water injection significantly influences the gasification process, controlling temperature in the reaction zone and promoting H2 production through steam gasification and water-gas shift reactions. Moreover, under the experiment and analysis conditions of this study, it is revealed that water injection up to an H2O/O2 molar ratio of 3.9 can effectively control the temperature of the gasification zone with enhancing H2 production.
Similar content being viewed by others
Introduction
Since the Industrial Revolution of the 18th and 19th centuries, modern society has been supported by the consumption of fossil fuels, such as coal, oil, and natural gas. According to the IEA report, as of the end of 2023, the ratio of reserves to production of oil and natural gas is approximately 50 years, while that for coal are approximately 130 years, making coal a more stable energy source than oil and natural gas1. Additionally, the IEA estimates that global coal demand in 2024 reached a record high of 8.74 billion tons (0.4% increase from the previous year)1. It is anticipated that emerging and developing countries, including China, India, and Indonesia, will continue to rely on coal to meet their electricity needs1. However, as coal demand increases, significant amounts of carbon dioxide are emitted from coal-fired power generation, steelmaking, and cement industries, raising concerns about environmental issues, such as global warming and rising sea levels. Therefore, there is a demand for coal resources with minimal environmental impact to achieve a decarbonized society. Furthermore, in recent years, there has been a growing movement towards decarbonization by utilizing hydrogen, which does not emit carbon dioxide during combustion or power generation, to reduce carbon dioxide emissions2.
Underground Coal Gasification (UCG) involves the direct gasification of unmined coal resources in situ to recover energy in the form of combustible gases, such as hydrogen, carbon monoxide, and methane. UCG involves adapting systems typically used for surface coal gasification in an underground environment, allowing the control of gas components recovered from coal through the regulation of injection agents and the temperature of the coal seam. For instance, injecting steam along with oxidants (oxygen-enriched air) into the combustion zone of a coal seam promotes steam gasification reactions and water-gas shift reactions, which can lead to a higher recovery of hydrogen3,4,5. Increasing the temperature of the coal seam can further enhance the coal gasification reactions, resulting in high-energy gases6. Additionally, in the UCG system, carbon dioxide produced alongside hydrogen can be separated, recovered, and stored in the numerous voids that are inevitably formed in the coal seam after gasification, offering the potential for greenhouse gas reduction from coal7,8,9,10,11,12. If there are abandoned mines nearby, CO2 slurry can also be stored in goaf roads and shafts13. Furthermore, since UCG systems do not require traditional underground manual labor, large-scale heading machines, or the construction of large gasification reactors, it is anticipated that the production costs associated with hydrogen could be significantly reduced, thereby addressing a major challenge in widespread hydrogen adoption12,14.
To date, artificial coal seam samples (550 mm × 600 mm × 2,740 mm) have been produced at the Mikasa Unused Coal Energy Research Facility in Hokkaido, Japan, and various UCG experiments have been conducted using a co-axial UCG system with horizontal wells (where gasification agents are injected and produced gas is recovered through a single boring hole). The research findings gained from these experiments include the ability to control the gasification zone by moving the injection pipe, the potential to recover a stable quality of produced gas by relocating the gasification zone to unreacted coal sections, and the ability to control gas components by regulating injection agents15,16,17. Although no accidents such as blowout occurred due to the movement of the injection pipe position in the previous experiments, it is necessary to monitor the gasification process during actual UCG operations. On the other hands, when the temperature in the coal seam during the UCG experiment exceeds approximately 1,300 °C, the high-temperature environment melted the steel injection pipe, affecting the supply of oxidants through the injection pipe to the coal seam reaction zone and the movement of the gasification zone owing to the displacement of the injection pipe. Gür, M. et al. investigated the effect of steam injection on the decrease of coal seam temperature and the increase in hydrogen production using a two-stage gasification method in lab-scale experiment18. It has been demonstrated through CFD simulations that combustible gases such as H2 and CO are generated by heterogeneous gasification reactions, such as boudouard and steam gasification reactions18. Alina Zogała et al. also showed through CFD simulation modeling of UCG that as the steam concentration in the gasification agent increases, the temperature of the UCG process decreases due to steam endothermic reactions19. However, considering the cost and effort required to produce steam, it is presumed that injecting a sufficient amount of water through the injection pipe can control the temperature of the injection pipe tip or combustion zone through the endothermic effect of water vaporization and the temperature rise of steam, potentially preventing the melting of the injection pipe. Furthermore, it has been shown that an increase in steam injection leads to an increase in the composition of hydrogen and the calorific value due to steam gasification reactions and water-gas shift reactions3,20,21. These studies suggest that injecting a sufficient amount of water into the gasification zone in a coal seam during the UCG process can increase the hydrogen component in the produced gas. Additionally, when injecting steam, it is anticipated that if the injection pressure or temperature of the steam is not sufficiently high compared to that of the coal seam, it will be difficult to inject it into the coal seam. It is generally known that gases, such as steam, expand at lower pressures. Because of that, steam requires high pressure and temperature when injected. However, when injecting water as the fluid for injection, this issue is expected to be less significant. Liquid water has low compressibility. Therefore, water can be injected relatively easily without significant changes in pressure or temperature. Nonetheless, to the best of the author’s knowledge, it remains unclear whether water injection during the UCG process can control both the temperature of the gasification zone and increase hydrogen production. Moreover, while it is desirable for water injection during UCG to decrease the gasification zone temperature and increase hydrogen production, excessive water injection may significantly lower the temperature of the gasification zone, and the temperature range required for the gasification reaction of UCG cannot be maintained. Therefore, this study aims to elucidate the effects of water injection on the temperature of the gasification zone and the produced gas by simulating a chemical reaction model of UCG using COMSOL Multiphysics software, based on temperature and produced gas results obtained from laboratory-scale artificial coal seam UCG experiment, and examine the temperature control of the gasification zone and hydrogen generation potential by water injection.
Chemical reactions during UCG process
The UCG process involves invisible phenomena occurring within underground coal seams, and is influenced by multiple factors such as the temperature of the coal seam, the type and composition of the gasification agents, and pressure, making it a highly complex phenomenon21. The chemical reactions occurring during the UCG process can be categorized into drying, pyrolysis, and char reactions, based on different temperature ranges22. First, in the drying zone, wet coal containing moisture is converted into dry coal at temperatures above 100 °C. Next, in the pyrolysis zone, the temperature increase of the coal causes dry coal to decompose23. In the pyrolysis zone, the dry coal loses weight and produces char and volatile substances, as indicated by Eqs. (1) and (2), and volatile gas is generated from porous coal in the temperature range of approximately 350–700 °C3,22,24.
The volatile substances generated by pyrolysis decompose into tar, volatile gas, and moisture22. The volatile gas consists of H2, CH4, C2H6, CO, CO2, NH3, and H2S. The main combustible gases are H2, CO, and CH4. These gas components are the primary constituents of UCG, although there are many other types of gas components present22. Finally, the char is converted into syngas containing combustible gases through oxidation and reduction reactions22. Char reactions involve complex chemical reactions between injected oxidants and carbon within the coal seam. These chemical reactions usually occur after pyrolysis completes22. The char process can be categorized into an oxidation reaction zone and a reduction reaction zone. Table 1 lists the parameters of the main chemical reaction equations of the UCG process. First, as shown in Eqs. (3) and (4), oxidation reactions of the carbon in coal (exothermic reactions) form a high-temperature region within the coal seam, ranging from 1,000 °C to over 1,200 °C. CO2 is produced at high temperatures in the oxidation reaction zone.
Next, reduction reactions occur in the high-temperature coal seam. In the reduction reaction zone, as shown in Eqs. (5)-(8), H2O and CO2, which are formed in the high-temperature oxidation reaction zone, are reduced, producing combustible gases such as H2, CO, and CH4. This occurs in the temperature range of approximately 700-1,000 °C. Reduction reactions are endothermic; they reduce the temperature of the coal seam whereas the oxidation reaction forms high-temperature regions in the coal seam.
Previous studies have shown that with an increase in the amount of steam in the gasification agent, steam decomposition reactions occur under high-temperature conditions (Eq. (5)), leading to an increase in the H2 content of the produced gas3,4,5,20,21,22. It has also been shown that excessive steam injection can absorb a large amount of heat through steam reduction reactions, as indicated in Eq. (5), reducing the high-temperature regions within the coal seam3,21.
Furthermore, when transitioning to relatively low-temperature conditions, the CO and H2O produced from the reduction reaction of CO2 (Eq. (6)) react to form a water-gas shift reaction, as shown in Eq. (7), increasing the H2 content in the produced gas3. Under high-pressure conditions, the hydrogasification reaction (Eq. (8)) is important; however, since artificial coal seams are not expected to be under high pressure as underground coal seams, this study does not consider it22. This study simulates the UCG process occurring in an artificial coal seam by introducing oxidation/reduction reactions (3), (5), (6), and (7), which are considered to be particularly important, based on the studies of Nourozieh et al. and Lanhe Yanga et al.3,22.
Materials and methods
UCG model experiment
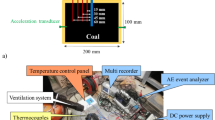
In this study, a laboratory-scale UCG model experiment is conducted using an artificial coal seam sample. Figure 1 shows a conceptual diagram of the UCG model experiment using an artificial coal seam sample. The experimental sample is a mixture of 1.2 tons of pulverized coal with cement and water at a weight ratio of 10:0.5:1. An artificial coal seam sample was prepared by filling the experimental mixture into a steel container with internal dimensions of 625 × 650 × 2,792 mm (H×W×L). Cement was added with the aim of preparing a uniform artificial coal seam throughout the entire coal sample. This was intended to expand the combustion and gasification zone within the artificial coal seam. To determine the optimal mixing ratio of pulverized coal, cement, and water, a preliminary combustion test was conducted. In the combustion test, cylindrical samples with a diameter of 50 mm and a height of 100 mm were prepared using seven different mixing ratios, as shown in Table 2. After the cylindrical samples were dried, their solidification state and combustibility were evaluated by heating the bottom surface. Based on the testing results, it is determined that a mixing ratio of 10:0.5:1 is appropriate from the perspectives of solidification and combustibility.
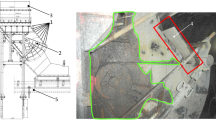
Pulverized coal was collected from the Bibai coal seam of the Sunago Mine, operated by Sunago Co., Ltd., Hokkaido, Japan. The collected pulverized coal had a calorific value of approximately 25 MJ/kg (6,000 kcal/kg) and contained approximately 10% ash. Pulverized coal was mixed with cement and water in the specified ratio and poured into the steel container to create an artificial coal seam sample. Table 3 lists the proximate and ultimate analysis values of the artificial coal seam sample used in the experiment, analyzed by air drying basis. The analyzed sample was obtained after mixing the coal powder with 5% by weight of cement. As a result, the proximate analysis of the artificial coal seam sample showed an ash content of 22% and a calorific value of 24 MJ/kg (5,800 kcal/kg). Figure 2 shows top and side views of the artificial coal seam sample. A co-axial horizontal well was used for ignition, oxidant injection, and product gas collection. The co-axial well, 2,600 mm in length and 76 mm in diameter, was installed 125 mm from the bottom surface of the artificial coal seam sample.
Figure 3 shows the experimental conditions during the course of the experiment. The experiment began by igniting the coal around the bottom of the horizontal co-axial well using a propane gas ignition device in the artificial coal seam. After confirming ignition inside the artificial coal seam, oxygen-enriched air (oxygen concentration: 30–35%) was continuously injected through the injection pipe at an injection pressure similar to atmospheric pressure to sustain the combustion and gasification of the coal sample. Initially, the tip of the injection pipe was placed 100 mm from the bottom of the co-axial well, but in order to extend the gasification zone to the unreacted coal part towards the inlet side of the coaxial well, the tip of the injection pipe was moved approximately 50–100 mm towards the inlet side every 5 h. In order to provide a stable gasification process, the oxygen-enriched air was continuously injected as shown in Fig. 3. At the start of the experiment, the oxidizer injection rate was 35 L/min (oxygen concentration: 33%), subsequently the rate was gradually increased to 120 L/min (oxygen concentration: 33%) by the end of the experiment. In the experiment, water was injected along with the oxidant at a rate of 20–30 mL/min. Water injection aims to prevent the injection pipe from melting because of the high temperature of the coal sample and to increase hydrogen production via the steam gasification and water-gas-shift reactions. However, during the 52–56 h period, water injection was temporarily halted to observe its impact on temperature and gas production. Water was injected by pouring water between the inner and outer pipes of the double-structured injection pipe. Figure 4 shows a conceptual diagram of the water injection device for the horizontal co-axial well. This device consists of a double-structured injection pipe (outer pipe: 3/4-inch stainless steel pipe, inner pipe: 1/2-inch stainless steel pipe), stainless-steel tube for water injection, mini pump, flow meter, and water tank. Additionally, a check valve and relief valve were attached to the stainless steel tube for water injection to accommodate the pressure increase caused by the rapid vaporization of the water inside the injection pipe.
During the experiment, the temperature inside the coal sample was measured using K-type thermocouples (SUS310S; Chino Corp.) and a data logger (GL220; GRAPHTEC Corp.). Figure 5 shows the arrangement of thermocouples. The thermocouples were inserted into holes drilled at specific depths from the top and side of the artificial coal seam sample and fixed with a gypsum solution. T11-T18, T21-T28, and T31-T38, each 550 mm long, were placed directly above the co-axial well, 150 mm above, and 300 mm above, respectively. T41-T44, each 350 mm long, were placed at different heights on the side of the artificial coal seam sample to measure the temperature changes in the portion beyond the co-axial well bottom. The gas produced during the UCG experiment was analyzed every hour using gas chromatography (INFICON Micro GC 3000 A) after removing the moisture and tar components with a purification system. The gases analyzed are oxygen (O2), nitrogen (N2), carbon dioxide (CO2), hydrogen (H2), carbon monoxide (CO), methane (CH4), ethylene (C2H4), ethane (C2H6), propylene (C3H6), and propane (C3H8). Before beginning the experiment, a standard gas containing a fixed amount of each gas was analyzed using gas chromatography for calibration purposes. The duration of the gasification experiment was 91 h. At the end of the experiment, the oxidant injection feed was stopped and carbon dioxide was injected (10 L/min) to extinguish the coal sample.
Numerical simulation
In this study, a chemical reaction model was developed using COMSOL Multiphysics ver6.2 (hereafter referred to as COMSOL) to reproduce the UCG chemical reactions occurring in the artificial coal seam UCG model experiment. The analysis focused solely on temporal changes in the chemical reactions. COMSOL is a general-purpose integrated simulation software program equipped with fully coupled multiphysics and single-physics analysis capabilities. In this study, a reaction engineering module was used to simulate the time-dependent progress of chemical reactions within a reactor, based on the reaction rate constant algorithm. The analysis flow scheme is illustrated in the Fig. 6.
Chemical Species Transport: Reaction Engineering (re) is used in this study. Generally, chemical reactions are governed by the reaction rate equation, as shown in Eq. (9). The reaction rate equation is the relationship between the reaction rate \(\:r\) (left side), concentration of reactants \(\:c\) (right side), and reaction rate constant \(\:k\). For chemical reactions, the reaction rate \(\:r\) is expressed as an exponential function, as follows:
where \(\:{r}_{j}\) is the reaction rate of reaction \(\:j\) (m3/(mol·s)), \(\:{k}_{j}\) is the reaction rate constant of reaction \(\:j\) (1/s), \(\:{c}_{i}\) is the concentration of species \(\:i\) (mol/m3), and \(\:{v}_{ij}\) is the stoichiometric coefficient (-). The rate constant \(\:k\) is temperature-dependent and governed by the Arrhenius equation, as shown in Eq. (10).
where \(\:A\) is the reaction frequency factor for reaction \(\:j\) (1/s), \(\:E\) is the activation energy for reaction \(\:j\) (J/mol), \(\:R\) is the gas constant (8.314 J/(mol·K)), and \(\:T\) is temperature (K). In this study, the UCG reaction process is simulated using (3) Oxidation, (5) Steam gasification, (6) Boudouard, and (7) Water-gas shift reaction, which are considered particularly important in UCG reaction process. The kinetic parameters for these oxidation/reduction reactions are set based on the studies by Lanhe Yanga et al. and Nourozieh et al.3,22. Equation (4) is excluded from the numerical simulation since the simulation assumes the artificial coal seam experiment. Reaction (4) is an exothermic reaction with relatively high heat of reaction (ΔH = 571 kJ/mol). In the artificial coal seam experiment, it is considered that less heat is generated compared to actual coal seams. In future research, when simulating UCG reactions assuming actual coal seams, Eq. (4) should be included. In the simulation conducted in this study, the effect of tar is not considered. In the steam gasification reaction and the water-gas shift reaction, the evaporation heat of water is subtracted from the reaction heat \(\:\varDelta\:H\) to account for water injection.
The chemical reaction model used in this study was constructed using a batch reactor. In the batch reactor model, all reactants are initially placed into the reactor, and the gasification reactions in UCG are simulated by providing the products after a specified time. In the batch reactor model, the reactants within the reactor are assumed to be in an ideal fully mixed state. Therefore, the concentrations and temperatures of the reactants in the batch reactor are homogeneous. Assuming that the gasification reactions occur within the horizontal co-axial well, the reactor volume was input as 1.18 × 10− 2 m3, corresponding to a co-axial well with a diameter of 7.6 × 10− 2 mm and a length of 2.6 m.
Table 4 shows the initial input parameters for the time-dependent numerical analysis. The numerical analysis in this study simulated the UCG process during the 34–40 h period, where the experimental results were relatively stable. The initial temperature was set at 1,180 °C, which is the average temperature during the 34–40 h period of the experiment. In Table 4, C represents the carbon content of coal, O2 and N2 represent oxygen-enriched air (33% oxygen concentration), and H2O represents water. The initial concentrations of O2, N2, and H2O were calculated based on the injection conditions during the 34–40 h period of the experiment. The concentration of C was calculated using the relationship between the oxygen injection flow rate and the amount of reactive carbon obtained experimentally. The concentration of H2O is classified into two types: the H2O concentration representing the water (moisture) contained in the coal, and the H2O concentration injected into the co-axial hole through the double-structured injection pipe. The gasifying agents reacting with the coal are O2 and H2O. N2 does not participate in the UCG chemical reactions. The model considered an experimental water injection rate of 30 mL/min. The time step was varied from 0.1 s to 10 s.
Results and discussion
UCG model experiment
Temperature
Figure 7(a) shows the two-dimensional temperature distribution in the artificial coal seam sample measured using the thermocouples. This figure presents the temperature distribution of the coal cross-section including the co-axial well during the 91-hour experimental period. The ignition point and tip position of the injection pipe are displayed at the bottom of each temperature profile. The temperature profile ranges from 200 °C to 1,400 °C, divided into 100 °C intervals. The temperature profile is classified as follows: 200–350 °C: coal; 350–750 °C: pyrolysis zone; 750–900 °C: second reduction zone; 900 °C-1,000 °C: first reduction zone; and above 1,000 °C: oxidation zone3. In the temperature profiles, a high-temperature region (red color) above 1,000 °C is formed, indicating that UCG gasification reactions are occurred. In the initial stages of the experiment (0–9 h), the UCG reaction region of 350 °C-1,200 °C is quite limited; however, as the experiment progresses, this gasification region expands, occupying most of the artificial coal seam sample in the later stages of the experiment (after 71 h). Furthermore, it can be confirmed that a high-temperature region above 1,000 °C is concentrated near the tip of the injection pipe directly above the co-axial well, and this high-temperature region moves towards the inlet side of the co-axial well (right side of the figure) as the injection pipe moves.
Figure 7(b) shows the temperature variations in the artificial coal seam sample measured by thermocouples T11-T18 during the experiment. The ignition time is set at 0 h, and the temperature variations of each thermocouple are shown from the start of the experiment to the end of the experiment at 91 h. In Fig. 7(b), it can be seen that thermocouple T11, which is closest to the ignition point at the start of the experiment, rises to 800 °C within one hour after ignition and reaches a maximum temperature of 1,300 °C after 5–10 h. Thermocouples T12-T18 subsequently reach a maximum temperature of approximately 1,200 °C as the injection pipe moves and the water injection rate increases. In all thermocouples, there is a tendency for the temperature to rise sharply, reach a peak temperature (approximately 1,200 °C), and then gradually decrease. This suggests that coal gasification area progressed towards the inlet of the co-axial well under stable high-temperature conditions because of the movement of the injection pipe. The decrease in maximum temperature (from 1300 °C to 1200 °C) can be attributed to the promotion of reduction reactions (endothermic), such as the steam gasification reaction and water-gas shift reaction, where the heat generated by coal combustion is utilized for the latent heat of vaporization of water and the endothermic effect of steam, thereby leading to thermal losses. Moreover, the maximum temperature at the end of the experiment (approximately 90 h) is approximately 100 °C lower than that at the early stage of the experiment (10 h). This is considered to be due to the significant reduction in unreacted coal regions at the end of the experiment, resulting in gasification occurring in a limited area16. From these temperature results, it becomes clear that in UCG, by moving the injection pipe position and injecting water, the gasification region could be stably formed and effectively advanced.
Product gas composition and calorific value
Figure 8 shows the composition and calorific value of the major produced gases analyzed by gas chromatography every hour. Table 5 presents the average composition and calorific value of the major gas components produced during the relatively stable experimental period of 34–40 h. The primary gases produced in the UCG process are nitrogen (N2), carbon dioxide (CO2), hydrogen (H2), carbon monoxide (CO), and methane (CH4). Among these gases, the combustible gas components are H2, CO, and CH4. Although N2 does not participate in the UCG chemical reactions, it is included in the oxidant (oxygen-enriched air) and, therefore, flows out without being involved in the UCG process. In Fig. 8, the calorific value serves as an indicator of the energy content of the combustible gases. It is calculated by multiplying its specific calorific value by the composition of each combustible gas obtained from the experiment. As shown in Table 5, the average values of the major gases recovered from 34 h to 40 h are 21.3% H2, 17.6% CO, 20.1% CO2, and 39.9% N2, with H2 and CO being the primary combustible components. Therefore, it is inferred that the combustible gases were mainly generated through steam gasification and water-gas shift reactions3. As shown in Fig. 8, the concentrations of each produced gas fluctuate markedly during the first 4 h of the experiment, indicating the occurrence of coal combustion and gasification. At approximately 9 h, increasing the oxygen concentration of the oxidant results in an increase in the H2 and CO compositions and the calorific value an hour later. This observation confirms, as noted in a previous study20, that the oxygen concentration significantly affects the gas composition and calorific value. Between 17 and 22 h, the composition of H2 and CO and the calorific value decrease, whereas the composition of CO2 increases, suggesting stagnation of gasification under a constant oxidant injection rate and oxygen concentration20. At approximately 22 h, a movement of the injection pipe and an increase in the oxidant injection rate lead to a significant increase in the composition of the combustible gases H2 and CO and the calorific value, while the concentration of CO2 decreases. In addition, the sharp drop in N2 composition at the 22-hour point indicates a significant increase in the volume of the produced gas. These results are attributed to the injection pipe being moved by 100 mm instead of the previous 50 mm intervals and an increase in the oxidant injection rate at the 22-hour point, which expanded the contact area between the oxidant and coal, resulting in more combustible gas production. Water injection was halted between 52 and 56 h in the experiment. This operation caused the H2 content to drop from 19.6 to 16.9%, while the CO content increased from 15.5 to 19.2%. The decrease in H2 composition and increase in CO composition due to the cessation of water injection can be attributed to the suppression of endothermic reactions, such as steam gasification and the water-gas shift reaction. Though the composition of H2 is relatively high during the 52–56 h period, it is observed that the stop of water injection led to changes in the composition of H2 and CO. The little change of the composition of H2 and CO is considered to be due to the movement of the injection pipe. Around 52 h of the experiment, the injection pipe was moved approximately 100 mm towards the inlet side in the artificial coal sample. The consequent contact of the oxidant with the unreacted coal area may have promoted the production of combustible gas. Alternatively, combustible gases may also have been produced by the intrusion of water outside the reaction zone (water contained in the artificial coal seam sample). After resuming water injection after 56 h, H2 increases to approximately 20%. Therefore, water injection during UCG process suggests the potential to enhance H2 production by promoting steam gasification and the water-gas shift reaction3,4,5,20,21,22. Furthermore, after 56 h, the water injection rate was reduced from 30 mL/min to 20 mLmin, leading to a decreasing trend in the H2 composition. Beyond 72 h, a notable decrease in the H2 composition and an increase in the N2 composition are observed. Considering the sharp decline in temperature near the co-axial well above, except for T18, and the decrease in maximum temperature compared to the initial stage of the experiment, it is suggested that the high-temperature region required for H2 production shrank after 72 h. This implies that the reduction in unreacted coal regions led to gasification reactions occurring within a limited high-temperature area, resulting in a decrease in the combustible gas components. CH4, a gas produced from coal pyrolysis occurring at approximately 600 °C, was detected at approximately 2% during the initial stage when the combustion-gasification region had not sufficiently expanded, but it was detected at less than 1% thereafter. Therefore, it is considered that CH4 does not significantly impact the composition and calorific value of the produced gas in this experiment. From the results of the produced gas, therefore, it is revealed that the conditions of oxygen concentration and water injection during the UCG process significantly influence the composition and calorific value of the produced gas.
Table 6 shows the results of the proximate analysis of coal and the product gas composition during the UCG experiment with steam/water injection. In Table 6, it is confirmed that the H2 gas composition produced in this experiment is lower than in previous studies. This can be attributed to the relatively low quality of the coal, such as higher ash content and lower fixed carbon. Moreover, considering that the maximum temperature in the gasification zone during this experiment reached approximately 1,200 °C, it is also possible that the amount of water injected was insufficient relative to the amount of coal sample. Steam gasification reaction that produces H2 gas are promoted in the temperature range of approximately 900-1,000 °C. Given that the endothermic effects of water injection can reduce the temperature in the gasification zone, it is suggested that increasing the amount of water injection could lead to an increase in H2 composition.
Gas production volume and gasification efficiency
In this study, the gas flow rate is determined from the N2 injection rate in the oxidizer and the N2 composition of the product gas analyzed by gas chromatography. Figure 9 shows the variation in the product gas flow rate from the start to the end of the experiment. The results shown in Fig. 9 represent the total flow rate of the product gas containing N2 per unit of time. Within 2.5 h of the start of the experiment, the product gas flow rate reaches approximately 7 m3/h. After 2.5 h, despite some fluctuations, the product gas flow rate tends to increase in response to the stepwise increase in the oxidizer injection rate. Additionally, although not as significant as the oxidizer injection rate, the movement of the injection pipe also leads to an increase in the product gas flow rate. This is attributed to reaction of the oxidant with unreacted coal by moving the injection pipe to the inlet side. At 56 h, when the water injection was resumed, there was a significant increase in the product gas flow rate. This probably indicates that the H2 production increased owing to the resumption of water injection. Furthermore, the product gas flow rate peaked at 10 m3/h at 77 h and then began to decrease. The decrease in the product gas flow rate is attributed to maintaining a constant oxidizer injection rate after 75 h. The results clearly indicate that the injection rates of the oxidizer and water, as well as the movement of the injection pipe, significantly influence gasification, and consequently, the product gas flow rate.
Table 7 shows the volume of product gas and the gasification efficiency in the experiments with steam/water injection. The results of the product gas volume are presented per unit of reacted coal and per unit of reacted carbon. Gasification efficiency is defined as the ratio of the energy recovered in the experiments to the total calorific value of the reacted coal. The product gas volume per unit of reacted coal is largest in the field test conducted by Huang et al., at 3.00 m3/kg. The experiment conducted by Hao et al., which is a laboratory experiment similar in scale to this study, shows a difference of approximately 0.66 m3/kg in the product gas volume per unit of reacted coal. Though several factors may account for this difference, this result can be explained by the fact that the coal used by Hao et al. contained approximately 80% carbon, and the oxidant injection rate was significantly higher than our study. Regarding gasification efficiency, our experiment achieves approximately 60% with water injection. This result is comparable to that obtained with steam injection, suggesting that water injection during UCG can similarly produce combustible gas components such as H2 and CO.
Assumption of reactive carbon by stoichiometry approach
In the experiment, the relationship between the amount of oxygen injected and the amount of reacted carbon was derived by determining the oxygen balance in the experiment and the amount of reacted coal from the stoichiometry approach using Eq. (11) and the results of the composition of the measured product gas26,27. The amount of reacted carbon refers to the quantity of carbon combusted and gasified per unit time, which is calculated using the amount of reacted coal and the ultimate analysis results of the coal.
where, \(\:m\) and \(\:n\) are the H/C and O/C ratios derived from the ultimate analysis of the coal, respectively. \(\:\alpha\:\) and \(\:\beta\:\) represent oxygen balance and water balance, respectively. \(\:\gamma\:\), \(\:\delta\:\), \(\:\epsilon\:\), and \(\:\eta\:\) are the outputs of representative gases from H2, CO, CO2, and CH4, respectively. The oxygen balance \(\:\alpha\:\) can be expressed as shown in Eq. (12), where the compositions of the produced gases H2, CO, CO2, and CH4 (dry basis, excluding N2) are denoted as \(\:p\), \(\:q\), \(\:r\), and \(\:s\), respectively.
As shown in Eq. (13), the sum of \(\:p\), \(\:q\), \(\:r\), and \(\:s\) is 1. Additionally, the amount of reacted coal can be determined from the oxygen injection amount and oxygen balance \(\:\alpha\:\), as shown in Eq. (14):
Here, \(\:C\%\) is the carbon content ratio (61.5%) obtained from the ultimate analysis, and the molar volume of the gas is 22.4 L (0.0224 m3). The product gas compositions \(\:p\), \(\:q\), \(\:r\), and \(\:s\) are derived from the average values of the product gas obtained by gas chromatography. All injected oxygen is assumed to be consumed in the UCG experiment.
Figure 10 shows the relationship between the oxygen injection and reacted carbon obtained from the experimental results. This figure illustrates the amount of reacted carbon calculated according to the amount oxygen injected during the experiment. A positive linear correlation is observed between oxygen injection and reacted carbon, which can be represented by Eq. (15). From this correlated relationship, the amount of reacted carbon per unit time can be estimated based on the amount of oxygen injected per unit time.
Numerical simulation
Model validation
To evaluate the performance of the UCG chemical reaction model developed in this study, the temperature and produced gas results were compared with those of the artificial coal seam experiment. The simulation results of the UCG chemical reaction model are shown in Fig. 11(a) to 11(d). The horizontal axis represents time, while the vertical axis shows gas composition, the temperature of the reaction zone, calorific value, and gas production flow rate, respectively. In Fig. 12(a) to 12(d), the final output values in Fig. 11 are compared with the average experimental values (34–40 h). Overall, the results of the model closely match the experimental results, indicating that the model accurately represents the gasification reactions that occurred in the UCG experiment. The difference in H2 composition between numerical simulation and experiment is approximately 10%. Due to the nature of the simulation model, potential gas leaks and the possibility of H2 gas being trapped within the production pipe during the experiment cannot be considered. Since hydrogen molecules are the smallest and tend to disperse easily, it is possible that some gas leakage occurred during the experiment. The difference between the model and the experimental hydrogen production amounts is approximately 650 L/h, equivalent to 8% of the total gas produced in the experiment. Assuming that approximately 3% of H2 was lost in the production or transport process, the difference between the model and experimental hydrogen production is reduced to approximately 5%28. In future research, it will be necessary to improve the simulated model by incorporating the geological structure of coal into UCG simulations. In addition, COMSOL software is based on thermodynamic equilibrium and does not take into account the kinetic aspects. Given the limited gasification time in the artificial coal seam experiment, the simulation results might differ from the experimental data.
Investigation of reaction temperature control and hydrogen production potential through water injection
The temperature control of the gasification zone and H2 production potential due to water injection are examined by discussing the numerical results when the water injection rate is increased. In this study, since water and oxygen are used as the gasification agents for UCG, the H2O/O2 molar ratio is introduced, as shown in Eq. (16). The H2O/O2 molar ratio is the ratio of H2O injection (mol/min) to O2 injection (mol/min). Table 8 lists the values of the H2O/O2 molar ratio introduced in this study. For example, the initial value of the H2O/O2 molar ratio, 1.6, represents the ratio of water injection to oxygen injection when the water injection rate is 30 mL/min in the experiment. The H2O/O2 molar ratios from 2.3 onward represent the ratio of water injection to oxygen injection when the water injection rate is gradually increased from 30 mL/min to 150 mL/min. At this time, the O2 injection flow rate is fixed at 24 L/min (1.07 mol/min) during the experimental period of 34–40 h. The reason for this is to examine the effect of increasing the water injection rate on the temperature of the gasification zone and hydrogen production. The actual input parameter for the COMSOL software is the H2O concentration, which considers both the H2O injection rate and coal moisture content calculated from the industrial analysis values of the coal.
In this section, we examine the potential for reaction temperature control and hydrogen production in UCG through water injection by analyzing the results obtained by varying the H2O/O2 molar ratio. The analyzed parameters include the temperature, syngas composition, calorific value, syngas flow rate, and H2O consumption.
Figure 13(a) shows the temperature variation with increasing H2O/O2 molar ratios. In this figure, the horizontal axis represents the H2O/O2 molar ratio and the vertical axis represents the temperature of the reaction zone. As the H2O injection rate increases, the temperature of the reaction zone decreases, reaching 860 °C at an H2O/O2 molar ratio of 7.8. This can be attributed to the heat generated from coal combustion being absorbed by the heat loss from endothermic reactions, such as the steam gasification reaction and the water-gas shift reaction, including the latent heat of vaporization when water changes to steam and the heat absorption effect due to the rising temperature of the steam. It has been demonstrated that as the amount of steam injected increases, the heat generated by the coal combustion is absorbed by the endothermic reaction of steam, resulting in a decrease in the temperature of the coal seam3,19,21. In this study, both experimental and analytical results suggest that the injection of water instead of steam into the UCG process could potentially be effective in controlling the temperature of the reaction zone. The optimal temperature range for the oxidation process is a high-temperature zone between 1,000 and 1,200 °C3,29. Based on the experimental temperature results (Fig. 7(b)) and the simulated results (Fig. 13(a)), it has been shown that water injection decreases the temperature in the coal gasification zone due to the latent heat of vaporization of water and the endothermic effect of steam. As a result, the steam gasification reaction increases the composition of H2 and CO within 900-1,000 °C temperature range3. Since the reduction reactions by water injection slow down at temperature range approximately 800 °C, it is necessary to maintain the temperature range of the gasification zone above 900 °C29. At the H2O/O2 molar ratio of 3.9 selected for this study, the gasification temperature is approximately 960 °C. Considering other factors affecting the produced gas included below, the results of this study suggest that at an H2O/O2 molar ratio of 3.9, temperature control can be effectively achieved. In future experiments, it will be necessary to verify whether increasing the H2O/O2 molar ratio will result in similar temperature ranges as show in Fig. 13(a). In addition, this result indicates that excessive water injection cannot maintain the temperature range necessary for combustible gas production because of the decrease in the temperature of the reaction zone. Therefore, it is clear that excessive water injection during the UCG process may prevent the gasification reaction from progressing because heat loss may prevent the coal seam sample from maintaining the temperature range necessary for the production of combustible gas.
Figure 13(b) shows the composition of syngas with increasing H2O/O2 molar ratios. In this figure, the horizontal axis represents the H2O/O2 molar ratio and the vertical axis represents the syngas composition. The syngas obtained from the analysis comprises four components: H2, CO, CO2, and N2. As shown in Fig. 13(b), the composition of H2 in the syngas increases with the water injection. As the H2O injection rate increases, the H2 composition gradually increases, reaching 36.0% at an H2O/O2 molar ratio of 3.9 and becoming the most predominant gas. The CO composition in the syngas decreases as the H2O injection rate increases. The CO2 composition in the syngas remains almost unchanged after gradually increasing the H2O injection rate. The N2 composition in the syngas decreases because of the water injection. As the H2O injection rate increases, the N2 composition gradually decreases, remaining almost constant at 35.2% from H2O/O2 molar ratio of 3.9 onwards. Previous studies have shown that an increase in the H2O (steam) supply results in an increase in the H2 composition and a decrease in CO concentration3,4,5,17,18,19,20. In this study, it was also confirmed that an increase in the H2O (water) injection rate resulted in an increase in the H2 composition and a decrease in the CO composition. This is considered to be due to the promotion of endothermic reactions such as the steam gasification reaction and water-gas shift reaction, resulting from increased H2O injection. Specifically, at high temperatures (above 900 °C), the steam gasification reaction (Eq. (5)) occurs, increasing both the H2 and CO compositions. As the H2O injection rate increases, the temperature of the reaction zone decreases (as indicated by the temperature results), causing the gasification zone to shift to a lower-temperature regime. Consequently, the water-gas shift reaction (Eq. (7)) leads to a decrease in the CO composition and a stagnation in the H2 composition. Therefore, under the experimental and simulated conditions of this study, it was revealed that water injection during UCG process can promote reduction reactions such as the steam gasification reaction and water-gas shift reaction, potentially affecting the H2 composition in the syngas.
Figure 13(c) shows the results of the calorific value of syngas with increasing H2O/O2 molar ratios. In this figure, the horizontal axis represents the H2O/O2 molar ratio and the vertical axis represents the calorific value. As the H2O injection rate increases, the calorific value gradually decreases, converging to approximately 5.0 MJ/m3 beyond an H2O/O2 molar ratio of 3.9. Since the calorific value is calculated using the composition of combustible gases, it is found that beyond an H2O/O2 molar ratio of 2.3 (influence of increased water injection), the decrease in CO concentration has an impact on the calorific value. The decrease in the calorific value with increasing H2O/O2 molar ratio is attributed to the reduction in reaction temperature due to the increased H2O injection rate and stagnation of H2 composition.
Figure 13(d) shows the results of the syngas production rate when increasing the H2O/O2 molar ratio. In this figure, the horizontal axis represents the H2O/O2 molar ratio and the vertical axis represents the syngas production rate. The actual total syngas production rate is three times the value on the vertical axis. Since N2 does not react with other chemical species during the UCG process, the syngas production rate can be determined from the N2 composition and the N2 injection flow rate. As the H2O injection rate increases, the H2 production rate gradually increases, making H2 the predominant gas at an H2O/O2 molar ratio of 3.9. Beyond an H2O/O2 molar ratio of 3.9, the H2 production rate keeps stable with increasing H2O injection rate, while the CO production rate continues to decrease. The total syngas production rate increases up to 8.1 m3/h at a H2O/O2 molar ratio of 3.9 due to increased H2 and CO2 production. Beyond an H2O/O2 molar ratio of 3.9, the total syngas production rate gradually decreased owing to the reduction in CO production. Therefore, under the experimental and simulated conditions of this study, it was revealed that increasing the H2O injection rate up to a H2O/O2 molar ratio of 3.9 has the potential to increase H2 production.
Figure 13(e) shows the results of H2O consumption with increasing H2O/O2 molar ratios. In this figure, the horizontal axis represents the H2O/O2 molar ratio and the vertical axis represents H2O consumption. The H2O consumption is the amount of water consumed in a series of UCG processes. The H2O consumption increased from 23.1 mol/m3 (H2O/O2 molar ratio = 1.6) to 31.0 mol/m3 (H2O/O2 molar ratio = 3.9) due to the increased water injection. This is attributed to the enhanced production of H2 through the promotion of reduction reactions, such as the steam gasification reaction (Eq. (5)) and the water-gas shift reaction (Eq. (7)). When the H2O/O2 molar ratio exceeds 3.9, the consumption of H2O stagnates. This suggests that, even with excessive H2O injection, the water remains unreacted with other reactants. This is most likely due to the shift of the gasification zone to relatively lower temperature regions with increased H2O injection, promoting the water-gas shift reaction (Eq. (7)). Therefore, under the experimental and simulated conditions of this study, it was revealed that increasing the H2O injection rate up to a H2O/O2 molar ratio of 3.9 has the potential to increase H2 production through the consumption of H2O in the gasification agency.
In this study, it is demonstrated that water injection during the UCG process is effective in controlling the reaction temperature and increasing H2 production up to an H2O/O2 molar ratio of 3.9. The results of our study contribute to the development of a strategy for the practical application of UCG, enabling the formation of a stable underground gasification zone and the recovery of high-efficiency H2 gas through appropriate water injection.
Conclusion
This study aims to elucidate the effects of water injection on the temperature of the gasification zone and the produced gas by simulating a chemical reaction model of UCG using COMSOL Multiphysics software, based on temperature and produced gas results obtained from laboratory-scale artificial coal seam UCG experiments, and examine the temperature control of the gasification zone and hydrogen generation potential by water injection. The obtained knowledge is as follows.
-
UCG model experiment demonstrated that the reaction temperature is approximately 1,200 °C under the injection of oxygen-enriched air (oxygen concentration: 30–35%) with water injection (20–30 mL/min). Injecting water together with an oxidant is effective to control the reaction temperature due to absorbed by the heat loss from endothermic reactions and the latent heat of vaporization.
-
In this study, approximately 60% gasification efficiency is achieved by water injection during the UCG model experiment. This result is comparable to the results observed with steam injection in previous studies. Therefore, it is clear that water injection in UCG process has the potential to produce combustible gases such as H2 and CO to the same extent as steam injection.
-
H2 and CO2 production increases with water injection while CO production decreases, meaning that H2 production is promoted with reduction reactions such as the steam gasification and water-gas shift reactions.
-
Under the experimental and simulated conditions of this study, it is revealed that water injection up to a H2O/O2 molar ratio of 3.9 can effectively control the temperature of the gasification zone and increase H2 production.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
International Energy Agency. 「Coal Mid-Year Update - July 2024」. https://www.iea.org/reports/coal-mid-year-update-july-2024 July (2024). (2024-09-08).
Stańczyk, K. et al. Dynamic experimental simulation of hydrogen oriented underground gasification of lignite. Fuel. 89 (11), 3307–3314 (2010).
Yang, L., Zhang, X., Liu, S., Yu, L. & Zhang, W. Field test of large-scale hydrogen manufacturing from underground coal gasification (UCG). Int. J. Hydrog. Energy. 33 (4), 1275–1285 (2008).
Liu, S. Q., Wang, Y. Y., Ke, Z. H. A. O., Ning, Y. A. & N. G Enhanced-hydrogen gas production through underground gasification of lignite. Min. Sci. Technol. (China). 19 (3), 389–394 (2009).
Seida, Y., Tsuchiya, N., Enomoto, H. & Chida, T. [Fundamental studies of underground coal gasification for hydrogen production] Sekitanchikagasuka Ni Yoru Suisoseizou Ni kansuru kisokennkyu (in Japanese). Shigen to sozai. (Journal Min. Mater. Process. Inst. Japan). 106 (12), 731–736 (1990).
Hamanaka, A. et al. Effect of injection flow rate on product gas quality in underground coal gasification (UCG) based on laboratory scale experiment: development of co-axial UCG system. Energies. 10 (2), 238 (2017).
Burchart-Korol, D., Krawczyk, P., Czaplicka-Kolarz, K. & Smoliński, A. Eco-efficiency of underground coal gasification (UCG) for electricity production. Fuel. 173, 239–246 (2016).
Yang, D., Koukouzas, N., Green, M. & Sheng, Y. Recent development on underground coal gasification and subsequent CO2 storage. J. Energy Inst. 89 (4), 469–484 (2016).
Khadse, A., Qayyumi, M., Mahajani, S. & Aghalayam, P. Underground coal gasification: a new clean coal utilization technique for India. Energy. 32 (11), 2061–2071 (2007).
Verma, A., Olateju, B., Kumar, A. & Gupta, R. Development of a process simulation model for energy analysis of hydrogen production from underground coal gasification (UCG). Int. J. Hydrog. Energy. 40 (34), 10705–10719 (2015).
Takyi, S. A. et al. Current status and technology development in implementing low carbon emission energy on underground coal gasification (UCG). Front. Energy Res. 10, 1051417 (2023).
Liu, H., Guo, W. & Liu, S. Comparative techno-economic performance analysis of underground coal gasification and surface coal gasification based coal-to-hydrogen process. Energy. 258, 125001 (2022).
Li, W., Ren, T. W., Su, E. L. & Cheng, Y. Is the long-term sequestration of CO2 in and around deep, abandoned coal mines feasible? Proc. Institution Mech. Eng. Part. A: J. Power Energy. 232 (1), 27–38 (2018).
Verma, A., Olateju, B. & Kumar, A. Greenhouse gas abatement costs of hydrogen production from underground coal gasification. Energy. 85, 556–568 (2015).
Su, F. Q. et al. Evaluation of a compact coaxial underground coal gasification system inside an artificial coal seam. Energies. 11 (4), 898 (2018).
Hamanaka, A. et al. Large-scale laboratory experiment on development of underground coal gasification with horizontal well. In ISRM Young Scholars Symposium on Rock Mechanics (pp. ISRM-YSRM). ISRM. (2019), December.
Su, F. Q. et al. Monitoring and evaluation of simulated underground coal gasification in an ex-situ experimental artificial coal seam system. Appl. Energy. 223, 82–92 (2018).
Gür, M. & Canbaz, E. D. Analysis of syngas production and reaction zones in hydrogen oriented underground coal gasification. Fuel. 269, 117331 (2020).
Żogała, A. & Janoszek, T. CFD simulations of influence of steam in gasification agent on parameters of UCG process. J. Sustainable Min. 14 (1), 2–11 (2015).
Huang, W. G., Wang, Z. T., Duan, T. H. & Xin, L. Effect of oxygen and steam on gasification and power generation in industrial tests of underground coal gasification. Fuel. 289, 119855 (2021).
Zagorščak, R. et al. Underground coal gasification–A numerical approach to study the formation of syngas and its reactive transport in the surrounding strata. Fuel. 253, 349–360 (2019).
Nourozieh, H., Kariznovi, M., Chen, Z. & Abedi, J. Simulation study of underground coal gasification in Alberta reservoirs: geological structure and process modeling. Energy Fuels. 24 (6), 3540–3550 (2010).
Lyczkowski, R. W. & Lyczkowski, R. W. IIT Code Begins Using Los Alamos’ K-FIX Code. The History of Multiphase Science and Computational Fluid Dynamics: A Personal Memoir, 161–174. (2018).
Anthony, D. B. & Howard, J. B. Coal devolatilization and hydrogastification. AIChE J. 22 (4), 625–656 (1976).
Chen, H., Qin, Y., Chen, Y., Dong, Z., Xue, J., Chen, S., … Zhao, Y. (2023). Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent. Energies,16(19), 6993.
Kaiho, M., Yamada, O. & Yasuda, H. [1-3-4 Estimation of chemical reaction process from composition of gas produced] 1-3-4 Seiseigasu sosei kara gasuka no kagakukatei wo suitei suru houhou (in Japanese) (1–3 Gasification 2, Session1 Coal, Heavy Oil, etc., Research Presentations). In Proceedings of the 20th Annual Meeting of the Japan Institute of Energy (pp. 18–19). The Japan Institute of Energy. (2011), August.
Su, F. Q. et al. Energy recovery evaluation and temperature field research of underground coal gasification under different oxygen concentrations. Fuel. 329, 125389 (2022).
Fan, Z. H. I. Y. U. A. N., Sheerazi, H. A. D. I. A., Bhardwaj, A., Corbeau, A. S.,Longobardi, K. A. T. H. R. Y. N., Castañeda, A. D. A. L. B. E. R. T. O., … Woodall,D. C. M. (2022). Hydrogen Leakage: a potential risk for the hydrogen economy. Columbia Center on Global Energy Policy: New York, NY, USA.
Stańczyk, K. et al. Experimental simulation of hard coal underground gasification for hydrogen production. Fuel. 91 (1), 40–50 (2012).
Acknowledgements
This work was supported by Mikasa City, Kyushu University Platform of Inter-/Transdisciplinary Energy Research (Q-PIT) through its “Module-Research Program”, The Iwatani Naoji Foundation, The Mining and Materials Processing Institute of Japan, JSPS KAKENHI Grant Number JP22K05005. The authors gratefully acknowledge their support and express particular appreciation to UCG project members.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.I., A.H. and K.I.; methodology, R.I. and G.D.; software, R.I., and A.H.; validation, T.S.; formal analysis, H.S.; investigation, J.K. and G.D.; resources, J.K.; data curation, G.D.; writing—original draft preparation, R.I. and A.H.; writing—review and editing, H.S.; visualization, R.I.; supervision, K.I. and J.K.; project administration, K.I. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Iriguchi, R., Hamanaka, A., Su, Fq. et al. Effects of water injection on reaction temperature and hydrogen production in horizontal co-axial underground coal gasification. Sci Rep 14, 25551 (2024). https://doi.org/10.1038/s41598-024-76966-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76966-x