Abstract
The self-healing of concrete through the use of bacteria is an ecological, sustainable, and environmentally friendly method that can replace time-consuming and costly repairs. The aim of this research was to isolate and select spore-forming bacilli with potential for concrete self-healing. Soil samples from the limestone mines of Simbal, Trujillo, La Libertad, Peru were used. These samples were processed to primarily isolate spore-forming bacteria. The self-healing potential of these bacteria was evaluated through their ability to produce the urease enzyme, grow at an alkaline pH, and produce calcium carbonate crystals in a culture medium with urea and CaCl2 at pH 8.0. The produced crystals were visualized with scanning electron microscopy and their calcium content was confirmed through EDS analysis. The isolated bacteria were identified by 16 S rRNA gene amplification by PCR as Bacillus sp and Streptomyces sp. It was observed that Bacillus sp. produces CaCO3 crystals with vaterite morphology, while Streptomyces sp. produces rhombohedral calcite crystals. It is concluded that the isolated bacteria have the potential to be used in concrete self-healing processes.
Similar content being viewed by others
Introduction
Concrete is an essential material in construction, valued for its strength and durability. However, it often faces issues such as crack formation and deterioration, which can compromise its structural integrity. In this context, research on the isolation and selection of bacteria becomes highly relevant, as concrete self-healing is an innovative process that uses bacteria to autonomously repair cracks, which can significantly prolong the lifespan of concrete structures.
The self-healing of concrete, through the precipitation of calcium carbonate by biologically induced mineralization, occurs when the metabolic activity of microorganisms creates favorable conditions for the formation and precipitation of these crystals. During this process, the cell walls facilitate the accumulation of crystals, acting as nucleation centers. The urease enzyme plays an important role in this process, as it hydrolyzes urea into ammonia and bicarbonate, which causes an increase in pH and provides the carbon ions for the precipitation of calcium carbonate1. This process is described in the following biochemical reactions:2.
In the process of carbonate precipitation by microorganisms, bacterial cells provide nucleation sites, which are very important for carbonate precipitation. The walls of the bacterial cells, which are negatively charged, act as adsorbents of divalent compounds such as calcium ions. When calcium ions are adsorbed on the bacterial cell, the reaction of the calcium cation and the carbonate anion occurs, and in this way, calcium carbonate precipitates on the surface of the bacteria2.
Previous research has explored the isolation and selection of microorganisms for concrete self-healing, identifying the bacteria S. pasturii, Bacillus subtilis and Pseudomonas putida3, Bacillus wiedmannii and Bacillus paramycoides4 and even some filamentous fungi species such as Trichoderma reesei and Aspergillus nidulans5, among which, Rhizopus oryzae, turned out to be a promising species for sealing concrete cracks with biometabolites6. These microorganisms managed to completely heal the cracks in the concrete, evidenced by the presence of calcite and CaCO3 crystals. Similarly, a medium has been used that optimizes the production of calcium carbonate, resulting in the formation of rhombohedral and spherical crystals containing vaterite and calcite6. Different methods have been used, from plate cultivation to molecular techniques, to identify bacteria with the ability to produce calcite, a mineral that can fill and repair cracks in concrete7. These studies have provided a solid foundation for the development of self-healing concrete, although there is still much to learn in this emerging field.
The self-healing of concrete through the precipitation of carbonates by microorganisms is a promising process that has emerged in the last two decades. It is presented as an innovative technology to increase the strength and sustainability of concrete structures8. However, the selection and identification of the appropriate bacteria for this process represent a challenge. This research focuses on how bacteria can be efficiently isolated and selected for use in concrete self-healing, with the aim of improving the strength and durability of concrete structures. This process seeks to identify bacteria capable of producing calcite, a mineral that can fill and repair cracks in concrete.
The research was conducted through the isolation of spore-forming bacilli from limestone soil in the district of Simbal, Trujillo, Peru, and their cultivation under controlled conditions. Subsequently, the bacteria that demonstrated the ability to produce calcite were selected. Later, these bacteria will be incorporated into concrete samples to evaluate their effectiveness in self-healing cracks. This study is of vital importance as it addresses a frequent problem in civil engineering and construction: cracks in concrete. By isolating and selecting bacteria for concrete self-healing, the strength and durability of structures can be improved, their lifespan can be extended, and maintenance and repair costs can be reduced. The importance of working with native bacteria lies in their adaptation to local environmental conditions, making them more effective in their function, ensuring constant and effective activity in concrete mixes under specific environmental conditions.
Materials and methods
Sample collection
Soil samples were collected in first-use polyethylene bags. Three samples of approximately 250 g each were obtained9 from the limestone mines of the district of Simbal, Trujillo, Peru, with UTM coordinates 17 M 0741676 9,118,679. The samples were collected aseptically at depths of 1 to 3 cm10 and transferred to the laboratory of the Dirección de Institutos y Centros de Investigación de la Universidad César Vallejo for processing.
Pasteurization of the samples
25 g of each sample were weighed and placed in flasks with 225 mL of sterile physiological saline solution (SSFe). They were then subjected to a temperature of 80 °C for 10 min in a Tom’s USA Science CDK-S24 water bath. The aim of this process was to eliminate vegetative cells and leave only spore-forming bacteria11.
Sample enrichment
To increase the number of spore-forming bacteria, 25 mL of the pasteurized sample was extracted and sown in 225 mL of nutrient broth. They were then incubated at a temperature of 20 ± 2 °C at 100 RPM in a Boeco brand orbital shaker for a period of 48 h12.
Isolation of spore-forming bacteria
In plates with sterile nutrient agar, a streak was sown from the enrichment medium aliquot and incubated at room temperature (20 ± 2 °C) for 24 to 48 h11. After the incubation period, the colonies were observed and their macroscopic characteristics, such as shape, color, size, appearance, texture, and edges (smooth or rough), among others, were recorded. A Gram stain was also performed and the microscopic characteristics, such as the shape of the bacteria, Gram staining, presence or absence of spores, and colony purity, were noted. Colonies that presented Gram-positive spore-forming bacilli were selected and streaked in tubes with Trypticase Soy Agar (TSB). The isolated bacteria were reserved for calcium carbonate production tests.
Selection of calcium carbonate-producing bacteria
Evaluation of urease production in urea agar tubes
The bacteria isolated in the previous step were sown in slanted tubes of Christensen’s urea agar, which contains: peptone (1.0 g/L), sodium chloride (5.0 g/L), glucose (1.0 g/L), potassium dihydrogen phosphate (0.8 g/L), disodium phosphate (1.2 g/L), urea (20 g/L), phenol red (0.012 g/L), and agar (15.0 g/L). The base medium, except for urea, was dissolved in distilled water and sterilized in an autoclave for 15 min at 121 °C. The urea was sterilized by filtration (20 g/L), added aseptically to the base medium, and distributed in the test tubes, which were allowed to solidify forming wedges. The bacteria were sown on the surface of the agar and incubated at 30 °C for 6–36 h. A bacterium with negative urease activity was used as a control.
After the incubation period, the degradation of urea was observed, evidenced by a color change in the culture medium. Phenol red acts as an indicator of urease activity, which causes an increase in pH, and causes the medium to change from pale yellow (negative) to pinkish red (positive) due to the increase in pH. On the other hand, the urease-negative bacteria showed no color change even after 48 h of incubation6,10,13.
Evaluation of growth under alkaline pH conditions
To evaluate the effect of pH on cell growth, flasks were prepared, each with 50 mL of 0.3% (w/v) TSB, with an initial pH of 8 and 10 (and a control of pH 6.5), The pH value was measured using a Hanna multiparameter potentiometer with an error of 0.001. They were inoculated with 1mL of approximately 1.2 × 109 cells/mL. Then, they were incubated at 30 °C in an orbital shaker at 200 rpm for 24 h. A sample was taken at the beginning and at 24 h to determine the cell density by plate count6,13.
Evaluation of the production of calcium carbonate (CaCO3) in urea-CaCl2 agar medium
The production capacity of CaCO3 was evaluated using Urea-CaCl2 agar plates (20 g/L of urea and 37 g/L of CaCl2). In these plates, each of the bacterial cultures was streaked by puncture. Subsequently, they were incubated at 30 °C for a period of 12 to 48 h and regularly observed to visualize the appearance of precipitation zones around the colonies, which correspond to the crystals of CaCO3. Microscopic observations of the colonies were made to detect polygonal crystals along with small spherical crystals6.
Tests of calcium carbonate precipitation
Flasks were prepared with 100mL of urea broth (20 g/L), supplemented with CaCl2 (37 g/L) at pH 8. They were inoculated with 1 mL of spores (1 × 109 spores/mL) and incubated at 30 °C for 48 h, with orbital agitation at 100 RPM. Once the incubation time was completed, the contents of each flask were centrifuged at 7000 rpm for 7 min. The sediment was placed on aluminum foil and dried at 50 °C for 8 h4. The morphology of the crystals (CaCO3) was characterized with the scanning electron microscope (SEM) of the Tescan brand, model Vega 3 LMU. For this, the samples were covered with a thin film of carbon fiber by sputtering for 90 s, with a current of 2.4 mA. The elemental composition of the crystals was analyzed by energy dispersive X-ray spectroscopy (EDS), using an EDS of the Brucker brand, with the software Quantas Esprit 1.9, equipped with the SEM.
To identify the structure of the CaCO2 crystals produced by the bacteria, a Bruker D8 ADVANCE ECO X-ray diffractometer was used. The tests were conducted in a 2 theta (2θ) range from 10° to 80° with a step size of 0.02 and a Cu K-alpha wavelength of 1.5418 Å.
Identification of calcium carbonate producing bacteria
The bacteria that produce calcium carbonate were selected and placed in tubes of Nutrient Agar. Subsequently, they were identified based on their biochemical characteristics, using the VITEK 2 equipment and the BCL cards for Gram positive spore-forming bacilli.
Molecular identification was carried out in the Inca Biotech SAC laboratory, by extraction of genomic DNA, amplification of the 16 S RNA gene by PCR, after amplicon purification and two-way sequencing, sequences were analysed in databases (GenBank of NCBI), by homology alignment using BLAST (Basic Local Alignment search tool).
Evaluation of the biological self-healing potential in sand columns
A mixture of 15 g of sterilized river sand, 100µL of nutrient broth with 2% urea and 2% calcium chloride, and 100µL of a suspension of Bacillus sp. and Streptomyces sp. (1 × 109 cells/mL) was prepared. The mixtures were placed in 10mL syringes, and for 10 days, 100µL of nutrient broth with 2% urea and 2% calcium chloride was added daily. After this period, the sand was left to dry inside the syringe at 50 °C for 2 days. Then, the sand column was removed from the syringe and left to dry at 37 °C for 4 days.
Results
Isolation of spore-forming bacteria
Six cultures of Gram-positive bacteria were isolated. Of these, three had coccus morphology and three had bacillus morphology. Their characteristics are described in Table 1.
Selection of urease-producing bacteria on urea agar plates
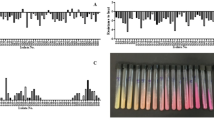
The production of the enzyme urease is an important factor for the production of calcium carbonate. On this occasion, only two of the isolated cultures showed urease activity. This is evidenced by the color change of the phenol red indicator, which goes from yellow to pink after 24 h, as can be seen in tubes 1 and 6 shown in Fig. 1(a). Cultures that are negative for urease do not show color change. The two cultures that are positive for urease correspond to Gram positive bacilli, whose micrographs are shown in Fig. 1(b) and (c).
Furthermore, it was observed that the activity of the urease enzyme remained even with pH changes, as can be seen in Fig. 2. Both the bacteria from culture 1 and culture 6 showed urease activity from a pH of 7 to a pH of 10. This is evidenced by the color change of the plates from yellow to pink.
Growth under alkaline pH conditions
One of the aspects to consider during the selection of bacteria with potential for concrete self-healing, through the production of crystals (CaCO3), is the ability to grow in alkaline pH, since concrete is highly alkaline14. The two isolated bacteria showed good growth in culture media with pH 8 and 10, as well as in the control medium (pH 6.5). An increase in the number of colony-forming units (CFU/mL) was observed at 24 h of cultivation, as can be seen in Fig. 3(a) and (b).
Production of calcium carbonate
The two cultures that showed urease activity and growth in alkaline pH formed crystals (CaCO3). Bacillus sp produced 5.86 g/L of medium, while Streptomyces sp produced 1.33 g/L. These calcium carbonate crystals show some differences in morphology. As can be seen in Fig. 4(a) and 4(c), the SEM micrographs are presented that show the morphology of the crystals. In the case of culture 1, they are spherical-shaped crystals that correspond to the form of vaterite, and in culture 6, they are rhombohedral-shaped crystals that correspond to the form of calcite crystals15. In Fig. 4(b) and (d), the basic composition of these crystals is presented, analyzed by EDS, where it can be seen that the highest peak corresponds to calcium.
In the diffractogram of Fig. 5, XRD of Bacillus sp. (a) shows the peaks of the Calcium Carbonate (CaCO₃) phase, which in the International Centre for Diffraction Data (ICDD) database corresponds to PDF 00-001-1033 known as Vaterite of hexagonal structure; (b) Streptomyces sp. shows diffraction peaks indicating the presence of the Calcium Carbonate (CaCO₃) phase, which in the ICDD database corresponds to PDF 01-081-2503 known as Vaterite, syn, of monoclinic structure.
Molecular identification of calcium carbonate producing bacteria
Evaluation of the biological self-healing potential in sand columns
Figure 6 shows the sand blocks formed by the cohesion of their particles due to the action of calcium carbonate (CaCO₃) in the form of calcite and vaterite crystals, produced by the bacteria Bacillus sp. (a) and Streptomyces sp. (b). These crystals deposit in the pores between the sand particles, acting as cementation bridges, which increases the cohesion and strength of the soil.
Discussion
This research was carried out with the purpose of isolating and selecting bacteria with potential for concrete self-healing, from samples of limestone soil. Six cultures of Gram-positive bacteria were isolated (Table 1), of which three presented the morphology of cocci and three the morphology of bacilli. Their macroscopic and microscopic characteristics pointed towards the genera Micrococcus, Bacillus, and Streptomyces. Similar results are reported in6, where Bacillus psichrodurans LC40 was isolated from a limestone cave. Other researchers interested in isolating bacteria with potential for the production of calcium carbonate crystals have reported isolations of bacteria of the genus Bacillus from adobe structures and deserts of Iran10, as well as bacteria of the genera Arthrobacter, Psychrobacillus, and Rhodococcus, isolated from concrete samples16. All these bacteria are characterized by coming from alkaline environments. The isolation of these bacteria is mainly focused on tolerance to alkaline pH and calcium tolerance17, hence limestone soil constitutes a good substrate for isolating these bacteria.
One of the important aspects during the isolation of bacteria with potential for concrete self-healing is the ability to produce the enzyme urease, as this allows for environmentally friendly biomineralization18. On this occasion, two of the isolated cultures showed urease activity, which is evidenced by the color change of the phenol red indicator. Since urease leads to an alkaline pH19, the indicator changes from yellow to pink after 24 h, as can be seen in tubes 1 and 6 presented in Fig. 1(a), which correspond to sporulated bacilli (Fig. 1(b)) and gram positive chain-forming bacilli (Fig. 1(c)). On the other hand, cultures negative for urease do not show a color change. The two cultures positive for urease, whose characteristics correspond to Bacillus and Streptomyces, are potential producers of CaCO3. The urease secreted by the bacteria catalyzes the hydrolysis of urea, leading to the production of CO2 and NH3. The CO2 balances in water forming bicarbonate, two moles of ammonium and two moles of hydroxide, which causes an increase in pH. This balance leads to the formation of carbonate ions. At this point, if the bacteria are surrounded by Ca2+, the negatively charged organic monolayer in the bacterial cells constantly chelates the Ca2+ to give rise to the deposition of CaCO3 crystals3,16,17. Therefore, bacteria that have high urease activity will result in high productivity of CaCO320, for which the alkalinization of the environment is fundamental21.
It was observed that the isolated bacteria retain the activity of the urease enzyme when they are in alkaline environments (Fig. 2(a) y (b)). This was demonstrated by performing the assay on Christensen’s urea agar plates with pH values from 7 to 10. After 96 h, the characteristic color change from yellow to pink was observed on all plates. This characteristic is of most importance, as, to be used in concrete self-healing, these bacteria must tolerate the highly alkaline pH that concrete presents22.
Another feature that bacteria with potential for concrete self-healing must have is the ability to grow in alkaline pH, since concrete is highly alkaline14. This feature was present in the two isolated bacteria. Their pH tolerance was demonstrated because they managed to develop in the culture media with pH values of 8 and 10. This became evident through the increase in the number of colony forming units (CFU/mL) at 24 h of culture (Fig. 3(a) y 3(b)). The experimental results resemble those obtained by19, who observed that B. sphaericus has a good pH tolerance, as it can grow and germinate in a wide range of alkaline pH. In addition, they found that high alkaline conditions (pH 10 ∼ 11) slow down, but do not stop, their growth and germination. On the other hand, in9 it was showed that Bacillus pasteurii has the ability to resist high levels of internal concrete pH (12 to 13 for conventional concrete) and continue to survive for a longer period.
Alkaline pH tolerance is an important factor, as it favors the production of calcium carbonate crystals. Other research11 also showed that when the pH increases up to 12, there was a 6.3-fold increase in CaCO3 precipitation compared to fermentation with uncontrolled pH. Likewise, when performing the morphological characterization, they observed that pH also influences the morphology of the CaCO3 crystals. On the other hand, in23 it was also established that for bacteria to be applied in the self-healing of concrete, it is very important that the microorganisms preserve their activity at different pH values. These bacteria must be able to survive a pH of 13, which is that of concrete, and be active at a pH of 9.5 to 10, which is estimated in the cracks.
The production of CaCO3 crystals by the isolated bacteria was confirmed by optical microscopy and SEM, where differences were observed in the morphology of the crystals produced by the two cultures (Fig. 4(a) and (c)). In the case of the Bacillus sp. culture, they are spherical-shaped crystals that correspond to the form of vaterite, and in the Streptomyces sp. culture, they are rhombohedral-shaped crystals that correspond to the form of calcite crystals15. In Fig. 4(b) and (d), the basic composition of these crystals is presented, analyzed by EDS, where it can be seen that the highest peak corresponds to calcium. It was confirmed that calcium carbonate is present in its vaterite and vaterite syn forms, with the phases shown in the diffractograms of Fig. 5 (a) and (b). These phases are precursors to calcite, which is the most stable and common form of calcium carbonate. These findings coincide with those of24, who found in the cracks of three mortar samples CaCO3 crystals of cubic and asymmetric rhombohedral shapes.
Biologically produced CaCO3 crystals can be classified into three types: calcite, vaterite, and aragonite. Among these, calcite and vaterite are the most commonly bacteria-induced polymorphs of CaCO3. Calcite is the thermodynamically most stable polymorph of CaCO3. Although vaterite has been reported to be stable, it eventually transforms into calcite. Vaterite, which has the highest density among these crystals, is a hexagonal crystal. Its pore-filling ability is superior to that of other CaCO3 crystals due to its large size2. Calcite, produced by microorganisms, is stable and combines with concrete materials17. Among the factors that influence the formation of this polymorphs are the bacterial species involved and the metabolic pathway, as these pathways control the amount of dissolved organic carbon, which is the main factor in the formation of these crystals25. Similarly, pH values play an important role in urease-mediated calcite precipitation, which generally occurs under alkaline conditions with a pH of 8.7 to 9.5, and the optimal temperature ranges from 20 to 37 °C26. When the qualitative test was conducted to evaluate the potential of these bacteria in the self-healing of concrete, it was observed that Bacillus sp. produced greater particle cohesion compared to Streptomyces sp. This is possibly due to Bacillus sp. having a higher number of nucleation sites than Streptomyces sp., allowing it to precipitate a greater amount of calcium carbonate and colonize the sand more deeply. This fact is reinforced by the XRD analysis, in which the presence of calcium carbonate in its crystalline form as vaterite, due to its hexagonal structure, has the potential to transform into other forms of CaCO₃, giving it the property of being a biocement.
As for the identification of the bacteria, VITEK 2 BCL cards were used for the spore-forming gram-positive bacilli. However, they could not be identified, apparently because, being species isolated from the environment, they are not in the VITEK 2 system databases. When the identification of bacteria was carried out through the amplification of the 16 S RNA gene, only the genus could be identified, as a %ID of 98.72% for Bacillus and 98.46% for Streptomyces was obtained (Table 2). According to the literature, to identify a species, the %ID must be ≥ 99%; in this case, it only fell within the range of ≥ 95% to ≥ 99%, which is considered to define a genus27. This may be due to the resolution power of 16 S RNA sequences not being sufficient to differentiate species within the same genus, due to the heterogeneity among the copies of the 16 S RNA gene within the genome28, or it could also be the case that it is a new species whose sequence has not yet been deposited in the gene bank27. As is known, bacteria of the genus Bacillus are gram-positive bacteria that have the ability to form spores when they are in hostile environments. This sporulation process helps them resist the mechanical stress and alkalinity of concrete, allowing them to remain inactive for extended periods of time12. To date, species of Bacillus subtillis, B. cohnii, B. pasteurii, B. pseudofirmus, and B. megaterium, among others, have been reported, which have shown great effectiveness for crack healing, while increasing the resistance to compression, bending, and traction in concrete29.
On the other hand, bacteria of the genus Streptomyces, which are also Gram-positive, form chain-like mycelia with many spores. Their natural habitat is soil, where they play an important role in the decomposition of organic matter30. In addition, it has been shown that bacteria of the genus Streptomyces have the ability to generate CaCO3 from the CO2 in the environment, as they also possess the enzyme carbonic anhydrase. This enzyme, like urease, contributes to the formation of well-defined calcite crystals31. As could be observed through SEM analysis, rhombohedral crystals were formed by Streptomyces sp and spherical vaterite crystals by Bacillus sp. Therefore, the two cultures isolated from limestone soils of the Simbal mines have potential to be applied in the self-healing of concrete.
Conclusions
Two cultures of native spore-forming Gram-positive bacteria from lime mines were successfully isolated. In addition to demonstrating their survival capacity in alkaline environments, they exhibited urease enzyme activity in alkaline pH and produced calcium carbonate crystals in the forms of vaterite and vaterite syn. The projection of calcium carbonate lies in filling cracks and pores to improve the structural integrity and durability of concrete. The self-healing potential of the bacteria was evidenced by the cohesion of particles in the sand columns. This is a preliminary qualitative assessment that will be complemented by ongoing research conducted on concrete samples. The importance of working with native bacteria lies in their adaptation to local environmental conditions, making them more effective in their function, ensuring constant and effective activity in concrete mixes under specific environmental conditions.
Data availability
This manuscript does not report data generation or analysis.
References
Hammes, F. & Verstraete, W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Re/Views Environ. Sci. Bio/Technol.. 1, 3–7 (2002).
Hemayati, M., Nikooee, E., Habibagahi, G., Niazi, A. & Afzali, S. F. New non-ureolytic heterotrophic microbial induced carbonate precipitation for suppression of sand dune wind erosion. Sci. Rep. 13, 5845 (2023).
Baidya, P., Dahal, B. K., Pandit, A. & Joshi, D. R. Bacteria-induced calcite precipitation for engineering and environmental applications. Adv. Mater. Sci. Eng. 2613209 (2023).
Mokhtar, G., Ahmed, A. A. E. A. & Reyad, A. M. The effect of isolated Bacillus ureolytic bacteria in improving the bio-healing of concrete cracks. Beni-Suef Univ. J. Basic. Appl. Sci. 10,10 (2021).
Van Wylick, A. et al. A review on the potential of filamentous fungi for microbial self-healing of concrete. Fungal Biol. Biotechnol. 8, 16 (2021).
Park, M. et al. Enzyme-mediated biocalcification by a novel alkaliphilic Bacillus psychrodurans LC40 and its eco-friendly application as a biosealant for crack healing. Sci. Total Environ. 802, 1–9 (2022).
Kanwal, M., Khushnood, R. A., Khaliq, W., Wattoo, A. G. & Shahid, T. Synthesis of pyrolytic carbonized bagasse to immobilize Bacillus subtilis; application in healing micro-cracks and fracture properties of concrete. Cem. Concr. Compos. 126 (2022).
Bagga, M. et al. Advancements in bacteria based self-healing concrete and the promise of modelling. Constr. Build. Mater. 358, 129412 (2022).
Metwally, G. A., Mahdy, M. & Abd El, A. E. R. H. Performance of bio concrete by using Bacillus pasteurii bacteria. Civ. Eng. J. 6, 1443–1456 (2020).
Farajnia, A., Shafaat, A., Farajnia, S., Sartipipour, M. & Khodadadi Tirkolaei, H. The efficiency of ureolytic bacteria isolated from historical adobe structures in the production of bio-bricks. Constr. Build. Mater. 317 (2022).
Seifan, M., Samani, A. K. & Berenjian, A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl. Microbiol. Biotechnol. 101, 3131–3142 (2017).
Saleem, B., Hussain, A., Khattak, A. & Khan, A. Performance evaluation of bacterial self-healing rigid pavement by incorporating recycled brick aggregate. Cem. Concr. Compos. 117, 103914 (2021).
Arias, D., Cisternas, L. A. & Rivas, M. Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile). Desalination. 405, 1–9 (2017).
Nodehi, M., Ozbakkaloglu, T. & Gholampour, A. A systematic review of bacteria-based self-healing concrete: Biomineralization, mechanical, and durability properties. J. Build. Eng. 49, 104038 (2022).
Silva-Castro, G. A. et al. Bioprecipitation of calcium carbonate crystals by bacteria isolated from saline environments grown in culture media amended with seawater and real brine. BioMed Res. Int. 816102 (2015).
Montaño-Salazar, S. M., Lizarazo-Marriaga, J. & Brandão, P. F. B. Isolation and potential biocementation of calcite precipitation inducing bacteria from Colombian buildings. Curr. Microbiol. 75, 256–265 (2018).
Jia, Q. & Wang, Y. Study on calcium carbonate deposition of microorganism bottom grouting to repair concrete cracks. Sustainability. 15, 3723 (2023).
Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 13, 59–67 (2018).
Reyad, A. M. & Mokhtar, G. Impact of the immobilized Bacillus cereus MG708176 on the characteristics of the bio-based self-healing concrete. Sci. Rep. 13, 500 (2023).
Wang, J., Jonkers, H. M., Boon, N. & De Belie, N. Bacillus sphaericus LMG 22257 is physiologically suitable for self-healing concrete. Appl. Microbiol. Biotechnol. 101, 5101–5114 (2017).
Fujita, Y., Ferris, F. G., Lawson, R. D., Colwell, F. S. & Smith, R. W. Subscribed content calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol. J. 17, 305–318 (2000).
Xu, J., Wang, X., Zuo, J. & Liu, X. Self-healing of concrete cracks by ceramsite-loaded microorganisms. Adv. Mater. Sci. Eng. (2018).
Erşan, Y. Ç. et al. Nitrate reducing CaCO3 precipitating bacteria survive in mortar and inhibit steel corrosion. Cem. Concr. Res. 83, 19–30 (2016).
Tziviloglou, E., Wiktor, V., Jonkers, H. M. & Schlangen, E. Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr. Build. Mater. 122, 118–125 (2016).
Dhami, N. K., Alsubhi, W. R., Watkin, E. & Mukherjee, A. Bacterial community dynamics and biocement formation during stimulation and augmentation: Implications for soil consolidation. Front. Microbiol. 8 (2017).
Anbu, P., Kang, C. H., Shin, Y. J. & So, J. S. Formations of calcium carbonate minerals by bacteria and its multiple applications. SpringerPlus. 5, 250 (2016).
Clarridge, J. E. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17, 840–862 (2004).
Chudzik, A., Jalkanen, K., Täubel, M., Szponar, B. & Paściak, M. Identification of environmental Actinobacteria in buildings by means of chemotaxonomy, 16S rRNA sequencing, and MALDI-TOF MS. Microbiol. Spectr. 12, e03596–e03523 (2024).
Muñoz-Pérez, S. P. & Carlos-Sánchez, J. Peralta-Sánchez, M. Influencia De las bacterias en la autocuración del concreto. Rev. UIS Ingenierías. 22, 69–86 (2023).
Komaki, H. Recent progress of reclassification of the genus Streptomyces. Microorganisms. 11, 831 (2023).
Sangeetha, M., Sivarajan, A., Radhakrishnan, M., Siddharthan, N. & Balagurunathan, R. Biosequestration of carbon dioxide using carbonic anhydrase from novel Streptomyces kunmingensis. Arch. Microbiol. 204, 270 (2022).
Acknowledgements
We express our appreciation to the Laboratorio de Investigación Multidisciplinario (LABINM) of the Universidad Privada Antenor Orrego (UPAO) for the facilities provided to carry out the SEM microscopy and EDS analysis.
Funding
This research has been funded by the Vicerrectorado de Investigación de la Universidad César Vallejo, RVI N°299-2022-VI-UCV.
Author information
Authors and Affiliations
Contributions
M.F. and N.M.O.Conception and design of the work; M.F., N.M.O.and L.C. analysis and data interpretation; M.F.; J.G.C and N.M.O.; formal examination, M.F. and N.M.O.; research, M.F.; N.M.O.; L.C.; resources, L. C.; drafting of the original draft, M.F. and N.M.O.; writing, proofreading and editing, M.F.; N.M.O.; L.C.; J.G.C. All authors have read and accepted the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Otiniano, N.M., Farfán-Córdova, M., Cabeza, J.G. et al. Isolation and selection of spore-forming bacilli with potential for self-healing of concrete. Sci Rep 14, 27223 (2024). https://doi.org/10.1038/s41598-024-77241-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-77241-9
Keywords
This article is cited by
-
Factors influencing bacterial-based precipitation, assessment of crack inducing, durability and characterization methods: a comprehensive review
Innovative Infrastructure Solutions (2025)