Abstract
Carbapenem-Resistant Acinetobacter baumannii (CRAB) outbreak in intensive care units (ICUs) is a significant problem for healthcare facilities. In this study, we aimed to investigate the occurrence of CRAB isolates among ICU-admitted patients during the three waves of the COVID-19 pandemic in Iran using Multiple-Locus Variable Number Tandem-Repeat Analysis (MLVA). We obtained 50 (A) baumannii isolates from tracheal aspirate and blood culture samples. In the disc diffusion method, all isolates were cefotaxime, ceftriaxone, and cefepime-resistant, while 98% (49/50) of isolates were resistant to piperacillin, piperacillin-tazobactam, ceftazidime, and ciprofloxacin. Levofloxacin and tobramycin resistance was found in 76% (38/50) of isolates. In the microbroth dilution test all isolates were resistant to imipenem, 98% (49/50) to meropenem, 68% (34/50) to colistin, and 20% (10/50) to polymyxin (B) Based on the PCR findings, all isolates harbored blaOXA−40, ISAba-1, and int-2 genes. There were no isolates found that have the blaOXA−58, blaOXA−143, blaVEB−1, blaVIM, and int-3 genes. Among Extended-spectrum beta-lactamases (ESBL) genes, blaCTX−M, blaTEM, blaSHV, blaGES, and blaPER−1 have a prevalence of 42% (21/50), 84% (42/50), 58% (29/50), 78% (39/50), and 54% (27/50), respectively. 74% (37/50) of the isolates had the blaOXA−23 gene, while all of the isolates carried the blaOXA−40 gene. Among MBL genes, blaIPM, blaGIM, blaSIM, and blaNDM−1 have a prevalence of 20% (10/50), 8% (4/50), 22% (11/50), and 60% (30/50), respectively. The prevalence of int-1 was documented as 74% (37/50). Accordingly, all isolates were identified as CRAB. The co-existence of blaOXA−23/int-2 and blaOXA−23/isaba-1 was 74% (37/50). The co-existence of blaNDM−1/ISAba-1 was observed in 30 (60%) isolates. Using an 80% similarity threshold on the dendrogram constructed through MLVA typing, all isolates were grouped into two clusters: cluster A with 9 isolates from wave 3, and cluster B with 41 isolates from waves 3, 4, and 5. Our study confirms a clonal transmission of CRAB during the study period and suggests using molecular typing methods like MLVA in healthcare settings to identify dominant clones, antibiotic resistance patterns, and transmission routes. This will help to better manage the emergence and spread of antibiotic-resistant strains in future outbreaks.
Similar content being viewed by others
Introduction
More than 6,433,794 people died during the six waves of the SARS-CoV-2 Pandemic, with 143,015 of them being Iranian as of August 17, 2022 (https://worldhealthorg.shinyapps.io/covid/). During the COVID-19 pandemic, many patients required mechanical ventilation and intubation and stayed in the intensive care unit (ICU) for an extended period [1]. Ventilator-associated pneumonia (VAP) is the most common infection in the ICU and significantly affects the morbidity and mortality of critically ill patients [2]. The gram-negative opportunistic pathogen Acinetobacter baumannii is a challenging nosocomial bacterium, commonly attributed to serious infections such as meningitis, hospital-acquired pneumonia, wound infections, urinary tract infections, and septicemia. Due to its propensity for acquiring resistance determinants and a scarcity of available medications, Carbapenem-Resistant Acinetobacter baumannii (CRAB) is categorized as an “urgent threat” pathogen. One major issue for healthcare institutions is the outbreaks of CRAB in ICUs. The incidence, handling, and containment of these outbreaks have been the subject of several research and publications [3–5] due to their high mortality rate, which varies between 45% and 70%. This emphasizes the seriousness and potentially fatal consequences of such infections [6]. On the other hand, the acquisition of infections caused by CRAB is correlated with substantial clinical and economic implications, such as increased healthcare expenditures and worse patient outcomes1.
Several research studies have revealed numerous major risk parameters associated with outbreaks of CRAB in ICU wards. Among the most relevant criteria are (i) patients 65 years of age or older, (ii) extended hospitalization, (iii) comorbidities, (iv) previous consumption of antibiotics, and (v) prior hospitalizations2,3,4. Prevention and management of these epidemics often entail rigorous infection control strategies, including strict compliance with protocols for infection control, strategies for the screening and isolation of patients, and disinfection of the environment5.
According to the Centers for Disease Control and Prevention (CDC), the spectrum of treatment alternatives available for infections caused by CRAB is extremely restricted since the pathogen exhibits resistance toward other classes of antibiotics6. Antibiotics such as colistin, tigecycline, and polymyxin B—which are not often used for other infections—are frequently prescribed for CRAB infections7,8. However, there is growing concern that the emergence of resistance to these medications may reduce their effectiveness8.
The field of molecular epidemiology is of paramount importance in understanding and addressing drug resistance. By analyzing the genetic content of pathogens, it is possible to identify transmission pathways, monitor the spread of resistant strains, and understand the mechanisms of resistance9,10. MLVA is a genotyping technique that is employed to compare strains and classify different bacterial species into subtypes. Multiple-Locus Variable Number Tandem-Repeat Analysis (MLVA) has shown a much higher level of discriminating ability compared to other typing techniques, such as multilocus sequence typing (MLST)11,12. It possesses the capability to discern genetic variations among strains of exceedingly homogeneous species and offers a substantial degree of discrimination12.
In this study, our goal was to investigate the occurrence of CRAB isolates among ICU-admitted patients during the three waves of the COVID-19 pandemic using MLVA. Additionally, we aimed to assess the antibiotic susceptibility of the isolates using disc diffusion and microbroth dilution methods. We also conducted screening for the presence of Extended-spectrum beta-lactamases (ESBLs), carbapenemases (CPs), metallo-beta-lactamases (MBLs), integrases (IN), and insertion sequences (IS) using conventional PCR.
Materials and methods
Sampling strategies
During the third wave of the COVID-19 pandemic, which occurred between October 5, 2020, and January 14, 2021, as well as the fourth wave from March 1, 2021, to June 18, 2021, and the fifth wave from July 6, 2021, to September 20, 2021, a total of 50 A. baumannii isolates were collected from the blood culture and tracheal aspirate culture of anonymous patients who were admitted to the ICU at Sina Educational Hospital in Hamadan, West of Iran. In the microbiology lab of Hamadan University of Medical Sciences, Hamadan, Iran, all A. baumannii isolates were subjected to typical microbiological examinations to further characterize them.
Characteristics of the isolates
We systematically collected samples from all COVID-19 patients admitted to the ICU who required respiratory support or exhibited signs of bloodstream infection. This approach ensured that our sample collection was neither random nor haphazard but followed a structured protocol aimed at capturing all instances of A. baumannii infections in this critical patient group.
The primary sources of samples were tracheal aspirates and blood cultures, given the high incidence of respiratory complications and bacteremia in severe COVID-19 cases. Respiratory samples are crucial for intubated patients13, while blood cultures are essential for detecting bacteremia and sepsis, which are serious complications in ICU patients14.
Tracheal aspirate samples were collected using sterile techniques to avoid contamination. A sterile suction catheter was inserted through the endotracheal tube, and tracheal secretions were aspirated into a sterile trap. Blood was collected aseptically from a peripheral vein using sterile syringes and immediately inoculated into blood culture bottles. Samples were immediately transported to the microbiology laboratory for processing.
Each isolate in our study was obtained from a unique patient. This patient-specific collection method ensured that our data reflected the true prevalence and distribution of A. baumannii infections among the ICU patients without duplication or bias.
All 50 isolates were identified as CRAB using disc diffusion testing with imipenem (IPM/10 µg) and meropenem (MEM/10 µg) from PadtanTeb, Iran, in clinical settings. Isolates were confirmed as CRAB through a microbroth dilution test with imipenem and meropenem in the university’s microbiology lab (material and methods, Sect. 2.4).
During our study period, there were no A. baumannii isolates obtained from other body sites such as urine or wounds. This could be attributed to the focused nature of sampling in patients with severe pulmonary manifestations of COVID-19, as well as the resource limitations and high workload in the laboratory during the pandemic.
Ethics approvals
The Hamadan University of Medical Sciences ethics committee provided approval for the current research, with the following code: IR.UMSHA.REC.1401.783. All experiments were conducted in compliance with the guidelines and regulations of the Hamadan University of Medical Sciences ethics committee. Informed consent was obtained from all participants and/or their legal guardians.
Disk diffusion and broth microdilution techniques
The Kirby-Bauer method15 was used to determine the antibiotic susceptibility patterns of the isolates. The antibiotic disc (Condalab/Spain) panel used in the study included the following antibiotics and their respective dosages: piperacillin (PIP/100 µg), piperacillin -tazobactam (TZP/100-µg/10 µg), ceftazidime (CAZ/30 µg), cefotaxime (CTX/30 µg), ceftriaxone (CRO/30 µg), cefepime (FEP/30 µg), ciprofloxacin (CIP/5 µg), levofloxacin (LVX/5 µg), amikacin (AN/30 µg), gentamicin (GM/10 µg), tobramycin (TM/10 µg), and trimethoprim-sulfamethoxazole (TMP-SXT/1.25–23.75 µg). In accordance with the Clinical & Laboratory Standards Institute (CLSI) recommendation16, the broth microdilution test was performed to determine the minimum inhibitory concentration (MIC) of isolates against imipenem (IPM), meropenem (MEM), colistin (CL), and polymyxin B (PB) (Sigma-Aldrich/USA).
Extended-spectrum beta-lactamases (ESBLs) phenotypic detection
The double-disk synergy test (DDST) was applied to detect ESBL producers among isolates. A 0.5 McFarland standard suspension of the test organism was applied to a Mueller-Hinton agar (MHA) (Merck, Germany) plate using a sterile swab. Two sets of discs containing CAZ (30 µg) /CAZ- Clavulanic acid (30/10 µg) and CTX (30 µg) /CTX-Clavulanic acid (30/10 µg) were placed on the plate and then incubated at 35–37 °C for 16–18 h. A ≥ 5-mm increase in zone diameter for either antimicrobial agent tested in combination with clavulanate versus the zone diameter of the agent when tested alone was considered positive17.
The modified Hodge test (MHT)
The carbapenemase enzyme was identified among the isolates using the MHT in compliance with CLSI guideline18. The control positive, control negative, and indicator organisms employed were K. pneumoniae ATCC BAA-1705, K. pneumoniae ATCC BAA-1706, and E. coli ATCC 25,922, respectively. A 0.5 McFarland standard suspension of E. coli ATCC 25,922, a strain sensitive to carbapenem, was prepared in sterile normal saline. It was then diluted 1:100 and cultured as a lawn on a MHA plate. After placing a meropenem disc in the center of the plate, 3–5 colonies of the test organism were spread from the edge of the disc to the periphery of the plate. The plate was then incubated at 35–37 °C for 16–18 h.
DNA extraction
The salting out method was employed to extract DNA, as previously described19. Briefly, 3–5 colonies of A. baumannii were introduced into 5 ml of Luria-Bertani (LB) broth (Merck, Germany) and incubated at 37 °C for 18 h. The cells were then pelleted by centrifuging 1.5 ml of the suspension at 4000 × g for 10 min. After discarding the supernatant, the pellets were resuspended in 567 µl of TE buffer. To achieve a final concentration of 100 µg/ml of proteinase K (SinaClon, Iran) in 0.5% sodium dodecyl sulfate (SDS), 30 µl of 10% SDS and 3 µl of 20 mg/ml proteinase K were added to the suspension. After thorough mixing, the suspension was incubated at 37 °C for one h. Following this, 100 µl of 5 M NaCl was added and thoroughly mixed. 80 µl of CTAB/NaCl solution were added, vigorously mixed, and then incubated at 65 °C for 10 min. After adding approximately an equal volume of chloroform/isoamyl alcohol and thoroughly mixing the solution, it was centrifuged at 6000 × g for 10 min. The aqueous phase was transferred to a new tube. Equal amounts of phenol/chloroform/isoamyl alcohol were added, thoroughly mixed, and then centrifuged for 5 min. The supernatant was transferred to a new tube, and then isopropanol (0.6 volumes) was added to precipitate the DNA. After washing the precipitate with 70% ethanol, the supernatant was removed and dried. The resuspended DNA participate in 100 µl of TE buffer was then stored at -20 °C.
PCR resistance gene detection
We used the primer sequences displayed in Table 1 to investigate the presence of the blaOXA− 51−like gene among the isolates, confirming their belonging to the A. baumannii species. Additionally, conventional PCR was used with the primers listed in Table 1 to identify the resistance genes blaOXA− 23, blaOXA− 40, blaOXA− 58, blaOXA− 143, blaTEM, blaSHV, blaCTX−M, blaGES, blaPER− 1, blaVEB− 1, blaIPM, blaGIM, blaSIM, blaVIM, blaNDM− 1, int-1, int-2, int-3, ISAba-1 and mcr-1 among the isolates. The thermal cycling conditions were as follows: The process started with an initial denaturation at 95 °C for 3 min, followed by 35 cycles at 95 °C for 30 s, annealing at the temperatures specified for each gene (listed in Table 1) for 1 min, and a final extension at 72 °C for 7 min. Following electrophoresis on a 1.2% agarose gel (Product No. A-6013, Sigma Aldrich), the amplicons were visualized using a UV-gel doc (UVP BioDoc-It™ Imaging System, Cambridge, UK).
Assessment of biofilm production
The ability of the isolates to produce biofilms was evaluated using a microtiter plate assay, as previously described by Stepanovic et al.20. Briefly, the isolates were grown on nutrient agar and incubated at 37 °C overnight. 180 µL of fresh LB broth was mixed with 20 µL of 0.5 McFarland-adjusted bacterial suspensions before being placed into wells. The plate was then incubated at 37 °C overnight. The only substance used in the wells for negative controls was LB medium. The wells were rinsed three times with normal saline solution, fixed with 150 µL of methanol for 20 min, and then stained with 200 µL of 0.1% crystal violet for 20 min at room temperature. To remove excess dye, the wells were washed repeatedly with normal saline. They then underwent a 20-min incubation in 200 µL of 95% ethanol. A microtiter-plate reader (ChroMate 4300) was used to measure the optical density (OD) at 570 nm for each well. The experiment was conducted in triplicate. The following criteria were applied to divide the isolates into four groups. OD cutoff = Mean OD negative control + (3 × Standard Deviation negative control). Mean OD ≤ OD cutoff = no biofilm producer; OD cutoff < Mean OD ≤ 2×OD cutoff = weak biofilm producer; 2×OD cutoff < Mean OD ≤ 4×OD cutoff= moderate biofilm producer; 4×OD cutoff < Mean OD = strong biofilm producer.
Phylogenetic analysis
The evolutionary relationships among the isolates were demonstrated using MLVA analysis with 8-loci variable number of tandem repeat (VNTR), following the procedure outlined by Pourcel et al.21. We used conventional PCR with the primer pairs listed in Table 2 to amplify VNTRs containing tandem repeats. The resulting amplicons were then electrophoresed on a 1.2% agarose gel (Sigma-Aldrich/USA). To achieve more precise band segregation of loci Abaum_0826, Abaum_0845, Abaum_2396, and Abaum_3468 we performed electrophoresis using 2% agarose and 2% metaphor powder (Biozym, Cambrex Bio Science Rockland, ME USA). The molecular mass of each amplicon was divided by its corresponding repeat unit after deducting the weight of the flanking region, in order to assign the copy numbers to their respective loci. Table 2 also provides the weights assigned to the flanking region and repeat unit for each locus. Subsequently, a dendrogram and minimum spanning tree (MStree) were constructed using the unweighted pair group method with arithmetic mean (UPGMA) algorithm in BioNumerics 7.1 software, developed by Applied Maths in Belgium.
Results
Study population
There were 16 A. baumannii isolates from wave 3, 18 isolates from wave 4, and 16 isolates from wave 5 of COVID-19 pandemic. A total of 46 A. baumannii isolates were obtained from tracheal aspirate samples, while 4 were obtained from blood cultures. Thirty two of the examined patients in this study were males and 18 were females. Wave 3 consisted of 6 female patients and 10 male patients, ranging in age from 48 to 92 years old. Wave 4 included 5 female patients and 13 male patients, with ages ranging from 23 to 81 years old. Wave 5 comprised 7 female patients and 9 male patients, aged between 56 and 89 years old. The minimum and maximum ages of the patients were 23 and 92, respectively.
Antibiotic susceptibility pattern
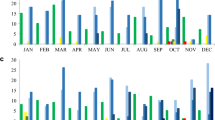
Out of 50 isolates, 48 were multidrug resistant (MDR), as determined by DDM and MIC findings. All the isolates in DDM exhibited resistance to CTX, CRO, and FEP. 98% (49/50) of the isolates showed resistance to PIP, TZP, CAZ, and CIP. Resistance to LVX and TM was detected in 76% (38/50) of the isolates. The percentages of resistance exhibited by the isolates against TMP-SXT, GM, and AN were 68% (34/50), 96% (48/50), and 84% (42/50), respectively. Figure 1 illustrates the comprehensive antibiotic susceptibility profile of the isolates generated using DDM. In the MIC test, all of the isolates were resistant to IPM, 98% (49/50) were resistant to MEM, 68% (34/50) were resistant to CL, and 20% (10/50) were resistant to PB. Accordingly, all isolates were identified as CRAB. Figure 2 shows the susceptibility profile of the isolates determined by the MIC.
ESBLs and carbapenemase producers
20% (10/50) of isolates were able to produce ESBLs according to the DDST findings. When interpreting the presence of a clover leaf as a positive outcome in the MHT, 98% (49/50) of the isolates were carbapenemase producers.
Distribution of resistance genes
All 50 isolates were discovered to possess the blaoxOXA−51−like gene, confirming that they belong to the A. baumannii species. All isolates harbored blaOXA−40, ISAba-1, and int-2 genes. There were no isolates found that have the blaOXA−58, blaOXA−143, blaVEB−1, blaVIM, and int-3 genes. All isolates carried at least one of the examined ESBL genes. Among ESBL genes, blaCTX−M, blaTEM, blaSHV, blaGES, and blaPER−1 have a prevalence of 42% (21/50), 84% (42/50), 58% (29/50), 78% (39/50), and 54% (27/50), respectively. 74% (37/50) of the isolates had the blaOXA−23 gene, while all of the isolates carried the blaOXA−40 gene. Among MBL genes, blaIPM, blaGIM, blaSIM, and blaNDM−1 have a prevalence of 20% (10/50), 8% (4/50), 22% (11/50), and 60% (30/50), respectively. The prevalence of int-1 and mcr-1 was documented as 74% (37/50) and 6% (3/50), respectively. The co-existence of blaOXA−23/int-1 was observed in 52% (26/50) of the isolates. The co-existence of blaOXA−23/int-2 and blaOXA−23/isaba-1 was 74% (37/50). The co-existence of blaNDM−1/ISAba-1 was observed in 30 (60%) isolates. Figure 3 presents a detailed profile of each isolate’s resistant genes.
Clonal relationship of the isolates. The dendrogram depicts the phylogenetic relationship of the isolates. Cluster A consists of nine isolates from wave 3 with 93.2% similarity, while Cluster B consists of 41 isolates from waves 3, 4, and 5 with 83.8% similarity. The blaOXA−51−like gene, specific to A. baumannii species, was present in all isolates but is not displayed on the dendrogram. The blaOXA−58, blaOXA−143, blaVEB−1, blaVIM, and int-3 genes, which were absent in all isolates, are also not shown.
Biofilm production profile
Out of the total isolates, 76% (38/50) were incapable of producing biofilm, whereas just one isolate showed a strong capacity to generate biofilm. In wave 3, 22% (11/50) of the isolates did not form biofilms, 4% (2/50) were weak biofilm formers, 4% (2/50) were moderate biofilm formers, and 2% (1/50) were strong biofilm formers. Wave 4 isolates exhibited the following characteristics: 28% (14/50) did not produce biofilms, 2% (1/50) formed weak biofilms, and 6% (3/50) formed moderate biofilms. Wave 5 isolates exhibited the following characteristics: 26% (13/50) did not produce biofilms, 4% (2/50) formed weak biofilms, and 2% (1/50) formed moderate biofilms.
Evolutionary relationship
By applying a similarity threshold of 80% to the dendrogram (Fig. 3), it was observed that all isolates were grouped into two distinct clusters. Cluster A consists of 9 isolates from wave 3, while Cluster B consists of 41 isolates from waves 3, 4, and 5. The MStree (Fig. 4) shows that isolates number 6 and 49 from wave 3 are the origins of the isolates obtained in wave 4 within pathway one. In pathway two, isolates number 5 and 58 from wave 3 are identified as the sources of isolates obtained in wave 4. The isolates identified in pathway one during wave 5 originated from isolates 113, 115, 108, 125, 139, and 150 from wave 4. Isolates 131, 110, 120, 138, 152, and 98 from wave 4 are identified as the origins of isolates obtained in wave 5 in pathway two.
Distribution routes of the isolates. The tree analysis indicates that there is a strong likelihood of isolates spreading through the two paths shown in the figure. Pathway one: The sources of the isolates collected in wave 4 were the isolates 6 and 49 from wave 3. The sources of the isolates collected in wave 5 were the isolates 113, 115, 108, 125, 139, and 150 from wave 4. Pathway two: The sources of the isolates collected in wave 4 were the isolates number 5 and 58 from wave 3. The sources of the isolates collected in wave 5 were the isolates 131, 110, 120, 138, 152, and 98 from wave 4. The A. baumannii isolates ID is represented by numbers on the nodes. 100% identical isolates are represented by numbers on a single node. The size of each node corresponds to the number of connected isolates. Nodes that differ by 1 locus are connected with a short, thick line, while nodes that differ by 2, 3, or 4 loci are connected with longer, thin lines. Nodes that differ by 4 loci are connected by a dotted line.
Discussion
The global incidence of CRAB has been on the rise, making it a significant and challenging nosocomial pathogen in the context of critically ill individuals. In some cases, it may even be necessary to temporarily close the ICU5.
During the COVID-19 pandemic, many healthcare systems, including those in Iran, experienced significant strain due to the high number of patients and the increased workload on laboratory staff. This situation often led to challenges in conducting comprehensive antimicrobial resistance surveillance, including the identification of CRAB isolates. Nevertheless, CRAB outbreaks have been well-documented in various countries, particularly in ICU settings, both before and during the COVID-19 pandemic. A study conducted in Italy during the COVID-19 pandemic reported a notable increase in CRAB infections among ICU patients. The study highlighted the challenges posed by the pandemic, including increased patient load and limited infection control resources, which contributed to the spread of CRAB22. One notable example occurred in Berlin, Germany, where three distinct CRAB outbreaks were identified in five ICUs between August 2020 and March 2021. These outbreaks affected 26 patients, with varying degrees of infection and colonization. Factors contributing to these outbreaks included shared patient rooms and the use of medical devices such as bronchoscopes23. In Iran, similar to other countries, the COVID-19 pandemic placed unprecedented demands on healthcare facilities. The high workload of laboratory staff and the urgent need to manage COVID-19 patients often limited the capacity to perform detailed antimicrobial resistance testing. Despite these challenges, previous studies in Iran have reported the presence of CRAB in healthcare settings. For instance, a study conducted before the pandemic found that 96.1% of A. baumannii isolates from ICU patients were carbapenem-resistant24. This indicates that CRAB was already a concern in Iranian hospitals before the additional burden of the pandemic.
Given the notable disparity in biofilm formation rates between our study and others, like the Krzyściak study25 where 80–98% of A. baumannii isolates were found to be biofilm producers, several factors could account for this inconsistency: (i) The COVID-19 patients in our study had a relatively short period of hospitalization in the ICU. Previous research has shown that the duration of hospitalization can influence the extent of biofilm formation by A. baumannii. Longer hospital stays may provide bacteria with more time and opportunities to form biofilms on medical devices and within the host. In contrast, the shorter stays in our study could have limited the bacteria’s ability to establish robust biofilms. A review by Del Pozo et al.26. supports the idea that prolonged exposure to the hospital environment and medical devices is associated with increased biofilm production. (ii) Various studies use different methods to evaluate biofilm production capability, and these methodological differences can significantly impact the results. The microtitre plate assay, while widely used, can vary in its execution. Factors such as the choice of staining agent (e.g., crystal violet, safranin), the duration of incubation, and the criteria for defining biofilm producers can lead to different outcomes. For example, a study by Peeters et al.27. highlights how these variations can affect the quantification of biofilms. (iii) The conditions under which bacteria are cultured can influence biofilm formation. Differences in nutrient composition, temperature, oxygen levels, and the presence of shear forces can all affect the ability of A. baumannii to form biofilms. A study by Stepanović et al.28. demonstrates how variations in culture conditions can lead to different biofilm formation capabilities among bacterial isolates. (iv) Different studies may use distinct statistical methods and interpretative criteria to analyze biofilm production. The thresholds for categorizing strong, moderate, weak, and non-biofilm producers can vary, leading to differences in reported prevalence rates. A study by Azeredo et al.29. provides insights into how different interpretative criteria can influence the classification of biofilm-forming bacteria. In light of these points, we propose that the lower biofilm formation rate observed in our study is likely influenced by above-mentioned factors. Future research could benefit from adopting standardized protocols and considering these variables to facilitate more direct comparisons across different studies.
In the context of COVID-19, respiratory and bloodstream infections are associated with higher morbidity and mortality, making them a priority for investigation30. During our study period, there were no A. baumannii isolates obtained from other body sites such as urine or wounds. This could be attributed to the focused nature of sampling in patients with severe pulmonary manifestations of COVID-19, as well as the resource limitations and high workload in the laboratory during the pandemic. Similar trends were observed in other countries heavily impacted by COVID-19. For instance, Adachi et al.31 reported that the majority of A. baumannii infections in COVID-19 patients were from respiratory and blood samples, with few isolates from other sites.
It is noteworthy that no CRAB outbreaks had been reported in the hospital before our study commenced. This absence of prior outbreaks suggests that our findings are likely not influenced by recent historical outbreaks and reflect the true incidence and risk factors for CRAB in the hospital setting. Our study, therefore, provides a novel insight into the epidemiology of CRAB in this clinical environment, emphasizing the need for continued surveillance and proactive infection control measures even in the absence of recent outbreaks.
According to our findings, the MHT data indicated that 98% of the isolates exhibit carbapenemase production. This finding is consistent with the results of PCR screening for carbapenemase-producing genes. On the other hand, the DDST test yielded positive results for only 20% of the isolates. However, the PCR results showed that each isolate had at least one ESBL gene. Previous studies have utilized clavulanic acid as an inhibitor and identified ESBL production in 2.08%, 4%, 21.4%, 28%, and 44% of Acinetobacter species isolates32,33,34,35,36. The prevalence of ESBL-producing Acinetobacter species varies significantly among different geographical areas and hospitals, as demonstrated in this study and other research. In certain studies, sulbactam was utilized as an inhibitory agent and detected ESBLs in 77% and 75% of the Acinetobacter isolates36,37. A significant difference (p < 0.01) in ESBL production rates was observed when comparing the two detection methods. This could be due to the presence of other resistance mechanisms in Acinetobacter species, which may obscure the detection of ESBL activity. This organism possesses chromosomally encoded inducible AmpC cephalosporinases capable of hydrolyzing all beta-lactam antibiotics. AmpC-producing organisms can act as concealed reservoirs for ESBLs. These isolates may exhibit high levels of AmpC enzymes when subjected to the clavulanic acid inhibition test. This can impede the inhibition of ESBLs, potentially resulting in a false negative outcome.
The occurrence of ESBL genes in CRAB differs across various geographical areas38. Farajnia et al.39 reported that 70% of A. baumannii isolates in the North-West of Iran produced ESBLs. A research conducted in Ethiopia revealed that between 45% and 67% of A. baumannii isolates obtained from both human and environmental origins exhibited ESBL production. Another research performed in Saudi Arabia by Alyamani et al., revealed that 94% of A. baumannii isolates were identified as ESBL producers40. Remarkably, all of the isolates obtained in our investigation harbored at least one ESBL gene.
The oxa genes in A. baumannii are important because they encode oxacillinases that break down carbapenems. These genes can be easily transferred through plasmids, spreading quickly in healthcare settings and among bacteria41,42. The PCR analysis demonstrated that the majority of the isolates had at least one oxa gene. Besides, the MIC data suggested that almost all of the isolates exhibited resistance to IPM or MEM. Based on this data, we have concluded that the prescription of carbapenem antibiotics was not effective and may have actually contributed to the increasing incidence of these isolates through selective pressure. A nationwide study conducted in Italy revealed elevated levels of blaOXA−23gene among A. baumannii isolates (81.7%) in ICUs, and surgical wards43. Atrouni et al., in Lebanon also revealed the widespread presence of blaOXA−23-producing CRAB strains (68.90%) in different hospitals44. Another investigation by Schuertz et al., found that blaOXA−23-producing strains of A. baumannii were associated with a 73.91% mortality rate45. Interestingly, A. baumannii strains linked to increased mortality rates in ICU patients have shown the coexistence of blaOXA−23 and ISAba-1. This implies that the severity of the disease may be exacerbated by the simultaneous presence of these genetic components46. The presence of ISAba-1 upstream of the blaOXA−23 gene increases its expression, leading to increased resistance to carbapenem antibiotics47. A study in Nepal found that all A. baumannii isolates carried both the ISAba-1 gene and the blaOXA−23 gene48. Additionally, a study by Huang et al.., in China revealed that 88.9% of the A. baumannii isolates carried blaOXA−23/ISAba-149. Similarly, we observed the presence of both blaOXA−23/ISAba-1in 74% of the isolates.
The frequency of blaNDM−1 in A. baumannii varies in research. Some studies indicate that 22% of isolates have blaNDM−1 among all species of Acinetobacter50, while other studies report that 13.6% of A. baumannii contain the blaNDM−1 gene48. In comparison, our analysis revealed a high prevalence of the blaNDM−1 gene (60%). The high prevalence of blaNDM−1 in our investigation is substantiated by the co-existence of blaNDM−1/ISAba-1, which is linked to the dissemination of A. baumannii isolates that carry blaNDM−1. Additionally, this implies the potential for a horizontal gene transfer48,50.
The global prevalence of colistin resistance among A. baumannii isolates varies, but it is generally lower than the 68% found in our study. For instance, a study from Egypt reported a colistin resistance rate of 52.9% in CRAB isolates51. Studies from other regions have shown lower resistance rates, such as 11.6% in Iran52 and 5–10% in countries like India and the United States53. However, resistance rates as high as 56% have been observed in Greece51, indicating that high resistance levels are not unprecedented, especially in countries facing antibiotic overuse and healthcare-associated infections. Previous reports from Iran showed varying levels of resistance. For example, a study conducted by Vakili et al. found only 11.6% colistin resistance in ICU isolates of A. baumannii52, while another study from Gorgan reported 94.36% of isolates being susceptible to colistin, though 20.45% exhibited heteroresistance53. These findings suggest that while colistin resistance is generally lower than our finding of 68%, there is still a concerning trend of increasing resistance, particularly in ICU settings during the COVID-19 pandemic.
Our molecular investigation revealed the presence of the mcr-1 gene in 3 out of 50 (6%) A. baumannii isolates. This finding is significant, as mcr-1 is a plasmid-mediated colistin resistance gene that has been infrequently reported in A. baumannii globally. The detection of mcr-1 in our isolates, albeit at a relatively low prevalence, suggests a concerning potential for horizontal transfer of colistin resistance among bacterial populations in our setting. Our results contrast with some previous studies, such as one from Egypt where only 1% of A. baumannii isolates were positive for mcr-154. However, they align more closely with findings from Pakistan, where mcr-1 was detected in 1.61% of total A. baumannii isolates and 16.6% of colistin-resistant isolates55.
Given the high resistance to polymyxins observed in our study, combination therapies might indeed offer a viable alternative for treating CRAB infections. Wistrand-Yuen et al. reported that combining polymyxin B with fosfomycin or rifampicin can result in synergistic effects against resistant Gram-negative pathogens56. While there is not much data available on CRAB specifically, these results indicate that combining polymyxins with either fosfomycin or rifampin could enhance treatment effectiveness for patients with CRAB infections. Considering the significant levels of polymyxin resistance we identified, these combinations may be particularly beneficial in situations where treatment choices are restricted.
The prevalence of the int-1 gene in A. baumannii isolates obtained from Iranian patients was found to be 55.2% in a systematic review and meta-analysis57. A different investigation performed in Isfahan, Iran revealed that 63.9% and 78.2% of the A. baumannii isolates harbored int-1 and int-2, respectively58. Our research found high prevalence rates for the int-1 and int-2 genes, with int-1 at 74% and int-2 at 100%.
The information presented on the dendrogram indicates that the ICU ward experienced a clonal transmission of A. baumannii during all three pandemic peaks. The isolates belonging to cluster B exhibit a considerable degree of similarity, including the majority of the isolates. In other words, the most dominant clone in this cluster has effectively established itself in the ICU. However, considering that the majority of the isolates were incapable of generating biofilm, it seems that the transmission occurred by other means. Some likely factors include extended use of immunomodulatory medications, employing non-ICU nurses due to overcrowding in ICUs, less compliance with infection control procedures, and challenges adhering to stringent personal protective equipment (PPE) regulations. The 74.5% genetic similarity between clusters A and B may be due to the lower amount of empirical antibiotic therapy prescribed at the beginning of the third wave of the pandemic.
The information provided in the MStree is crucial for identifying where the connector isolates are located between the waves. Disrupting the transmission cycle can be achieved by identifying and correcting any deficiencies in the hospital’s antibiotic stewardship and infection prevention programs by closely examining the characteristics of these connector isolates, including their antibiotic susceptibility pattern, prescribed antibiotics, isolation date, etc. Maciel et al., conducted a study to investigate the clonal dissemination of CRAB in a neonatal intensive care unit (NICU). Their research provided insights into the potential association between A. baumannii colonization and infection in NICUs59. A molecular epidemiology analysis of A. baumannii strains from patients and environmental contamination in a neurosurgical ICU in a Chinese hospital showed genetic similarities, suggesting possible clonal transmission within the ICU60. In 2021, Boral et al.., studied the relationship between A. baumannii infections and the COVID-19 pandemic in an ICU in Turkey. They found clonal isolates with a clonality rating of 85% or higher, suggesting potential A. baumannii transmission within ICUs during the pandemic61.
Research indicates that A. baumannii is highly resilient and can survive in hospital settings, contributing to outbreaks through environmental reservoirs such as surfaces and medical devices. The ability of this pathogen to form biofilms enhances its survival and complicates eradication efforts62,63. Unfortunately, despite our best efforts, we were unable to obtain non-clinical samples. This was due to several factors, such as the excessive use of disinfectants and the implementation of strict regulations resulting from quarantine restrictions during the COVID-19 pandemic. Future studies should prioritize environmental sampling to better understand the dynamics of A. baumannii in hospital settings and its potential contributions to infection spread. By addressing these environmental reservoirs, we can develop more effective infection control strategies and treatment protocols. We did not screen the isolates for genes that confer resistance to aminoglycosides, quinolones, and sulfonamides. This information could provide better insight into the isolates’ antibacterial resistance profile. In the future, we will focus on identifying the factors that contribute to the spread of the connector isolates, as indicated on the MStree graph.
Based on our study results, we recommend using molecular typing methods such as MLVA in healthcare settings to identify dominant clones, their antibiotic resistance patterns, and transmission routes. This will help in effectively managing the emergence and proliferation of antibiotic-resistant strains through the administration of suitable antibiotics.
Conclusion
CRAB outbreaks in ICUs present a significant challenge to healthcare facilities, particularly during public health crises like the COVID-19 pandemic. In this study, we investigated the prevalence and genetic characteristics of CRAB isolates among ICU-admitted patients across three waves of the pandemic in Iran by MLVA. Our findings underscore the pervasive antibiotic resistance profiles among the 50 A. baumannii isolates collected from tracheal aspirate and blood culture samples, revealing widespread resistance to multiple antibiotics, including carbapenems and fluoroquinolones. The genetic analysis highlighted the prevalence of blaOXA−40, ISAba-1, and int-2 genes in all isolates, with significant proportions carrying ESBL genes such as blaCTX−M, blaTEM, blaSHV, blaGES, and blaPER−1. Using MLVA, we identified two distinct clusters (A and B) with varying distribution across different pandemic waves. The predominance of cluster B across waves 3, 4, and 5 indicates clonal transmission within the ICU environment. Our study emphasizes the critical role of molecular typing methods such as MLVA in healthcare settings to characterize dominant clones, understand antibiotic resistance patterns, and trace transmission routes of CRAB. These insights are pivotal for implementing targeted infection control measures and optimizing treatment strategies to mitigate future outbreaks of antibiotic-resistant pathogens in ICU settings.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CRAB:
-
Carbapenem-resistant Acinetobacter baumannii
- MLVA:
-
Multiple-locus variable number tandem-repeat analysis
- ICU:
-
Intensive care unit
- ESBL:
-
Extended-spectrum beta-lactamases
- MStree:
-
Minimum spanning tree
- MBl:
-
Metallo-beta-lactamases
- VAP:
-
Ventilator-associated pneumonia
- CDC:
-
Centers for disease control and prevention
References
Saleh Ahmed, M., Abdulrahman, Z. F. A. & Taha, Z. M. A. Risk factors of clonally related, multi, and extensively drug-resistant Acinetobacter baumannii in severely Ill COVID-19 patients. Can. J. Infect. Dis. Med. Microbiol. https://doi.org/10.1155/2023/3139270 (2023).
Blanco, N. et al. Risk factors and outcomes associated with multidrug-resistant Acinetobacter baumannii upon intensive care unit admission. Antimicrob. Agents Chemother. https://doi.org/10.1128/aac.01631-17 (2018).
Jung, J. Y. et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect. Dis. 10, 1–11. https://doi.org/10.1186/1471-2334-10-228 (2010).
Sheng, W.-H. et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int. J. Infect. Dis. 14, e764–e769. https://doi.org/10.1016/j.ijid.2010.02.2254 (2010).
Ben-Chetrit, E. et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: long-term impact for the ICU and hospital. Crit Care. 22, 1–10. https://doi.org/10.1186/s13054-018-2247-y (2018).
C.f.D. Control, Prevention, CDC’s antibiotic resistance threats in the United States, 2019, Centers for Disease Control and Prevention, Atlanta, GA. (2019)
Viehman, J. A., Nguyen, M. H. & Doi, Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 74, 1315–1333. https://doi.org/10.1007/s40265-014-0267-8 (2014).
Giannella, M. & Viale, P. Treating carbapenem-resistant Acinetobacter baumannii infections. Lancet Infec.t Dis. https://doi.org/10.1016/S1473-3099(23)00203-7 (2023).
Genovese, C. et al. Molecular epidemiology of antimicrobial resistant microorganisms in the 21th century: a review of the literature. Acta bio-medica. 91, 256. https://doi.org/10.23750/abm.v91i2.9176 (2020).
Lipworth, S. et al. Molecular epidemiology and antimicrobial resistance phenotype of paediatric bloodstream infections caused by Gram-negative bacteria. Commun. Med. 2, 101. https://doi.org/10.1038/s43856-022-00161-0 (2022).
Benitez, A. J. et al. Multilocus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical isolates from 1962 to the present: a retrospective study. J. Clin. Microbiol. 50, 3620–3626. https://doi.org/10.1128/JCM.01755-12 (2012).
Dahyot, S. et al. Multiple-locus variable number tandem repeat analysis (MLVA) and tandem repeat sequence typing (TRST), helpful tools for subtyping Staphylococcus lugdunensis. Sci. Rep. 8, 11669. https://doi.org/10.1038/s41598-018-30144-y (2018).
Zhang, G. et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan China. J. Clin. Virol. 127, 104364. https://doi.org/10.1016/j.jcv.2020.104364 (2020).
Giacobbe, D.R., Battaglini, D., Ball, L., Brunetti, I., Bruzzone, B., Codda, G., Crea, F., De Maria, A., Dentone, C., Di Biagio, A. Bloodstream infections in critically ill patients with COVID-19. 50: e13319, https://doi.org/10.1111/eci.13319 (2020).
Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. ASM. 15, 55–63 (2009).
W.P.C.a.L.S.I. (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI supplement M100. 2021.
Litake, G. M., Ghole, V. S., Niphadkar, K. B. & Joshi, S. G. Phenotypic ESBL detection in Acinetobacter baumannii: A real challenge. Am. J. Infect. Dis. 11, 48. https://doi.org/10.3844/ajidsp.2015.48.53 (2015).
W.P.C.a.L.S.I. (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. 2018.
Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 56, 241–245. https://doi.org/10.1002/0471142727.mb0204s56 (2001).
Stepanović, S. et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 115, 891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x (2007).
Pourcel, C. et al. Identification of variable-number tandem-repeat (VNTR) sequences in Acinetobacter baumannii and interlaboratory validation of an optimized multiple-locus VNTR analysis typing scheme. J. Clin. Microbiol. 49, 539–548. https://doi.org/10.1128/jcm.02003-10 (2011).
D. Mangioni, V. Fox, L. Chatenoud, M. Bolis, N. Bottino, L. Cariani, F. Gentiloni Silverj, C. Matinato, G. Monti, A. Muscatello, A. Teri, L. Terranova, A. Piatti, A. Gori, G. Grasselli, N. Stocchetti, C. Alteri, Genomic Characterization of Carbapenem-Resistant Acinetobacter baumannii (CRAB) in Mechanically Ventilated COVID-19 Patients and Impact of Infection Control Measures on Reducing CRAB Circulation during the Second Wave of the SARS-CoV-2 Pandemic in Milan, Italy. 11: e0020923, https://doi.org/10.1128/spectrum.00209-23 (2023).
Schlosser, B. et al. Risk factors for transmission of carbapenem-resistant Acinetobacter baumannii in outbreak situations: results of a case-control study. BMC Infect. Dis. 24, 120. https://doi.org/10.1186/s12879-024-09015-7 (2024).
Shoja, S. et al. Genotyping of carbapenem resistant Acinetobacter baumannii isolated from tracheal tube discharge of hospitalized patients in intensive care units Ahvaz, Iran. Iran. J. Microbiol. 5, 315–322 (2013).
Krzyściak, P., Chmielarczyk, A., Pobiega, M., Romaniszyn, D. & Wójkowska-Mach, J. Acinetobacter baumannii isolated from hospital-acquired infection: Biofilm production and drug susceptibility. APMIS. 125, 1017–1026. https://doi.org/10.1111/apm.12739 (2017).
Del Pozo, J. L. Novel treatment dynamics for biofilm-related infections. Expert Rev. Anti-infect. Ther. 19, 1443–1456. https://doi.org/10.1080/14787210.2021.1917993 (2021).
Peeters, E., Nelis, H. J. & Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods. 72, 157–165. https://doi.org/10.1016/j.mimet.2007.11.010 (2008).
Stepanović, S. et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 115, 891–899. https://doi.org/10.1111/j.1600-0463.2007.apm_630.x (2007).
Azeredo, J. et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 43, 313–351. https://doi.org/10.1080/1040841x.2016.1208146 (2017).
Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region Italy. JAMA. 323, 1574–1581. https://doi.org/10.1001/jama.2020.5394 (2020).
Adachi, Y. et al. Predicting recurrence of respiratory failure in critically ill patients with COVID-19: A preliminary study. J. Infect. 82, e33–e35. https://doi.org/10.1016/j.jinf.2021.01.016 (2021).
Jazani, N., Babazadeh, H., Sohrabpour, M., Zartoshti, M. & Ghasemi Rad, M. The prevalence of extended spectrum beta-lactamases in Acinetobacter baumannii isolates from burn wounds in Iran. Internet J. Microbiol. 9, 1–7 (2011).
Bhattacharjee, A., Sen, M., Prakash, P., Gaur, A. & Anupurba, S. Increased prevalence of extended spectrum βlactamase producers in neonatal septicaemic cases at a tertiary referral hospital. Indian J. Med. Microbiol. 26, 356–360 (2008).
Sinha, M., Srinivasa, H. & Macaden, R. Antibiotic resistance profile & extended spectrum beta-lactamase (ESBL) production in Acinetobacter species. Indian J. Med. Res. 126, 63–67 (2007).
Hashemizadeh, Z., Emami, A. & Rahimi, M. Acinetobacter antibiotic resistance and frequency of ESBL-producing strains in ICU patients of Namazi Hospital (2008–2009). J. Inflam. Dis. 14, 47–53 (2010).
Singla, P., Sikka, R., Deeep, A., Gagneja, D. & Chaudhary, U. Co-production of ESBL and AmpC β-lactamases in Clinical isolates of A. baumannii and A. lwoffii in a tertiary care hospital from Northern India. J. Clin. Diagnos. Res. JCDR. 8, 16. https://doi.org/10.7860/JCDR/2014/8008.4289 (2014).
Kansal, R., Pandey, A. & Asthana, A. K. β-lactamase producing Acinetobacter species in hospitalized patients. Indian J. Pathol. Microbiol. 52, 456–457. https://doi.org/10.4103/0377-4929.55035 (2009).
Al-Sheboul, S. A. et al. Molecular characterization of carbapenem-resistant Acinetobacter baumannii isolated from intensive care unit patients in Jordanian hospitals. Antibiotics. 11, 835. https://doi.org/10.3390/antibiotics11070835 (2022).
Farajnia, S. et al. Prevalence of PER and VEB type extended spectrum betalactamases among multidrug resistant Acinetobacter baumannii isolates in North-West of Iran. Iran. J. Basic Med. Sci. 16, 751 (2013).
Alyamani, E. J. et al. Molecular characterization of extended-spectrum beta-lactamases (ESBLs) produced by clinical isolates of Acinetobacter baumannii in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 14, 1–9. https://doi.org/10.1186/s12941-015-0098-9 (2015).
Colquhoun, J. M. et al. OXA-23 β-lactamase overexpression in Acinetobacter baumannii drives physiological changes resulting in new genetic vulnerabilities. MBio. 12, e03137-e3221. https://doi.org/10.1128/mBio.03137-21 (2021).
Brown, S. & Amyes, S. OXA β-lactamases in Acinetobacter: The story so far. J. Antimicrob. Chemother. 57, 1–3. https://doi.org/10.1093/jac/dki425 (2006).
Principe, L. et al. Epidemic diffusion of OXA-23-producing Acinetobacter baumannii isolates in Italy: Results of the first cross-sectional countrywide survey. J. Clin. Microbiol. 52, 3004–3010. https://doi.org/10.1128/JCM.00291-14 (2014).
Al Atrouni, A. et al. Wide spread of OXA-23-producing carbapenem-resistant Acinetobacter baumannii belonging to clonal complex II in different hospitals in Lebanon. Int. J. Infect. Dis. 52, 29–36. https://doi.org/10.1016/j.ijid.2016.09.017 (2016).
Schuertz, K. F. et al. Bacteremia and meningitis caused by OXA-23-producing Acinetobacter baumannii–molecular characterization and susceptibility testing for alternative antibiotics, Braz. J Microbiol. 49, 199–204. https://doi.org/10.1016/j.bjm.2018.04.002 (2018).
da Silva, K. E. et al. A high mortality rate associated with multidrug-resistant Acinetobacter baumannii ST79 and ST25 carrying OXA-23 in a Brazilian intensive care unit. PloS one. 13, e0209367. https://doi.org/10.1371/journal.pone.0209367 (2018).
Turton, J. F. et al. The role of IS Aba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 258, 72–77. https://doi.org/10.1111/j.1574-6968.2006.00195.x (2006).
Joshi, P. R. et al. Co-existence of bla OXA-23 and bla NDM-1 genes of Acinetobacter baumannii isolated from Nepal: Antimicrobial resistance and clinical significance. Antimicrob. Resist. Infect. Control. 6, 1–7. https://doi.org/10.1186/s13756-017-0180-5 (2017).
Huang, Z.-Y. et al. Co-existence of bla OXA-23 and bla VIM in carbapenem-resistant Acinetobacter baumannii isolates belonging to global complex 2 in a Chinese teaching hospital. Chin. Med. J. 132, 1166–1172. https://doi.org/10.1097/CM9.0000000000000193 (2019).
Chatterjee, S. et al. Carbapenem resistance in Acinetobacter baumannii and other Acinetobacter spp. causing neonatal sepsis: focus on NDM-1 and its linkage to IS Aba125. Front. Microbiol. 7, 1126. https://doi.org/10.3389/fmicb.2016.01126 (2016).
N.S. Fam, D. Gamal, S.H. Mohamed, R.M. Wasfy, M.S. Soliman, A.A. El-Kholy, P.G. Higgins, Molecular characterization of Carbapenem/Colistin-resistant Acinetobacter baumannii clinical isolates from Egypt by whole-genome sequencing, Infection and Drug Resistance. (2020) 4487–93,
Vakili, B. et al. Detection of colistin sensitivity in clinical isolates of Acinetobacter baumannii in Iran. J. Res. Med. Sci. J. Isfahan Univ. Med. Sci. 19, S67 (2014).
Ezadi, F., Jamali, A., Heidari, A., Javid, N. & Ardebili, A. Heteroresistance to colistin in oxacillinase-producing carbapenem-resistant Acinetobacter baumannii clinical isolates from Gorgan, Northern Iran. J. Glob. Antimicrob. Resist. 21, 380–385. https://doi.org/10.1016/j.jgar.2019.11.010 (2020).
Seleim, S. M., Mostafa, M. S., Ouda, N. H. & Shash, R. Y. The role of pmrCAB genes in colistin-resistant Acinetobacter baumannii. Sci. Rep. 12, 20951. https://doi.org/10.1038/s41598-022-25226-x (2022).
Hameed, F. et al. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: first report from Pakistan. Rev. Soc. Bras. Med. Trop. 52, e20190237. https://doi.org/10.1590/0037-8682-0237-2019 (2019).
Wistrand-Yuen, P. et al. Evaluation of polymyxin B in combination with 13 other antibiotics against carbapenemase-producing Klebsiella pneumoniae in time-lapse microscopy and time-kill experiments. Clin. Microbiol. Infect. 26, 1214–1221. https://doi.org/10.1016/j.cmi.2020.03.007 (2020).
Ghazalibina, M. et al. Prevalence of integrons and antibiotic resistance pattern in Acinetobacter baumannii isolated from clinical samples of Iranian patients: A systematic review and meta-analysis. Ethiop. J. Health Sci. https://doi.org/10.4314/ejhs.v29i5.15 (2019).
Halaji, M., Rezaei, A., Zalipoor, M. & Faghri, J. Investigation of class I II, and III integrons among Acinetobacter baumannii isolates from hospitalized patients in Isfahan, Iran, Oman. Med. J. 33, 37. https://doi.org/10.5001/omj.2018.07 (2018).
Maciel, W. et al. Clonal spread of carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. J. Hosp. Infect. 98, 300–304. https://doi.org/10.1016/j.jhin.2017.10.015 (2018).
Ying, C., Li, Y., Wang, Y., Zheng, B. & Yang, C. Investigation of the molecular epidemiology of Acinetobacter baumannii isolated from patients and environmental contamination. J. Antibiot. 68, 562–567. https://doi.org/10.1038/ja.2015.30 (2015).
Boral, J. et al. The association between Acinetobacter baumannii infections and the COVID-19 pandemic in an intensive care unit. Sci. Rep. 12, 20808. https://doi.org/10.1038/s41598-022-25493-8 (2022).
Fahy, S., O’Connor, J., Lucey, B. & Sleator, R. Hospital reservoirs of multidrug resistant acinetobacter species—the elephant in the room!. Br. J. Biomed. Sci. 80, 11098. https://doi.org/10.3389/bjbs.2023.11098 (2023).
Adewoyin, M. A. & Okoh, A. I. The natural environment as a reservoir of pathogenic and non-pathogenic Acinetobacter species. Rev. Environ. Health. 33, 265–272. https://doi.org/10.1515/reveh-2017-0034 (2018).
Bali, E. B., Acik, L. & Sultan, N. Phenotypic and molecular characterization of SHV, TEM, CTX-M and extended-spectrum beta-lactamase produced by Escherichia coli, Acinobacter baumannii and Klebsiella isolates in a Turkish hospital, Afr. J. Microbiol. Res. 4, 650–654 (2010).
Lal, P., Kapil, A., Das, B. K. & Sood, S. Occurrence of TEM & SHV gene in extended spectrum β-lactamases (ESBLs) producing Klebsiella sp. isolated from a tertiary care hospital. Indian J. Med. Res. 125, 173–178 (2007).
Kiratisin, P., Apisarnthanarak, A., Laesripa, C. & Saifon, P. Molecular characterization and epidemiology of extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates causing health care-associated infection in Thailand, where the CTX-M family is endemic. Antimicrob. Agents Chemother. 52, 2818–2824. https://doi.org/10.1128/aac.00171-08 (2008).
Poirel, L. et al. Extended-spectrum β-lactamase-producing strain of Acinetobacter baumannii isolated from a patient in France. J. Antimicrob. Chemother. 43, 157–158. https://doi.org/10.1093/jac/43.1.157 (1999).
Zafer, M. M., Al-Agamy, M. H., El-Mahallawy, H. A., Amin, M. A. & Ashour, M.S.E.-D. Antimicrobial resistance pattern and their beta-lactamase encoding genes among Pseudomonas aeruginosa strains isolated from cancer patients. Biomed. Res. Int. https://doi.org/10.1155/2014/101635 (2014).
Khorsi, K., Messai, Y., Hamidi, M., Ammari, H. & Bakour, R. High prevalence of multidrug-resistance in Acinetobacter baumannii and dissemination of carbapenemase-encoding genes blaOXA-23-like, blaOXA-24-like and blaNDM-1 in Algiers hospitals. Asian Pac. J. Trop. Med. 8, 438–446. https://doi.org/10.1016/j.apjtm.2015.05.011 (2015).
Kobs, V. C. et al. The role of the genetic elements bla oxa and IS Aba 1 in the Acinetobacter calcoaceticus-Acinetobacter baumannii complex in carbapenem resistance in the hospital setting. Rev. Soc. Bras. Med. Trop. 49, 433–440. https://doi.org/10.1590/0037-8682-0002-2016 (2016).
Amudhan, S., Sekar, U., Arunagiri, K. & Sekar, B. OXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J. Med. Microbiol. 29, 269–274. https://doi.org/10.4103/0255-0857.83911 (2011).
Handal, R. et al. Characterization of carbapenem-resistant Acinetobacter baumannii strains isolated from hospitalized patients in Palestine. Int. J. Microbiol. https://doi.org/10.1155/2017/8012104 (2017).
Hujer, K. M. et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 50, 4114–4123. https://doi.org/10.1128/aac.00778-06 (2006).
Poirel, L., Walsh, T. R., Cuvillier, V. & Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70, 119–123. https://doi.org/10.1016/j.diagmicrobio.2010.12.002 (2011).
Koeleman, J. G., Stoof, J., Van Der Bijl, M. W., Vandenbroucke-Grauls, C. M. & Savelkoul, P. H. Identification of epidemic strains of Acinetobacter baumannii by integrase gene PCR. J. Clin. Microbiol. 39, 8–13. https://doi.org/10.1128/jcm.39.1.8-13.2001 (2001).
Ploy, M.-C.C., Denis, F. O., Courvalin, P. & Lambert, T. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob. Agents Chemother. 44, 2684–2648. https://doi.org/10.1128/aac.44.10.2684-2688.2000 (2000).
Acknowledgements
The authors express their gratitude to the Vice-chancellor of Research and Technology of Hamadan University of Medical Sciences, Hamadan, Iran, for advocating this research.
Funding
This study was funded by the Vice-chancellor of Research and Technology of Hamadan University of Medical Sciences, Hamadan, IRAN, under Grant numbers: 140110279098.
Author information
Authors and Affiliations
Contributions
MYA designed and supervised the study. MYA, MA, and AB performed data interpretation; MA, SS, MSA, PM, LA, SM, and AR were responsible for data collection and doing experiments; MA analyzed the statistical results of the study; All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethics Review Board of the Hamadan University of Medical Sciences, Hamadan, Iran approved the present study (Ethical approval code: IR.UMSHA.REC.1401.783). Ethical Review Board approved informed consent taken from all the participants, and all experiments were performed in accordance with relevant guidelines and regulations.
Consent for publication
All authors and acknowledged individuals have provided their consent for publication.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Azimzadeh, M., Bahador, A., Shiralizadeh, S. et al. A single-center analysis of clonal transmission of carbapenem-resistant Acinetobacter baumannii among intensive care unit patients during the COVID-19 pandemic. Sci Rep 14, 25897 (2024). https://doi.org/10.1038/s41598-024-77238-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77238-4