Abstract
Uremic Encephalopathy results from the elevation of toxins and blood-brain barrier (BBB) disruption. Renal Ischemia/Reperfusion (I/R) injury is the principal cause of acute kidney injury and brain tissue injury. The present study was crafted to estimate the restorative impact of platelet-rich plasma (PRP) and exosome injection before the reperfusion phase on the kidney following renal I/R injury and its influence on brain tissue by tracking the histopathological, biochemical, and Doppler ultrasonography alternations in both kidney and brain tissue. Forty mature male rats were divided into five groups as follows: control, I/R, PRP, exosome, and Exosome + PRP. Renal Doppler ultrasonography was traced for all rats. Serum kidney functions and acetylcholine esterase enzyme (AchE) were evaluated. Both Gamma-aminobutyric acid (GABA) and glutamate were assessed in brain tissues. The oxidative stress (malondialdehyde), anti-oxidative (glutathione and catalase), and pro-inflammatory (Tumor necrosis factor- α and interleukin-6) markers were estimated in renal tissues. Additionally, morphometric histological examination was performed in both renal and brain tissues. Both PRP and exosome-received rats exhibited a significant improvement in both serum kidney functions and AchE compared to I/R rats. There was a 3.39-fold increase in GABA and a 2.27-fold decrease in glutamate levels in the brain tissue of PRP rats compared to the I/R rats. A significant elevation (P ≤ 0.0001) of glutathione and catalase besides a significant reduction in the expression of TNF-α and IL-6 was observed in renal tissue compared to I/R rats. A significant severe reduction (P < 0.0001) in the number of Purkinje cells, pyramidal cells in the cerebellar cortex, and the CA1 region in the hippocampus was observed in I/R rats which was significantly alleviated by both PRP and exosome. Furthermore, there was a significant improvement in Doppler parameters. PRP exerted a significant superior impact on the restoration of kidney functions and repairing uremic-induced damage in brain tissue.
Similar content being viewed by others
Introduction
Renal I/R injury is a major risk factor that leads to acute kidney injury (AKI), which in turn is the eminent cause of rising rates of morbidity and mortality during major surgeries such as cardiac surgery, partial nephrectomy, kidney transplantation, vascular surgery, hemorrhagic shock, and other urological problems1. Renal I/R injury is defined as two conjugated complex stages: ischemia is a sudden cessation of blood flow, oxygen, and nutrients to kidney tissues, which stimulates the bioaccumulation of toxic nitrogenous wastes such as creatinine, urea, sodium, calcium, and a huge building up of reactive oxygen species (ROS) that is followed by parenchyma cell damage and likewise endothelial and tubular epithelial cells2. The renal ischemia phase is followed by the reperfusion phase, which is characterized by a huge influx of blood, re-oxygenation, and increasing PH that adversely causes severe tissue damage and leads to inflammation, oxidative stress, impairment of energy metabolism, apoptosis, and tubular necrosis3.
The remote effect of renal I/R injury extends to other distant organs such as the pancreas, lungs, liver, and brain. Uremic encephalopathy results from uremia toxins elevation, an increase in serum sodium concentration that leads to a rising level of osmolality in brain tissue, activation of ROS expression, and suppression of the defense of antioxidant enzymes. All these successive events eventually result in BBB disruption, brain receptors, and neurotransmitter disarray4,5.
Platelet-rich plasma (PRP) is a pivotal autologous product extracted by centrifugation of a fresh blood sample to separate highly concentrated platelet-rich supernatant by an efficient, simple, and natural method6. Owing to the unique and double features of a huge number of effective growth factors included in PRP such as hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), and epidermal growth factor (EGF) that carry multifactorial features in tissue regeneration, repair of renal tubules, proliferation of endothelial cells, curbing tubular necrosis, and renal healing after ischemia. Furthermore, neuroprotective effects of growth factors released from PRP express their impact by their ability to cross the blood-brain barrier7, for example, vascular endothelial growth factor (VEGF) that reported its role in blood-brain barrier permeability8, brain-derived neurotrophic factor (BDNF)9, nerve growth factor (NGF) via receptor-mediated transport10, and IGF-111; So using PRP as a treatment tool in uremic encephalopathy is an important event.
Exosomes are a unique subtype of extracellular vesicles (ECVs), defined as heterogenous biovesicles with about 30–150 nanometers in diameter12. Exosomes are found in various biological fluids, such as urine, saliva, and breast milk. Almost all normal cell types can produce exosomes such as umbilical vein endothelial cells, T cells, Nature killer cells, and mesenchymal stem cells (MSC). Owing to its special cargo profile that is equipped with cellular signaling molecules such as lipids, proteins, and nucleic acid (mi RNA) and carries an anti-inflammatory, anti-apoptotic, and pro-angiogenic feature13, using MSC-derived exosomes is considered an attractive biological instrument in renal injury and neurodegenerative diseases compared to treatment with mesenchymal stem cells alone14. Consequently, this study aims to assess the potential ameliorative effect of PRP and exosome injection before the reperfusion phase on the kidney following renal ischemia or reperfusion injury and, subsequently, the distant influence on brain tissue.
Material and method
Ethical statement and sample size
All applications, treatments, and experimental procedures in the study were approved and adopted by the ethics of the Institutional Animal Care and Use Committee, Zagazig University, Zagazig, Egypt, with registration number ZU-IACUC/1/F/126/2023, which met the ARRIVE guidelines.
The achieved power of our study was calculated using G* Power (Germany) software version 3.1.9.4 using six experimental groups with a total sample size of 56 by post hoc power analysis depending on significant standards of numerator df = 5, denominator df = 50, α err prob = 0.05, and a critical F-value = 2.891 using ANOVA: one-way (one independent variable). This study’s effect size (f) was calculated from the results of all measurable tested variables, depending on the partial eta squared of each variance, and it ranged from 1.37 to 9.07. Results showed that the achieved power (1-β err prob) of this study equals 100%. Thus, the sample size of N = 56 is adequate to detect the hypothesis of the tested variables.
Experimental design
A total of 56 mature male Sprague-Dawley rats, weighted (mean ± SD) 211.36 ± 5.32 g, were used in this controlled randomized experimental study. They were purchased and housed under standard conditions of a 12-h light/12-h dark cycle at 22 ± 2 °C and relative humidity of 65–70% at the Medical Experimental Research Centre (MERC), Faculty of Medicine, Mansoura University, Mansoura, Egypt. Animals were fed on a standard laboratory diet (from the department of nutrition, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt) with free access to water and food. A period of 7 days was allowed for rats to acclimatize to the new housing conditions. Donor rats (n = 8) were used for PRP preparation. Experimental rats (n = 48) were randomized into normal control rats (n = 8), sham rats (n = 8) that were subjected to laparotomy along with right nephrectomy without left renal clamping, and the whole rats exposed to I/R (n = 32) where left renal I/R injury along with right nephrectomy were performed, and later on, they were subdivided according to the received treatment into 4 groups (n = 8 each); I/R: received no treatment, PRP: received PRP before renal reperfusion, Ex.: rats received exosome before renal reperfusion, and Ex.+PRP: received PRP and exosome mixture before renal reperfusion Fig. 1.
Preparation of exosome
Exosome® is a sterile, standardized concentration of lyophilized mesenchymal stem cell-derived exosome that were purchased from the Stem Vie® Company, Al Haram, Giza, Egypt. The exosome was diluted using the excipient Garamycin vial (2 ml), and thus, each 1 ml contains lyophilized concentrates of exosomes obtained from 3 × 106 mesenchymal stem cells and a protein concentration of BCA 500 µg/ml (+/- 10%).
Platelet-Rich plasma (PRP) Preparation
Eight mature male Sprague-Dawley rats were used to prepare PRP, and platelets were counted according to the technique15,16,17. Briefly, fresh blood samples were collected under all aseptic conditions and anesthesia. To prevent clotting, samples were drawn into tubes containing 4% sodium citrate. The samples were then centrifuged using a laboratory centrifuge at 160 Xg for 20 min at an environmental temperature (22℃) to separate blood cellular elements, resulting in two layers: a lower red cell fraction and an upper straw-yellow turbid plasma fraction. The straw-yellow turbid supernatant layer was transferred to a clean tube and centrifuged once more at 400 Xg for 15 min, resulting in two components, two-thirds of the supernatant, platelet-poor plasma (PPP), that was discarded and the PRP retained. The number of platelets in PRP was counted to be 987,000 platelets/µl using CBC analyzer Sysmex XP300/Germany.
Surgical procedures
All procedures were performed by the same trained and skilled surgeons (A.S. and E.A.) simultaneously at 10 AM to avoid the influence of both surgery variables and circadian rhythm on the results18,19.
The surgical plane of general anesthesia was achieved via intraperitoneal injection of a mixture of 5 mg kg-1 Xylazine Hcl (20 mg/ml, XYLAJECT, ADWIA CO, Egypt) and 75 mg kg-1 Ketamine Hcl (50 mg/ml, KETAMINE, SIGMA TECH CO, Egypt)20. To induce left renal ischemia Figs. 2 and 3, the left renal pedicle was approached, isolated, clamped using a non-traumatic micro-vascular clamp, and reinserted in its position in the retroperitoneal cavity1. The abdominal skin incision was temporarily closed using small towel clamps to maintain ischemia for 45 min.
The surgical procedures used for the induction of renal ischemia/reperfusion injury. After laparotomy and approaching the left kidney (a), the renal pedicle (yellow arrow) was isolated (b) and clamped using a non-traumatic vascular bulldog (green arrow, c). Renal ischemia was maintained for 45 min by reinsertion of the clamped pedicle into its original situation (d) and covering the incisional line (e). Notice the careful subcapsular injection (blue arrow) before reperfusion in the congested kidney (f). A right nephrectomy was performed via double ligation of its pedicle and cutting in between (g and h). (blue K: left kidney, yellow RK: right kidney).
The left renal subcapsular injection with PRP and/or exosome was applied according to the treatment groups; PRP group (0.3 ml of PRP), Ex. group (0.3 ml of exosome), and Ex.+PRP group (0.15 ml PRP + 0.15 ml exosome) at 15 min before renal reperfusion. Through the same incision line, a right nephrectomy was performed 5 min before left renal reperfusion to prevent the right kidney from contributing to the results. Left renal reperfusion was achieved by careful de-clamping of the renal pedicle. Finally, the laparotomy incision was closed routinely by continuous patterns using 3/0 polyglycolide sutures (MAXON, COVIDIEN CO., USA), and the skin incision was closed with a 4/0 prolene suture Fig. 3.
Postoperative care
After the operation, rats were kept in an incubator at 37 °C for the next 6 h and then transferred into clean cages (2 rats per cage), with full access to food and water. Rats received an intramuscular injection of both the amoxicillin antibiotic (Flumox, EIPICO, Egypt) in a dose of 50 mg/kg and the anti-inflammatory meloxicam (Anti-cox II, 15 mg/3 ml, ADWIA, Egypt) in a dose of 5 mg/kg, two hours before the procedures and once a day afterward for the next three days. The wounds were dressed daily with povidone-iodine.
Doppler ultrasonography imaging
Half an hour before euthanasia, each rat was prepared for ultrasound inspection by induction of general anesthesia using ketamine Hcl 75 mg kg-1 ip, shaving the entire abdominal area, and applying coupling gel. Non-invasive color Doppler ultrasound (Esaote MyLab 30X Vision, Esaote, Genova, Italy) was performed in all rats by the same person (A.S.) with a multi-frequency probe (6–12) with a filter of 100 Hz, pulse repetition frequency of 4,500 Hz, power of 50%, and a Doppler angle ranging from 0 to 40.
Rats were examined and assessed transabdominal as previously mentioned21. Doppler signals of the left renal artery (before its division) were identified laterally close to the aorta and almost horizontally to the left kidney, posteriorly to the left renal vein, and at just the origin of the mesenteric cranial artery. The duration of each examination was about 5–10 min. The Doppler parameters: Resistance index (RI), Pulsatility index (PI), Peak systolic velocity (PSV), Time-average mean velocity (TAMV) and variables were determined and traced automatically. Two Doppler mages were traced and recoded from each rat.
Collection of biological samples
After 3 days of reperfusion, rats were allowed to be placed in metabolic cages, and aseptically 24-hour urine samples were collected to evaluate the level of urinary parameters. Then all rats were sacrificed by a single overdose of thiopental sodium injected intra-peritoneally at 7.5 mg/100 g BW22. Using a sterile syringe, the whole blood of rats was obtained via cardiac puncture in a plain tube without anti-coagulant to allow the tubes to clot and centrifuged at 3000 rpm/min for 5 min. The upper serum was collected carefully and stored at -80 °C for further biochemical analysis. The left renal tissue was harvested and rinsed with cold saline to remove any clots or any red blood cells, then was cut into halves; one of the pieces was fixed in the buffered 10% formalin solution for histological procedures, and the other was stored at -80 °C for biochemical analysis. Briefly, renal tissues were prepared by washing with 1× PBS to remove excess blood, homogenized in 20 mL of 1× PBS, and then stored overnight at ≤ -20℃. After two freeze-thaw cycles, the homogenate was centrifuged at 5000×g for 5 min.
Brain tissue was removed, washed with cold saline, and then was cut into halves identically. The first portion was fixed in the buffered 10% formalin solution for histological examination, and the second one was frozen in liquid nitrogen and stored at -80 °C for further use. The tissue samples were homogenized after adding PBS (pH 7.4). Using centrifugation for 20 min at 2,000–3,000 rpm, the supernatant was collected carefully for the ELISA assay.
Assessment of kidney function indicators
Serum levels of creatinine, uric acid (U.A.), and blood urea nitrogen (BUN) were analyzed using commercial kits (SPINREACT, Girona, Spain) by spectrophotometry and colorimetric assay at wavelengths of 492 nm, 340 nm, and 520 nm, respectively, according to the manufacturer’s protocol.
Assessment of Acetylcholine esterase enzyme level (AchE)
AchE level was measured in serum using a rat-specific enzyme-linked immunosorbent assay, and the optical density was determined using a microplate reader at 450 nm (CUSABIO, Houston, U.S.).
Determination of lipid peroxidation in renal tissue
The malondialdehyde level was measured using a commercial ELISA kit (MyBioSource, Vancouver, Canada) spectrophotometrically at wavelength 450 nm.
Measurement of antioxidant biomarkers in renal tissue
Antioxidant parameters were measured using a commercial Rat Glutathione ELISA kit (CUSABIO, Houston, U.S.) and a commercial Rat Catalase ELISA kit (MyBioSource, Vancouver, Canada) spectrophotometrically at wavelength 450 nm.
PCR analysis of TNF-α and IL-6 gene expression in renal tissue
The expression of IL-6 and TNF-α was measured using a real-time polymerase chain reaction (RT-PCR). The SYBR® Green PCR Master Mix (Applied Biosystems, USA) was prepared according to the manufacturer’s instructions. Total RNA was isolated using a commercial kit (Promega, USA), Then mRNA was reverse transcribed into single-stranded cDNA using a cDNA synthesis kit (Meridian Bioscience, USA). Finally, the expression level of the relative mRNA was evaluated by calculating the values of \(\Lambda\)cycle threshold (\(\Lambda\)Ct) by normalizing the average values of Ct to the control housekeeping gene (GAPDH) and then calculating \(2^{{ - \Lambda \Lambda Ct}}\) values.
Measurement of neurotransmitters in brain tissue homogenate
A commercial ELISA kit (Bio Vision, Zurich, Switzerland) was used to estimate GABA concentration. Glutamate concentration was measured using a commercial ELISA kit (Abnova, Taiwan) at a wavelength of 450 nm using a microplate reader.
Histological examination
After isolating the left kidney and brain from each rat, tissues were fixed in a 10% buffered formalin solution, paraffin-embedded, and sectioned into 3 μm-thick sections according to standard procedures. Gradually, the sections were deparaffinized, hydrated, and finally stained with hematoxylin and eosin (H&E). Then the samples were examined using a light microscope at ×400 magnification power by an experienced pathologist.
Morphometry count
The morphometric change in left renal tissue was performed to evaluate the scoring of inflammation and congestion in the cortex and medulla of kidney tissue by counting degenerated tubules and casts formed after renal ischemia per 10 non-overlapping fields in each sample at ×400 magnification power using a light microscope by an experienced pathologist.
Correspondingly, the morphometric change in brain tissue was performed by counting the Purkinje cells in the cerebellar cortex and pyramidal cells in the CA1 region in the hippocampus per 10 non-overlapping fields in each sample at ×400 magnification power using a light microscope by an experienced pathologist.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). The Statistical Package for the Social Sciences (SPSS) software, version 23.0 (Chicago, Illinois, USA), was used to perform all statistical analyses. The normality of the quantitative variables was determined using the Shapiro-Wilk test. A pairwise comparison of the tested quantitative variables between different groups was performed using a one-way analysis of variance (ANOVA), followed by Tukey’s post hoc correction. Whereas a difference with a chance probability of P < 0.05 was accepted as statistically significant. All graphs were carried out using GraphPad Prism software, version 8.0 (CA, USA).
Results
Doppler ultrasonography results
In the term of Doppler ultrasonography, results Fig. 4 showed a significant increase (P < 0.0001) in both renal RI and PI readings along with a significant decrease (P < 0.0001) in PSV and TAMV in I/R rats. Our finding showed that the entire treatment group exhibited significant (P < 0.0342) improvements in all Doppler parameters compared to those of the I/R group. PRP-treated rats revealed the most significant improvement in all Doppler parameters, and interestingly, both RI and PI of rats that received PRP were non-significant compared to those of the sham group (P = 0.9556, and 0.4069, respectively). Concerning both PSV and TAMV, compared to the sham group, results showed significant (P ≤ 0.0101) increases in their measurements in all treatment groups.
The effect of PRP and exosome administration on the level of RI (A), PI (B), PSV (C), and TAMV (D) of the renal artery. Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
Serum biochemical analysis
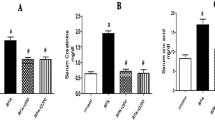
As described in Fig. 5, compared with the sham group, creatinine, BUN, and uric acid serum levels were significantly (P < 0.0001) elevated in rats subjected to renal I/R injury. All treatment groups showed a significant (P < 0.0001) improvement in all tested serum kidney function parameters compared to the I/R group. PRP group revealed the most significant decrease (P < 0.0001) in serum creatinine, BUN, and uric acid than the other treatment groups, where it was non-significant (0.430-0.9942) from the sham group in the levels of creatinine and uric acid. Results revealed a non-significant effect between both Ex. and Ex.+PRP groups in enhancing serum creatinine, BUN, and uric acid.
The effect of PRP and exosome administration on the levels of serum kidney function parameters. Statistical analysis using a one-way ANOVA followed by Tukey’s post-hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
A significant (P < 0.0001) 4.2-fold elevation was recorded in rats of I/R group compared with the sham group. All treatment groups showed a significant (P < 0.0001) improvement in serum AchE measurement compared to the I/R group. Among the entire treatment groups, rats that received PRP were observed to have a significant (P < 0.0001) improvement in serum AchE level. Results revealed a non-significant effect between both Ex. and Ex.+PRP groups in enhancing serum AchE level Fig. 6.
Results of oxidative and anti-oxidative statuses of renal tissue
As shown in Fig. 7, a significant (P < 0.0001) 12.85-fold increase in the oxidative stress marker MDA was observed in I/R rats compared with the sham rats. All treatment groups showed a significant (P < 0.0001) improvement in MDA measurement compared to the I/R group where both PRP and Ex.+PRP groups were non-significant (P = 0.4892–0.9841) from the sham group. Conversely, significant (P < 0.0001) 7.11- and 20.46-fold decreases were observed in GSH and CAT levels respectively in I/R groups compared to the sham rats. All treatment groups showed a significant (P < 0.0001) improvement in GSH and CAT measurement compared to the I/R group where PRP rats were non-significant (P = 0.0679) from the sham group in expression of CAT marker.
The effect of PRP and exosome administration on the levels of renal oxidative stress and antioxidant parameters. Statistical analysis using a one-way ANOVA followed by Tukey’s post-hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
Results of mRNA gene expression (IL-6 and TNF- α) in renal tissue
A significant (P < 0.0001) 4.35-fold, and 4.14fold increase in the mRNA expression levels of both pro-inflammatory mediators; IL-6, and TNF- α was observed in I/R rats compared with sham rats. All treatment groups showed a significant (P < 0.0001) improvement in IL-6, and TNF- α mRNA expression levels compared to the I/R group. PRP group revealed the most significant decrease (P < 0.0001) in IL-6 mRNA expression level than the other treatment groups, where it was non-significant (P = 0.0575) from the sham group as illustrated in Fig. 8.
Real-time PCR analysis for IL-6 (A) and TNF- α (B) gene expression in kidney tissue. Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
Results of neurotransmitter level (glutamate and GABA) in brain tissue
As shown in Fig. 9, a significant (P < 0.0001) 7.028-fold increase in the level of glutamate was observed in I/R rats compared with the sham rats. All treatment groups showed a significant (P < 0.0001) improvement in glutamate level compared to the I/R group. Conversely, significant (P < 0.0001) 4.74-fold decreases were observed in GABA level respectively in I/R groups compared to the sham rats. All treatment groups showed a significant (P < 0.0001) improvement in glutamate and GABA measurement compared to the I/R group.
The levels of Glutamate (A) and GABA (B) neurotransmitters in the brain homogenate after administration of PRP and exosome. Statistical analysis using a one-way ANOVA followed by Tukey’s post-hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
Histopathological results
After examination of the renal cortex and medulla using light microscopy at (H&E, x400), it appeared that the renal cortex of a rat from the control group (distilled water and diet) shows a Malpighian corpuscle containing glomerulus (G) with many capillaries (c) and is surrounded by Bowman’s space (S), which is clear of debris. Bowman’s capsule reveals its parietal layer with flat squamous cells (arrow). Proximal (PT) and distal convoluted tubules (DT) appear with rounded vesicular nuclei, as explained in Fig. 10a. Regarding the sham-operated without inducing ischemia, the renal cortex of a rat shows a Malpighian corpuscle containing glomerulus (G) with many capillaries (c) and surrounded with Bowman’s space (S) which is clear of debris. Bowman’s capsule reveals its parietal layer with flat squamous cells (arrow). Proximal (PT) and distal convoluted tubule (DT) appear with rounded vesicular nuclei as shown in Fig. 10b. In the I/R group, the renal cortex of a rat shows enlarged Malpighian corpuscles with large, segmented glomeruli (G), congested capillaries (c), and is surrounded by a wide Bowman’s space (S) with debris. Bowman’s capsules reveal its parietal layer with flat cells (arrow). Nearly all tubules (T) appear with darkly stained nuclei (n), while a few tubules have rounded vesicular nuclei (N). Another corpuscle with atrophic glomeruli (G1) surrounded by a very wide Bowman’s space (S) is seen. Bowman’s capsules reveal its parietal layer with flat cells (arrow). Nearly all the tubules (T) appear with darkly stained nuclei (n) and vacuolated cytoplasm (v). Extensively dilated tubules (D) and numerous exfoliated cells in a tubule (arrowhead). Large acidophilic materials or casts (asterisks) fill their lumens, as observed in Fig. 10c, d, e.
Regarding the I/R rats injected with exosomes, the renal cortex shows a Malpighian corpuscle containing a glomerulus (G) with many capillaries (c) and is surrounded by a wide Bowman’s space (S) with debris. Bowman’s capsules reveal its parietal layer with flat cells (arrow). Proximal (PT) and distal convoluted tubule (DT) appear with rounded pale nuclei, while others have vacuolated cytoplasm (v), darkly stained nuclei (n), luminal exfoliated cells (arrowhead), or casts (asterisk), as demonstrated in Fig. 10f. The renal cortex section from the I/R rat injected with PRP reveals nearly normal tissue with little damage. Malpighian corpuscle containing glomerulus (G) with many capillaries (c) and surrounded with Bowman’s space (S) which is clear of debris. Bowman’s capsules reveal its parietal layer with flat squamous cells (arrow). Proximal (PT) and distal convoluted tubule (DT) appear with rounded nuclei (N) while others with darkly stained nuclei (n) and luminal exfoliated cells (arrowhead). Small capillaries (tailed arrows) are observed in Fig. 10g.
In I/R rats injected with PRP + Exosome, the renal cortex shows nearly normal tissue with little damage. Malpighian corpuscle containing glomerulus (G) with many capillaries (c) and surrounded by a wide Bowman’s space (S) with little debris. Bowman’s capsules reveal their parietal layer with cuboidal to flat cells (arrow). Proximal (PT) and distal convoluted tubules (DT) appear with rounded nuclei, while others have darkly stained nuclei (n), luminal exfoliated cells (arrowhead), or casts (asterisk). Wide congested capillaries (tailed arrows), as shown in Fig. 10h.
Concerning the renal medulla, rats in the control group (distilled water and diet) show a small group of loops of Henle (LH) with flat squamous lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow), and a few small capillaries (c), as found in Fig. 11a. For the sham-operated without inducing ischemia rats, the renal medulla shows a small group of loops of Henle (LH) with flat squamous lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow) as noticed in Fig. 10b. Rats in the I/R group (ischemia procedure) show a small group of loops of Henle (LH) with flat squamous lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow). Nearly all tubules reveal darkly stained nuclei (n) and casts (ca.). Acidophilic or colloidal materials (asterisk) in between tubules and congested small capillaries (c), as noticed in Fig. 11c.
Regarding I/R rats-injected exosomes, it shows a group of loops of Henle (LH) with flat squamous lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow). Numerous congested small capillaries (c), some tubules with vacuolated cytoplasm (v), and casts (ca.) are noticed in Fig. 11d. The renal medulla of the I/R rat-injected PRP shows a small group of loops of Henle (LH) with flat-lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow). Few tubules with darkly stained nuclei (n) or casts (ca.) and small capillaries (c) are noticed in Fig. 11e.
In the I/R rats injected with PRP + Ex., the renal medulla shows a small group of Henle loops (LH) with flat squamous lining cells (arrowhead). Most of the section shows collecting tubules or ducts (CT) with cuboidal lining cells (arrow). Some tubules with darkly stained and exfoliated nuclei (n) or casts (ca.) and congested small capillaries (c) are noticed. Little acidophilic or colloidal materials (asterisks) in between tubules can be demonstrated in Fig. 11f.
On a light microscopic examination of the cerebral cortex at (H&E, × 400), the rat from the control group (distilled water and diet) shows closely adherent pia matter (arrow). It reveals the molecular layer and cellular external cortical layers (CL). Cortical neurons (Cn) with vesicular nuclei and basophilic cytoplasm are observed in these external layers. Intercellular neuropil shows neuroglia (g) and blood vessels with narrow perivascular spaces (Bv), as shown in Fig. 12a. Concerning the rats in the sham group, the cerebral cortex of the brain shows closely adherent pia matter (arrow). It reveals molecular layer cellular external cortical layers (CL). Cortical neurons (Cn) with vesicular nuclei and basophilic cytoplasm are observed in the external layers. Intercellular neuropil shows neuroglia (g) and blood vessels with narrow perivascular spaces (Bv) as observed in Fig. 12b. Regarding the rats in the I/R group, the cerebral cortex of a rat shows widely separated irregular pia matter (arrow). The outer thick molecular layer with different types of neuroglia (g), some inflammatory cells (f), and vacuolated neuropil (v), the external cortical layer. Cortical neurons (Cn) with vesicular nuclei and basophilic cytoplasm, numerous darkly stained shrunken neurons (arrowhead), and blood vessels with wide perivascular spaces (Bv), vacuolated neuropil (v), and different types of neuroglia (g) are observed in Fig. 12c, d, e.
In the Ex. group, the cerebral cortex of a rat shows separated pia matter (arrow) and the thick molecular layer (M) with some inflammatory cells (f) and a vacuolated neuropil (v). The external cortical layer (CL) shows cortical neurons (Cn) with vesicular nuclei and basophilic cytoplasm and numerous darkly stained shrunken neurons (arrowhead). Blood vessels with wide perivascular spaces (Bv) and different types of neuroglia (g) are observed in both the molecular and outer cortical layers, as illustrated in Fig. 12f. Regarding the I/R group of rats injected with PRP, the cerebral cortex of a rat shows nearly normal tissue. The closely adherent pia matter (arrow) is seen. Vacuolated neuropil (v) in the molecular layer (M) and cellular external cortical layers (CL). Most cortical neurons (Cn) appear with vesicular nuclei and basophilic cytoplasm, while a few darkly stained neurons (arrowheads) in the external layers are observed. Numerous different types of neuroglia (g) and blood vessels with narrow perivascular spaces (Bv) are found in Fig. 12g.
The cerebral cortex of I/R rats injected with PRP + Ex reveals more or less normal tissue. The slightly separated pia matter (arrow) is seen. The outer molecular layer (M) shows vacuolated neuropil (v) and blood vessels with wide perivascular spaces (Bv). The external cortical layers show most cortical neurons (Cn) with vesicular nuclei and basophilic cytoplasm and a few darkly stained neurons (arrowhead). Different types of neuroglia (g) are observed in this all-layers, as demonstrated in Fig. 12h.
Regarding the control group of rats (distilled water and diet), the CA1 region of the hippocampus reveals both molecular (M) and polymorphic (Pm) layers, with some glial cells with pale nuclei (g) and blood vessels with narrow perivascular spaces (Bv). 5–6 compact layers of pyramidal neurons with centrally vesicular nuclei (N) and a few glial cells with elongated, darkly stained nuclei (arrow) are observed in the pyramidal layer (Py) in Fig. 13a. For sham-operated rats, the CA1 region of the hippocampus shows polymorphic (Pm) layers having some glial cells with pale nuclei (g) and blood vessels with narrow perivascular spaces (Bv). 5–6 compact layers of pyramidal neurons with centrally vesicular nuclei (N) and a few glial cells with darkly stained nuclei (arrow) are observed in the pyramidal layer (Py) as shown in Fig. 13b. In the I/R rats, the CA1 region of the hippocampus is shown in the pyramidal layer (Py); 2–3 layers of few normal neurons with centrally vesicular nuclei (N), others shrunken with darkly stained nuclei (arrowhead), and numerous glial cells with elongated darkly stained nuclei (arrow). Both molecular (M) and polymorphic (Pm) layers show numerous different types of glial cells (g) and also blood vessels with wide perivascular spaces (Bv), as noticed in Fig. 13c, d.
The CA1 region of the hippocampus from the group of I/R rats injected with exosomes shows a few hypocellular layers of pyramidal neurons with centrally vesicular nuclei (N) and numerous other neurons with darkly stained nuclei (arrowheads) in the pyramidal layer (Py). Both molecular (M) and polymorphic (Pm) layers show numerous glial cells with pale nuclei (g) and blood vessels with wide perivascular spaces (Bv), as shown in Fig. 13e. Moreover, in I/R rats injected with PRP, the CA1 region of the hippocampus shows nearly normal tissue. 3–5 layers of pyramidal neurons with centrally vesicular nuclei (N) and numerous glial cells with elongated, darkly stained nuclei (arrow) are observed in the pyramidal layer (Py). Both molecular (M) and polymorphic (Pm) layers show numerous glial cells with pale nuclei (g) and blood vessels with narrow perivascular spaces (Bv), as observed in Fig. 13f.
In PRP + Ex. injected group of rats, the CA1 region of the hippocampus shows nearly normal tissue. 3–5 layers of pyramidal neurons with centrally vesicular nuclei (N) and numerous glial cells with elongated, darkly stained nuclei (arrow) are observed in the pyramidal layer (Py). Both molecular (M) and polymorphic (Pm) layers show numerous glial cells with pale nuclei (g) and also blood vessels with wide perivascular spaces (Bv), as stated in Fig. 13g.
Morphometry results
After examination and counting 10 non-overlapping fields at 400X magnification power using a light microscope by an experienced pathologist, it was recorded that the number of renal degenerated tubules in both cortex and medulla was increased compared to the sham rats, owing to the fact that the rats in renal I/R groups were subjected to 45 min ischemia that ultimately leads to congestion and inflammation of renal tissue. Concerning the formation of casts, a high increase in their number after renal I/R injury was recorded. The formation occurred due to disruption in renal tubules, then enlargement in Malpighian corpuscles and a wide Boman’s capsule, which in the end led to a low filtration rate and the accumulation of toxins and casts Figs. 14 and 15.
The effect of PRP and exosome administration on the change of the number of degenerated tubules and casts in the renal cortex. Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
The effect of PRP and exosome administration on the change of the number of degenerated tubules and casts in the renal medulla. Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
As illustrated in Fig. 16, after examination and counting 10 non-overlapping fields at 400X magnification power using a light microscope by an experienced pathologist, it was recorded that the number of Purkinje cells, pyramidal cells in the cerebellar cortex, and the CA1 region in the hippocampus, respectively, was highly reduced compared to the sham group (P < 0.0001).
The effect of PRP and exosome administration on the change of the number of Purkinje cells in the cerebellar cortex and pyramidal cells in the CA1 region in the hippocampus of brain tissue. Statistical analysis using a one-way ANOVA followed by Tukey’s post hoc test. Variables were presented as the mean ± standard deviation (SD). Groups with different small superscript letters are significantly different at P < 0.05.
Discussion
The main target of the present study was to investigate the protective effects of renal sub-capsular injection of PRP and MSC-derived exosome for the first time especially at the pre-reperfusion phase on the remote impact of brain functions following a renal I/R injury. Treatment with PRP in this model was associated with a superior improvement in both renal and distant effects on brain biomarkers and functions. In contrast, rats treated with exosomes show the significantly weakest effect among the treatment groups in both kidney and brain functions. The combination group exhibited a significant improvement in the bio-physiological markers of both the kidney and brain compared to the sham one, but this improvement was significantly weaker than the PRP group.
Nephrotoxicity not limited only by induction renal I/R model but also there are various potent medicals that had various side effects such as Methotrexate23, and Cisplatin6,24, the later one mentioned has been reported from previous studies its role in nephrotoxicity after accumulation in renal proximal tubules that converted to other toxic reactive metabolite, a cysteine conjugate, that act as a terrible promoter for cellular kidney injury. MSCs had reported its effect as immunomodulatory, anti-oxidant, anti-inflammatory tool in injured kidney tissue whether injury induced by cisplatin24 or establishment a renal I/R injury model13.
Renal ischemia/reperfusion triggered a complicated sequence of events initiated in the ischemic phase by deprivation of the tissue from nutrients and oxygen supply, then switching off the aerobic cellular metabolism that turns into anaerobic conditions, consequently reducing the rate of ATP production, Na+/K+ ATPase dysfunction, and ATPase pumping that leads to Na+ accumulation in the cytoplasm and ultimately hypertension25. In the present study, our kidney function results revealed that induction renal I/R markedly increased levels of serum creatinine, BUN, and uric acid. Thus, consistent with previous studies mentioned above, our findings have proved that rats in both groups were injected with PRP and Ex. +PRP before the reperfusion phase revealed more improvement in all biochemical parameters and histological tissues owing to the unique features of PRP than exosomes alone.
Whereas rats injected with PRP before reperfusion exhibited a superior significant enhancement in serum kidney function parameters by decreasing levels of serum creatinine, BUN, and uric acid, and ameliorates hypertension, compared with I/R rats. Gradually, the improvement was recorded but by a less significant degree in Ex. +PRP rats than others in the PRP treated rats before reperfusion.
The levels of serum kidney function parameters, creatinine, BUN, and uric acid, were reduced. As the concerned group of rats was injected with exosomes only, and owing to the rapid clearance, low retention, and low encapsulation capacity features of exosomes that impede the effect of injected exosomes26,27. The rats received a single dose of exosomes before reperfusion, which revealed the weakest effect on both kidney function parameters.
Furthermore, during the reperfusion phase, the aerobic metabolism resumed with rapid elevation in O2 and PH normalization, Ca++ overload that causes damage in the mitochondrial membrane, and overproduction of reactive oxygen species (ROS) that causes an imbalance between the antioxidant defense system by decreasing GSH content and catalase enzyme levels and increasing oxidative cellular stress, including high levels of MDA as a final by-product of lipid peroxidation during β-oxidation of unsaturated fatty acids28. Alongside previous studies, it was recorded that in renal homogenate of I/R group, enhancement of lipid peroxidation by elevation level of MDA as well as decreasing the level of both antioxidants CAT enzyme and GSH. Our results proved that rats in both groups were injected with PRP, and Ex. +PRP before the reperfusion phase revealed more improvement in all antioxidant and oxidative stress parameters. Correspondingly, a great elevation was noticed in the antioxidant defense system by increasing both levels of CAT enzyme and GSH.
Lipid peroxidation in renal tissue was significantly reduced by decreasing the level of MDA in the renal tissue of PRP-treated rats compared with the I/R rats group. Progressively, the improvement was reduced in the group of rats injected with a mixture of platelet-rich plasma and exosomes before reperfusion compared to others in the group of rats injected with PRP before reperfusion; the level of CAT antioxidant enzyme and GSH level significantly increased after injection rats with PRP + Ex. before reperfusion, on the other hand, the oxidative stress was ameliorated by decreasing the level of MDA. Regarding the I/R rats injected with exosomes, only the weakest effect was shown owing to the limitations mentioned above based on previous studies; CAT antioxidant enzyme and GSH level increased significantly after injection with exosomes before reperfusion compared to I/R rats. Additionally, the level of MDA as a biomarker for lipid peroxidation increased significantly.
Owing to the successive damage induced by the period of ischemia in renal I/R tissue, the inflammatory reactions begin through the high expression of proinflammatory cytokines TNF-α and IL-6 in I/R renal tissue29. In line with these studies, our findings were recorded that in renal homogenate, there was a markedly increased mRNA expression level of the anti-inflammatory cytokines TNF-α and IL-6 by promoting oxidative stress and a high expression of adhesion molecules in renal tissue30,31. By virtue of the superior efficiency of injection PRP before reperfusion, our results proved that renal I/R injected with PRP had highly superior effects by reducing the elevated mRNA expression level of pro-inflammatory cytokines TNF-α and IL-6 compared with the I/R group. For the group of rats injected with Ex. +PRP, the elevated mRNA expression level of pro-inflammatory cytokines TNF-α and IL-6 was reduced, but to a lesser degree than in rats injected with a single dose of PRP. Relative to a Ex. -treated rats before reperfusion, the levels of proinflammatory mediators TNF-α and IL-6 were significantly reduced by the weakest degree among other groups.
At the same time, renal histological deterioration was exhibited by the enlargement of the Malpighian corpuscles with large, segmented glomeruli, congested capillaries, and a wide Bowman’s space with a reduction glomerular filtration rate, leading to the accumulation of uremic toxins and damage to renal proximal TECs because of their high demand for O2 supply in the cortex and medulla32,33. In our study, it was proven that renal histological deteriorations were enhanced and restored to normal rats in the control group after renal subcapsular injection with PRP, owing to its superior regeneration power for its growth factors. Moreover, the morphometry examination was a vital confirmatory tool in counting the number of degenerated tubules and casts in both the renal cortex and medulla after their extremely elevating level in I/R rats.
Our findings explained that in rats subjected to renal I/R without treatment, distant inflammatory damage to brain tissue was evidenced by increasing levels of serum acetylcholine esterase enzyme, neurotransmitter derangement by increasing levels of glutamate, and decreasing levels of GABA in brain homogenate. Regarding the PRP-treated rats injected with before reperfusion, an enhancement in brain biochemical parameters was observed by a significant reduction in the hyperactivity of the serum acetylcholine esterase enzyme, the level of glutamate in brain homogenate, and the increasing level of GABA in brain tissue compared to I/R rats.
Subsequently, the amelioration observed in rats injected with Ex. +PRP to a low degree in the brain was achieved by decreasing the increased levels of both serum acetylcholine esterase and glutamate levels in brain tissue and increasing the level of GABA in brain homogenate compared with the I/R group. Due to the limitations faced by exosome injection, the weakest effect was recorded in the rats injected with a single dose of exosome before reperfusion: a significant decrease in serum acetylcholine esterase level, glutamate in brain homogenate, and a noticeable increase in GABA level.
The distant effect of renal I/R injury extends to other organs such as the pancreas34, lungs35, liver36, and brain5,37 have been reported in various previous studies. In harmony with these findings, our results were interpreted as follow by elevating levels of uremic nitrogenous wastes, including guanidino compounds, creatinine, urea, and uric acid, as well as an increasing level of serum Na+, oxidative stress, and inflammatory mediators with suppression of anti-oxidant defense enzymes. All these factors contribute to brain tissue inflammation and neuronal loss by disrupting the integrity of the blood-brain barrier and inhibiting the permeability of the blood-brain barrier38,, increasing acetylcholine esterase activity that responsible for degradation acetylcholine neurotransmitter which exerts a reno-protective effect39,40, inhibiting cholinergic activity which associated with blocking GABA ergic receptors41, making a hyper-stimulatory effect on glutamate receptors that is associated with the loss of Purkinje neurons in the cerebellar cortex, hyper excitatory in cornu Ammonis (CA1) region in the hippocampus that is responsible for memory and learning that ultimately leads to death of pyramidal neurons (major GABAergic neurons) via necrosis4.
Brain histological damage was recorded after examination of the cerebellar cortex and CA1 region in the hippocampus by loss of Purkinje and pyramidal neurons in both regions, respectively. As well, our results also proved that, after using a supportive confirmatory tool, morphometry examination in the cerebellar cortex and CA1 region in the hippocampus revealed that several Purkinje and pyramidal neurons, respectively, were increased by a greatly significant degree after PRP injection compared to a positive ischemic group of rats. Based on previous studies that proved brain tissue is adversely affected following renal I/R injury, especially in the CA1 region in the hippocampus, which therefore causes hyper-excitatory in the CA1 region and loss of the number of pyramidal neurons4, and loss of the number of Purkinje cells that are known as master GABAergic neurons, it considered experienced results in line with our biochemical findings that refer to diminishing the GABA neurotransmitter level in brain homogenate41.
In the present study, non-invasive color-doppler ultrasonography was used to assess renal hemodynamic conditions of the renal microcirculation after 45 min of renal ischemia, as recommended by many authors42,43,44 who declared its role as a prognostic tool in predicting revascularization success after ischemia is relieved. Results of Doppler ultrasonography showed a significant increase in both renal RI and PI (renal vascular resistance indicators) in ischemic rats, which could be attributed to both acute tubular necrosis and interstitial edema within the ischemic solitary kidney, as explained by Değirmenci et al.45 who declared that both PI and RI afford an estimate of the degree of interstitial edema within the kidney graft after transplantation.
Darabont et al.46 added that renal RI and PI depend mainly on the renal arterial blood flow during the diastolic phase, which is disturbed by increased intrarenal resistance by renal inflammation and intrarenal microvascular damage, the state of I/R injury. Histopathological examination of the 45-minute ischemic kidney in the I/R group confirmed this explanation. Köger et al.43 mentioned that high resistive indices (> 0.8) in renal transplant recipients are associated with an increased risk of graft loss and death. Additionally, the elevated RI in I/R rats could be attributed to the impaired creatinine clearance along with elevated creatinine concentrations in such rats, as reported by47 who found an inverse correlation between renal resistance index and a reduction in creatinine clearance.
In the same manner, doppler ultrasonography showed a significant decrease in renal artery flow velocity (PSV and TAMV) in 45-minute ischemic kidneys, which are used as early indicators of renal parenchymal resistance and acute kidney injury, as mentioned48 who found a moderate negative correlation and a greater than 80% accuracy between a reduction in the magnitude of TAMV and a higher degree of tubular degeneration. Fortunately, the entire treatment group showed significant improvements in all biochemical, histopathological, and creatinine clearance tests, which explain the improvement of all Doppler ultrasonography indices and velocities.
Conclusion
In summary, we concluded that injection of PRP only or a mixture of PRP and EX. before the reperfusion phase exerts a significantly superior effect on kidney and brain biochemical parameters and enhancement in the regeneration of kidney and brain tissue that nearly comes close to that of normal rats, owing to the distinctive incredible growth factors of PRP. Single-dose injection with exosomes doesn’t exhibit a positive physiological change more than the other two groups. Furthermore, injection before the reperfusion phase exhibited an obvious physiological positive change by enhancing the level of biochemical and histological parameters in both kidney and brain tissue by diminishing the elevation of inflammatory reactions induced by the imbalance between the antioxidant defense enzyme and oxidative stress due to the excessive sudden return of blood flow and re-oxygenation.
Data availability
The experimental data and the simulation results that support the findings of this study are available in Mendeley Data repository https://data.mendeley.com/datasets/52z7gz55fg/1 , Abdelsalam, Hani (2024), “Exosome & PRP data”, Mendeley Data, V1, doi: https://doi.org/10.17632/52z7gz55fg.1.
Abbreviations
- BBB:
-
Blood brain barrier
- I/R:
-
Ischemia/Reperfusion
- AKI:
-
Acute kidney injury
- PRP:
-
Platelet rich plasma
- Ex.:
-
Exosome
- CA:
-
Cornu ammonis
- AchE:
-
Acetylcholine esterase
- ELISA:
-
Enzyme linked immunosorbent assay
- CAT:
-
Catalase
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- GABA:
-
Gamma aminobutyric acid
- PCR:
-
Polymerase chain reaction
- TNFα:
-
Tumor necrosis factor-alpha
- IL6:
-
Interleukin-6
- RI:
-
Resistance index
- PI:
-
Pulsatility index
- PSV:
-
Peak systolic velocity
- TAMV:
-
Time average mean velocity
References
Güvenç, M. et al. Nobiletin protects from Renal Ischemia-Reperfusion Injury in rats by suppressing inflammatory cytokines and regulating iNOS-eNOS expressions. Inflammation. 43 (1), 336–346 (2019).
Ahmadvand, H. et al. The protective role of gallic acid pretreatment on renal ischemia-reperfusion injury in rats. Rep. Biochem. Mol. Biology. 8 (1), 42 (2019).
Liu, Z. et al. Neutrophil membrane-enveloped nanoparticles for the amelioration of renal ischemia-reperfusion injury in mice. Acta Biomater. 104, 158–166 (2020).
Firouzjaei, M. A., Haghani, M. & Moosavi SM. Renal ischemia/reperfusion induced learning and memory deficit in the rat: insights into underlying molecular and cellular mechanisms. Brain Res. 1719, 263–273. (2019).
Lee, B. et al. Effect Renal ischemia reperfusion brain neuroinflammation 10(11): 2993. (2022).
Salem, N., Helmi, N. & Assaf, N. Renoprotective effect of platelet-rich plasma on cisplatin-induced nephrotoxicity in rats. Oxid Med Cell Longev, 9658230 (2018).
Bayat, M. et al. Effects of platelet-rich plasma on the memory impairment, apoptosis, and hippocampal synaptic plasticity in a rat model of hepatic encephalopathy. Brain Behav. 12 (1), e2447 (2022).
Nakayama, M. & P. Berger, Coordination of VEGF receptor trafficking and signaling by coreceptors. Exper. Cell Res. 319(9) 1340–1347 (2013).
Pan, W. et al. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 37(12) 1553–1561 (1998).
Friden, P. M. J. N. Receptor-mediated transport of therapeutics across the blood-brain barrier. Neurosurgery. 35(2) 294–298 (1994).
Duffy, K. R., Pardridge, W. M. & Rosenfeld, R. G. Human blood-brain barrier insulin-like growth factor receptor. Metabolism 37(2): 136–140 (1988).
Camussi, G. et al. Human extracellular microvesicles from renal tubules reverse kidney ischemia-reperfusion injury in rats. Plos One. 13 (8), e0202550 (2018).
Zahran, R. et al. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia–reperfusion injury in a rat model. Int. J. Urol. 27 (11), 1039–1049 (2020).
Li, L. et al. Exosomes derived from mesenchymal stem cells ameliorate renal ischemic-reperfusion injury through inhibiting inflammation and cell apoptosis. Front. Med. 6, (2019).
Okuda, K. et al. Platelet-Rich plasma contains high levels of platelet-derived growth factor and transforming growth Factor-β and modulates the proliferation of Periodontally related cells in Vitro. J. Periodontol. 74 (6), 849–857 (2003).
Ehrenfest, D. M. D., Rasmusson, L. & T. Albrektsson, Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trend Biotechnol. 27(3), 158–167 (2009).
Mohammadi, R. et al. Effect of platelet rich plasma combined with chitosan biodegradable film on full-thickness wound healing in rat model. Bull. Emergency Trauma 4(1), 29 (2016).
McCulloch, P. et al. Randomised trials in surgery: problems and possible solutions. BMJ. 324(7351), 1448–1451 (2002).
Hibberd, T. J. et al. Circadian rhythms colonic function. Front. Physiol. 14. (2023).
Samy, A. et al. The potential protective and therapeutic effects of platelet-rich plasma on ischemia/reperfusion injury following experimental torsion/detorsion of testis in the albino rat model. Life Sci. 256, 117982 (2020).
Fisch, S. et al. Early detection of drug-induced renal hemodynamic dysfunction using sonographic technology in rats. J. visualiz. Exper. 109, e52409 (2016).
Close, B. et al. Recommendations for euthanasia of experimental animals: Part 1. Lab. Animals 30(4), 293–316 (1996).
Amini, N. et al. Dose-dependent renoprotective effect of vanillic acid on methotrexate-induced nephrotoxicity via its anti-apoptosis, antioxidant, and anti-inflammatory properties. Naunyn-Schmiedeberg’s Arch. Pharmacol. 397(6), 4195–4204 (2024).
Amini, N. et al. Combined effect of naringin and adipose tissue-derived mesenchymal stem cell on cisplatin nephrotoxicity through Sirtuin1/Nrf-2/HO-1 signaling pathway: A promising nephroprotective candidate. Cell Tissue Res.. 1–12 (2024).
Lima, N. K. et al. Renal ischemia-reperfusion leads to hypertension and changes in proximal tubule na + transport and renin-angiotensin-aldosterone system: Role of NADPH oxidase. Life Sci. 266, 118879 (2021).
Imai, T. et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicl. 4(1), 26238 (2015).
Liu, Y. et al. Enhanced therapeutic effects of MSC-derived extracellular vesicles with an injectable collagen matrix for experimental acute kidney injury treatment. Stem Cell Res. Therapy 11(1), 1–12 (2020).
Aboutaleb, N. et al. Lavender oil (Lavandula angustifolia) Attenuates Renal ischemia/reperfusion Injury in rats through Suppression of Inflammation, Oxidative Stress and Apoptosis. Biomedi. Pharmacotherapy 110, 9–19 (2019).
Yousefi-Manesh, H. et al. Protective effects of hydroalcoholic extracts from an ancient apple variety ‘Mela Rosa dei Monti Sibillini’ against renal ischemia/reperfusion injury in rats. Food Functi. 10(11), 7544–7552 (2019).
Patel, N. S. et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J. Pharmacol. Exper. Therapeut. 312(3), 1170–1178 (2005).
Aal-Aaboda, M. S., Abu Raghif, A. R. & Hadi, N. R. Renoprotective potential of the ultra-pure lipopolysaccharide from rhodobacter sphaeroides on acutely injured kidneys in an animal model. Arch. Razi. Inst. 76(6), 1755–1764 (2021).
Gholampour, F. et al. The role of nitric oxide in the protective action of remote ischemic per-conditioning against ischemia/reperfusion-induced acute renal failure in rat. Iran. J. Basic Med. Sci. 21(6), 600–606 (2018).
Han, F. et al. Cordycepin protects renal ischemia/reperfusion injury through regulating inflammation, apoptosis, and oxidative stress. Acta Biochim. Biophys. Sin. (Shanghai) 52(2), 125–132 (2020).
El Agaty, M. S., Ibrahim Ahmed, A. Pathophysiological and immunohistochemical analysis of pancreas after renal ischemia/reperfusion injury: protective role of melatonin. Arch. Physiol. Biochem. 126(3), 264–275 (2020).
Hassoun, H. T. et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am. J. Physiol.-Renal Physiol. 293(1) F30–F40 (2007).
Serteser, M. et al. Changes in hepatic TNF-α levels, antioxidant status, and oxidation products after renal ischemia/reperfusion injury in mice. J. Surg. Res. 107(2), 234–240 (2002).
Amini, N. et al. Naringin and trimetazidine improve baroreflex sensitivity and nucleus tractus solitarius electrical activity in renal ischemia-reperfusion injury. Arqu. Brasileiros de Cardiol. 117, 290–297 (2021).
Azarkish, F. et al. The influence of renal ischemia-reperfusion injury on remote organs: the histological brain changes in male and female rats. Brain Circulat. 7(3), 194 (2021).
Inoue, T. et al. Non-canonical cholinergic anti-inflammatory pathway-mediated activation of peritoneal macrophages induces Hes1 and blocks ischemia/reperfusion injury in the kidney. Kidney Internat. 95(3), 563–576 (2019).
Xu, H. et al. Association between cholinesterase inhibitors and kidney function decline in patients with Alzheimer’s dementia. Kidney Internat. 103(1): pp. 166–176. (2023).
Natale, G. et al. Effects of uremic toxins on hippocampal synaptic transmission: implication for neurodegeneration in chronic kidney disease. Cell Death Discov.. 7(1), 295 (2021).
Aschwanden, M. et al. Rapid adaptation of the intrarenal resistance index after living donor kidney transplantation. Nephrol. Dialysis Transplant. 24(4), 1331–1334 (2009).
Köger, P. et al. Association of intrarenal resistance index and systemic atherosclerosis after kidney transplantation. In Vivo 35(6), 3369–3375 (2021).
Kong, H. Y. et al. Intrarenal resistance index for the assessment of acute renal injury in a rat liver transplantation model. BMC Nephrol. 14(1), 1–8 (2013).
Değirmenci, N. A. et al. Evaluation of the renal resistive index and pulsatility index in patients with pleural effusion by duplex Doppler ultrasonography. Japanese J. Radiol. 31, 39–44 (2013).
Darabont, R., Mihalcea, D. & Vinereanu, D. Current insights into renal resistive index significance in kidney and cardiovascular disease. 13(10), 1687 (2023).
Radermacher, J., Ellis, S. & Haller, H. Renal resistance index and progression of renal disease. Hypertension 39(2 Pt 2), 699–703 (2002).
Gasser, B. et al. Clinical and ultrasound variables for early diagnosis of septic acute kidney injury in bitches with pyometra. Sci. Report. 10(1), 8994 (2020).
Acknowledgements
This study wouldn’t be complete without acknowledging those who contributed to its realization. I’d like to express my thanks to my supervisors for their unwavering encouragement and their support.
Funding
No applicable.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.S. H.A. M.S. and E.A. designed the experiment and performed all surgical procedures. H.A., M.S. and E.A. carried out the biochemical, and physiological studies, contributed to the sequence alignment, and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All applicable international, national, and/ or institutional guidelines for the care and use of animals were followed. The study is reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelsalam, H.M., Samy, A., Mosaleem, E.E.A. et al. The ameliorative potential of platelet-rich plasma and exosome on renal ischemia/reperfusion-induced uremic encephalopathy in rats. Sci Rep 14, 26888 (2024). https://doi.org/10.1038/s41598-024-77094-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77094-2