Abstract
In this population-based observational cross-sectional study, we investigated retinal peripheral refraction in Chinese adults with myopia. We categorized 1511 Chinese adults with myopia (18 − 55 years) into low (LM), moderate (MM), and high myopia (HM) groups. Axial length, central corneal thickness, steep keratometry, flat keratometry, and intraocular pressure were measured. Refractive difference values (RDVs) for different eccentricities (RDV0–53) and the superior (RDV-S), inferior (RDV-I), temporal (RDV-T), and nasal (RDV-N) retinal regions were measured using multispectral refractive topography. The hyperopic defocus was higher for the MM group than for the LM group in RDV20–53, RDV-S, and RDV-T and HM group in RDV20–53, RDV-S, RDV-T and RDV-N. The hyperopic defocus was higher for the HM group than for the LM group in RDV20-53, RDV-S and RDV-T but lower for the LM group in RDV-N. RDV-N decreased with age (R2 = 0.0191, slope = − 0.01, p < 0.0001), whereas RDV-S (R2 = 0.0112, slope = 0.01,p < 0.0001) and RDV-T increased (R2 = 0.0038, slope = 0.01, p = 0.0160). RDV correlated with central spherical equivalent and axial length. Flat keratometry explained the most RDV variation (RDV20–30: β = 0.0714, p < 0.0001; RDV-N: β = 0.1801, p < 0.0001; RDV-S: β = 0.1426, p < 0.0001; RDV-T: β = 0.1239, p < 0.0001). Reference values for peripheral retinal defocus are provided for Chinese adults with different myopia ranges.
Similar content being viewed by others
Introduction
The prevalence of myopia, a widespread health concern globally, is increasing rapidly. A meta-analysis1 showed that, in the absence of effective interventions, nearly 50% of the global population may have myopia by 2050. The reasons for myopia development are not entirely clear. However, increasing evidence suggests that its onset and progression are associated with peripheral visual signals from the retina, with patients with myopia mostly showing relative peripheral hyperopic defocus and patients with hyperopia mostly showing relative peripheral myopic defocus2,3,4. Nevertheless, few studies have focused on the refractive states of different retinal eccentricities and their relationship to myopia. The conventional equipment used in such studies is complicated to operate and has a small area for detecting peripheral refraction. In addition, the procedure requires a high degree of patient cooperation, takes a long time to perform, and is challenging to adapt to clinical practice5,6. In this study, we used a new examination device to measure the refractive status of the peripheral retina, and multispectral refractive topography. It mainly utilizes single-optical light rays of different wavelengths to collect fundus images sequentially. Then, by developing computer algorithms, we performed comparative analysis on the lens-compensated multispectral images and calculated and summarised the actual refractive values of each pixel to draw corresponding topographic maps. The advantages of this method over traditional methods are that the measurement is more accurate as it is not affected by changes in ocular muscle contraction and accommodation and does not require the cooperation of multiple gaze points for a single shot. In addition, the device can quantify the relative peripheral refraction difference value (RDV) of the retina over a field of view of 0° to 53° within 3 s, and its accuracy and reproducibility have been verified7,8.
Current studies on this topic usually have small sample sizes, and the effects of age on retinal peripheral defocus remain unclear. Therefore, we included a large sample to investigate the distribution of relative retinal peripheral refraction in adults with different myopia ranges in different eccentricity regions and to explore the relationships between RDV and factors such as age, axial length (AL), spherical equivalent (SE), and keratometry value. Our results provide reference values for peripheral retinal defocus in Chinese adults with myopic refractive error.
Materials and methods
General information
This study included patients who underwent preoperative examinations for myopia correction surgery at Beijing Ming Vision and Ophthalmology, the teaching base of the In Eye Hospital of Chengdu University of Traditional Chinese Medicine (TCM), between October 2022 and June 2023. Ethical approval was obtained from the medical ethics committee of the In Eye Hospital of Chengdu University of TCM (Approval No. 2022yh-022). All examinations were performed in accordance with the principles of the Declaration of Helsinki, and written informed consent was obtained from all participants following a detailed explanation of the nature and possible consequences of the study.
This was an observational cross-sectional study of Chinese adults with myopia, aged 18–55 years. The inclusion criteria were (1) age ≥ 18 years; (2) corrected distance visual acuity logMAR ≤ 0.10; (3) central spherical dioptre < − 0.50 D and − 2.50 D ≤ cylindrical dioptre ≤ 0 D; and (4) no other ocular disease. The exclusion criteria were (1) fundus lesions, cataracts, or any other eye disease; (2) systemic diseases that may cause ocular pathology; and (3) a history of ocular surgery. The right eyes of the participants were included; their left eyes were included if clear images of the right eye were not available. The participants were divided into three groups based on their central SE refractive error: low myopia (LM, -3.00 D ≤ SE<-0.50 D), moderate myopia (MM,-6.00 D ≤ SE<-3.00 D), and high myopia (HM, >−6.0 D).
Examination
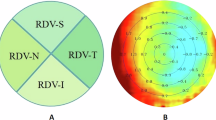
Before the study, well-trained optometrists and ophthalmologists performed routine ocular examinations to ensure the eye health of each participant and ascertain refractive status. Steep keratometry (Ks), flat keratometry (Kf), and average keratometry (Ave-K) were performed using a topographic modeling system (TMS-4; Tomey, Japan). Intraocular pressure (IOP) was measured using a non-contact tonometer (CT-800; TOPCON Co., Japan). AL and central corneal thickness (CCT) were measured using Haag-Streit diagnostics (LS900, Haag-Streit AG, Switzerland). RDV was measured using multispectral refractive topography (MSIC2000, ShengDa TongZe, Shenzhen, China) and recorded in five retinal eccentricity ranges: 0–10°, 10–20°, 20–30°, 30–40°, and 40–53° (RDV0–10, RDV10–20, RDV20–30, RDV30–40, and RDV40–53, respectively; Fig. 1A). Defocus values were recorded for the four sectoral quadrant regions of the retina (Fig. 1B)—temporal (RDV-T), inferior (RDV-I), superior (RDV-S), and nasal (RDV-N). The retinal RDV in the total range (0–53°) of the macular fovea centralis was recorded as total RDV (TRDV). Positive values were used to denote hyperopic defocus RDVs, and negative values were used to denote myopic defocus RDVs. Furthermore, 0.5% tropicamide was administered to induce cycloplegia, and the refractive power was measured using computerized optometry and subjective refraction. The SE was calculated as SE = dioptre sphere + 1/2 dioptre cylinder.
Statistical analysis
All statistical methods were performed using R (version 4.3.0). The Kolmogorov–Smirnov normality test was utilized to assess whether the data were normally distributed. In the baseline analysis and three-group analysis of variance, one-way analysis of variance and TukeyHSD range test were used for normally distributed data, while the Kruskal–Wallis and Wilcoxon Rank Sum Test (FDR adjusted) were used for non-normally distributed data. For sex data, the chi-squared test was used. Correlation and linear regression analyses were performed to further analyze the relationship between retinal defocus status and ocular biological parameters. Pearson’s and Spearman’s rank correlation coefficients were used for normally and non-normally distributed data. Simple linear and multiple regression analyses were used to establish equations for possible relationships between them. Normally and non-normally distributed data are presented as mean ± standard deviation and median (Q1, Q3). P < 0.05 was considered statistically significant.
Results
Descriptive characteristics
A total of 1755 adults with myopia were recruited, but 244 participants were excluded during screening: 27 were found to have ocular diseases, 25 were excluded due to the inability to obtain clear fundus images owing to small pupils, and 192 were excluded to level the baseline ocular biological parameters (IOP, CCT, Kf, Ks, and Ave-K) to ensure that the retinal peripheral defocus data of the three groups were comparable. After rigorous screening, 1511 eligible participants (946 women and 565 men, aged 18–55 years) were finally enrolled. Of the 1,511 eyes, 426, 578, and 507 were categorized as LM, MM, and HM, respectively. Among the three refractive groups, there were no significant differences in age, IOP, CCT, Ks, Kf, or Ave-k; however, gender, SE, and AL were significantly different (all p < 0.05) (Table 1).
RDV at different eccentricities among the three refractive groups
All three refractive groups exhibited slight myopic defocus in the central region of the 0–20° eccentricity range. In the 20–53° eccentricity range, the LM, MM, and HM groups all had relative hyperopic defocus, and RDV increased with increasing eccentricity. In the 20–53° range, the RDV increased with myopia severity in LM and MM, but decreased in HM (Fig. 2).
(A) Retinal refraction difference values (RDVs): the centremost region corresponds to RDV0–10; the first annulus is RDV10–20; the second annulus is RDV20–30; the third annulus is RDV30–40; and the fourth annulus is RDV40–53. Positive values denote relative hyperopic defocus, and negative values denote relative myopic defocus. (B) The defocus values of the four sectoral quadrant regions of the retina are denoted as RDV-Superior (RDV-S), RDV-Inferior (RDV-I), RDV-Temporal (RDV-T), and RDV-Nasal (RDV-N).
Comparison of RDV among the three refractive groups
The RDVs of the three groups and their comparisons are shown in Table 2; Fig. 3, respectively. There was no significant difference among the three groups in RDV0-10 and RDV-I (p > 0.05; Table 2; Fig. 3A, G); however, there were significant differences among them in RDV10–53, RDV-S, RDV-N, and RDV-T (p < 0.05; Table 2). In RDV10–20, The myopic defocus was significantly higher in the LM group than in the MM and HM groups, and myopic defocus was significantly higher in the HM than in MM group (p < 0.05; Fig. 3B). The hyperopic defocus of the MM group was significantly higher than that of the LM group in RDV20–53, RDV-S, and RDV-T (p < 0.05; Fig. 3C–F, H) and was significantly higher than that of the HM group in RDV20–53, RDV-S, RDV-T and RDV-N (p < 0.05; Fig. 3C- F, H, I); the hyperopic defocus of the HM group was significantly higher than that of the LM group in RDV20-53, RDV-S and RDV-T (p < 0.05; Fig. 3C, D, E, F, H), and was significantly lower than that of the LM group in RDV-N (p < 0.05; Fig. 3I).
The RDV 0–10 (A), RDV10-20 (B), RVD20-30 (C), RDV30-40 (D), RVD40-53 (E), RVD-S (F), RDV-I(G), RDV-T(H) and RDV-N(I) of different eccentricities and quadrants in the three refractive groups. RDV, refraction difference values; RDV-I, RDV-Inferior; RDV-N, RDV-Nasal; RDV-S, RDV-Superior; RDV-T, RDV-Temporal; LM, low myopia; MM, moderate myopia; HM, high myopia; Ns: p ≥ 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Correlation analysis between SE and RDV in the different refractive groups (Table 3) showed the following: in the LM group, SE was negatively correlated only with RDV-S; in the MM group, SE was negatively correlated with RDV20–53 and RDV-T; and in the HM group, SE was positively correlated with RDV-N.
Relationships between RDV and biological parameters
Age was positively correlated with RDV-S, RDV-I, and RDV-T and negatively correlated with RDV-N (p < 0.05). SE was positively correlated with RDV-N and RDV0–10 (p < 0.05) and negatively correlated with RDV10–53, RDV-S, RDV-T and TRDV(p < 0.05). CCT was negatively correlated with RDV0–40 and RDV-I (p < 0.05). IOP was negatively correlated with RDV-I (p < 0.05). Kf was positively correlated with RDV30–53, RDV-S, RDV-T, and TRDV (p < 0.05). Ave-K was positively correlated with RDV-S (p < 0.05). AL was positively correlated with RDV20-30, RDV-I and RDV-T, and negatively correlated with RDV-N (p < 0.05) (Fig. 4).
Correlation analysis of RDV with age and ocular biometric parameters. RDV, refraction difference values; RDV-S: RDV-Superior; RDV-I: RDV-Inferior; RDV-T: RDV- Temporal; RDV-N: RDV-Nasal; SE, spherical equivalent; AL, axial length; IOP, intraocular pressure; CCT, central corneal thickness; Kf, flat keratometry; Ks, steep keratometry; AK, average keratometry. *p < 0.05, **p < 0.01, ***p < 0.001.
Linear relationships among AL, SE, and RDV
In the simple linear regression analysis, RDV-N decreased significantly with AL (R2 = 0.0242, slope = − 0.09, p < 0.0001) (Fig. 5A); whereas RDV-T (R2 = 0.0166, slope = 0.08, p < 0.0001) (Fig. 5B) and RDV-I (R2 = 0.0064, slope = 0.04, p = 0.0018) (Fig. 5C) increased significantly with AL.
Simple linear regression analysis of RDV-N (A), RDV-T (B), and RDV-I (C) with AL. Simple linear regression analysis of RDV-N (D), RDV-T (E), and RDV20-30 (F) with SE. RDV, refraction difference value; RDV-I, RDV-Inferior; RDV-N, RDV-Nasal; RDV-T, RDV-Temporal; SE, spherical equivalent; AL, axial length.
RDV-N increased significantly with SE (R2 = 0.0193, slope = 0.04, p < 0.0001) (Fig. 5E); RDV-T (R2 = 0.0233, slope = − 0.04, p < 0.0001) (Fig. 5D) and RDV20–30 (R2 = 0.0189, slope = − 0.01, p < 0.0001) (Fig. 5F) decreased significantly with SE.
Linear relationship between age and RDV
In the simple linear regression, RDV-N decreased significantly with age (R2 = 0.0191, slope = − 0.01, p < 0.0001) (Fig. 6A), while RDV-S (R2 = 0.0112, slope = 0.01, p < 0.0001) (Fig. 6B) and RDV-T (R2 = 0.0038, slope = 0.01, p = 0.0160) (Fig. 6C) increased.
Multivariate analysis of RDV and biological parameters
In the multivariate analysis of RDV using multiple linear regression, biological parameters (age, CCT, IOP, Ks, Kf, and AL) were included as independent variables (Fig. 7). RDV20–30 was significantly correlated with Ks, Kf, IOP and AL (Fig. 7A); RDV-N was significantly correlated with age, CCT, IOP, Ks, Kf, and AL (Fig. 7B); RDV-S was significantly correlated with age, CCT, Ks, Kf and AL (Fig. 7C); and RDV-T was significantly correlated with Ks, Kf, and AL (Fig. 7D). Kf accounted for the most variation in RDV (unstandardized coefficient; RDV20–30: β = 0.0714, p < 0.0001; RDV-N: β = 0.1801, p < 0.0001; RDV-S: β = 0.1426, p < 0.0001; RDV-T: β = 0.1239, p < 0.0001) (Fig. 7A–D).
Multiple linear regression analysis of RDV20-30(A), RDV-N(B), RDV-S(C) and RDV-T(D) with biological parameters. RDV, refraction difference value; RDV-N, RDV-Nasal; RDV-T, RDV-Temporal; RDV-S: RDV-Superior; AL, axial length; IOP, intraocular pressure; CCT, central corneal thickness; Kf, flat keratometry; Ks, steep keratometry; VIF: variance inflation factor.
Discussion
This is the first study to examine relative retinal peripheral refraction in different eccentricity and sector quadrant regions of the retina in a large sample of healthy Chinese adults with myopia (1511 participants). Some researchers believe that peripheral hyperopic defocus may result from eye growth rather than a precursor9,10. By contrast, others believe that it is a significant factor in axial myopia development11,12,13. Smith et al.3 found that when the defocus signal around the retina contradicted the center, it dominated the process of eye growth and development. Wallman et al.14 pointed out that the refractive state of the peripheral retina had a greater impact on the eye growth and refractive development than the refractive state of the macular fovea, mainly because there are more optic nerve fibers in the peripheral retina than in central region. When the retina receives visual signals from both the central and peripheral retinas simultaneously, signals from the relatively large number of neurons in the peripheral retina may inhibit those from the central retina, thereby directly regulating refractive development and eye growth. Mutti et al.15 measured peripheral refraction at 30° of the visual field in 820 children aged between 5 and 15 years old and found that the peripheral relative hyperopic defocus was greater in myopic (+ 0.80 ± 1.29)D than in emmetropic (-0.41 ± 0.75)D and hyperopic (-1.09 ± 1.02)D children. Although the relative hyperopic defocus observed in the study was in the smaller range, this level of defocus status may have subtle but long-term effects on the ocular growth and refractive status. Although, the association between peripheral refraction and myopia remains controversial. However, many effective interventions to control myopia currently rely on reducing retinal peripheral hyperopic defocus or even reversing it to myopic defocus to slow the growth of AL. Common interventions include orthokeratology lenses, defocused soft lenses, defocused framed glasses, and multifocal soft contact lenses16,17,18. Understanding peripheral refraction holds significant clinical implications for myopia control. On the one hand, by discussing the potential risks of hyperopic defocus, the public, parents and ophthalmologists can raise the importance of myopia prevention and control, and promote early intervention and scientific management. On the other hand, the results of this study provide basic data for further investigation of the relationship between peripheral defocus and myopia development, encouraging future studies to explore in depth the specific mechanisms of myopia development at different defocus levels, in different age groups, and in different genetic backgrounds, aiming to provide a scientific basis for the development of more precise myopia prevention and control strategies.
Our study examined peripheral refraction in adults and found that all three refractive groups exhibited slight myopic defocus in the central region of the eccentricity range (0–20°). Within the 20–53° range, patients in the LM, MM, and HM groups showed relative hyperopic defocus and RDV increased with eccentricity. Owing to the large sample size, these results can provide a reference standard for peripheral refractive observation. In addition, good peripheral vision is required owing to the specific scenarios of detecting motion and orientation while driving or on the move19,20. The examination using MRT can to some extent reflect the peripheral retinal refraction of the population, takes only 3 s, is fast and noninvasive, and therefore, this examination may become a valuable complementary test for assessing peripheral vision.
Central refraction influences the refractive state of the peripheral retina. Both children and adults with myopia tend to exhibit a greater degree of hyperopic defocus in their peripheral retinas. Furthermore, as axial myopia increases, peripheral retinal hyperopic defocus severity also increases21,22,23,24. However, our study found that the peripheral hyperopic defocus of HM was lower than that of MM in many regions and even lower than that of LM in the nasal quadrant. This finding differs from that in previous studies. Therefore, we performed correlation analysis between SE and RDV in different refractive groups and found that, in the LM group, RDV SE was negatively correlated only with RDV-S; in the MM group, SE was negatively correlated with RDV20–53 and RDV-T; and in the HM group, SE was positively correlated with RDV-N. Therefore, we hypothesized that from − 3.0 to − 6.0 D, hyperopic RDV increases with increasing refraction; however, in HM (>–6.0 D), RDV in the nasal quadrant decreases with increasing myopia. Therefore, we propose this conjecture: is the application of the defocus principle for myopia control in adolescents with low to moderate myopia more helpful than in patients with high myopia? We intend to further observe and validate myopia control in adolescents.
In future studies, it is necessary to observe peripheral defocus in a large sample of children and to perform a side-by-side comparison of peripheral defocus between adults and children with similar myopia ranges to understand the differences and commonalities in peripheral defocus between the two populations, so that the results of the study can be utilized to better serve the prevention and control of myopia in adolescents.
Many studies have shown that SE is negatively correlated with peripheral hyperopic defocus while AL is positively correlated with it21,22,25. This study showed similar results, but the relationship between peripheral defocus and myopia in different regions of the retina (0–53° and in different quadrants) was analyzed in more detail.Lu et al.7 found that the repeatability of MRT measurements in the nasal, temporal, superior, and inferior quadrants was not as good as that in the central and peripheral annular regions. The RDV-S was the most susceptible, mainly considering that eyelid pressure may cause changes in corneal morphology, which may affect peripheral refractive measurements. To minimize the impact of this factor on the measurements in this study, we fully exposed the area of the cornea to the upper and lower corneal rims during the MRT examination and reduced the impact of measurement error by averaging multiple measurements. Despite the limitations of measurement repeatability, our study provided some meaningful findings. In the simple linear regression analysis, RDV-T and RDV20–30 decreased with increasing SE (SE was negative) (Fig. 5D, F), and RDV-T increased with increasing AL (Fig. 5B). Multivariate regression analysis also showed that AL was positively correlated with RDV20–30 and RDV-T (Fig. 7A, D). Unlike previous studies, we found that RDV-N decreased significantly with AL and increased significantly with SE. This suggests a strong relationship between myopia and RDV in the horizontal direction (nasal and temporal retinal quadrants) and the 20–30° eccentricity ring region.
A complex regulatory relationship may exist between the degree of myopia and ocular growth patterns during myopia development. Atchison et al.26 investigated 116 individuals (18–35 years) and found that myopia affected peripheral refraction in the horizontal field more than in the vertical field. The morphological features of myopia are primarily enlargement of the sagittal plane of the eye and, to a lesser extent, the coronal plane, which results in thinning of the retina at the equator, a decrease in the density of the retinal pigment epithelium, and thinning of the choroid and sclera at the posterior pole27. The Bruch’s membrane (BM) opening in the inner layer of the optic nerve head layer moves in a temporal direction in MM eyes (AL < 26.5 mm), resulting in the BM overhanging the intrapapillary compartment on the nasal side of the optic disc and an absence of the BM at the border of the temporal optic disc. In HM eyes (AL > 26.5 mm), the BM opening enlarges as the AL lengthens, resulting in enlargement of the delta zones, parapapillary gamma, and optic disc, elongation and thinning of the lamina cribrosa and peripapillary scleral flange, and elongation of the optic disc–fovea distance27,28. This may explain why retinal hyperopia, which defocuses on the nasal side of the macula, decreases with increasing AL and increases with increasing SE. It has been suggested that hyperopic defocus induces axial increase while myopic defocus inhibits axial growth and that the human eye can recognize defocus signals; this affects AL by inducing changes in choroidal thickness29,30. However, as this was a cross-sectional study, it was not possible to determine whether the degree of peripheral retinal defocus in these regions is the cause or effect of myopia. The mechanism by which the refractive state of the peripheral retina affects AL growth requires further investigation.
RDV-N decreased significantly with age in this study, whereas RDV-S and RDV-T increased. To our knowledge, this is the first study to reveal the relationship between age and peripheral retinal defocus. Chang et al.31 explored fundus changes in patients with myopia and found that the prevalence of staphyloma and choroidal atrophy lesions, the most common fundus findings in Asian adults with HM, increased with age. However, in adolescents with HM, staphyloma and choroidal atrophy were rare. Thus, fundus changes in patients with myopia are also influenced by age. A study exploring the effects of age and myopic shift on retinal development revealed that the thickness of the retinal layer in the central recess increased in children (< 10 years) but was unchanged or decreased in adolescents (> 13 years) and that changes in retinal thickness decreased with age32. Both the eye optics and the retina’s shape affect the retina’s peripheral refraction and expansion during myopia development, which also leads to changes in peripheral refraction33. Some studies have shown that, as myopia increases, the length of the eyeball grows faster than its height and width; therefore, the eye can change from an oblate/spherical shape to a prolate one34. Using computerized digital processing of magnetic resonance images of the eye, Atchison et al.35 discovered that as myopia progresses, the size of all ellipsoids increased with age. Additionally, they observed that axial dimensions increased more than vertical dimensions, which in turn increased horizontal dimensions (increases in approximate ratios of 3:2:1), and that these changes decreased the oblateness of the retinal shape. As the participants in this study were adults with stabilized refractive errors, we hypothesized that irregular dilation was associated with increasing age and myopia. The presence of unbalanced development of the eye in both the vertical and horizontal directions, as well as changes in the thickness of the retinal choroid, led to corresponding changes in peripheral retinal defocus.
In multivariate regression analysis, Kf was significantly positively associated with RDV20–30, RDV-N, RDV-T, and RDV-S, and Ks was significantly negatively associated with RDV20–30, RDV-N, RDV-T, and RDV-S. Kf explained most of the variation in RDV, indicating that changes in Kf significantly affect retinal peripheral defocus. Our research team observed changes in retinal peripheral refraction before and after small-incision lenticule extraction (SMILE) surgery. The retinal peripheral defocus values 3 months postoperatively were significantly lower than preoperative values36. By performing peripheral refraction tests on adult patients undergoing femtosecond laser-assisted laser in situ keratomileusis (LASIK), Q-guided LASIK, SMILE, and implantable Collamer lens (ICL), Lin et al.37 found that a corneal refractive surgery group showed significantly less relative peripheral refraction of the retina postoperatively than preoperatively. By contrast, the ICL group showed no significant changes in peripheral defocus after surgery. As ICL implantation does not change the keratometry value, the peripheral refraction change after surgery is insignificant. However, corneal refractive surgery]37 corrects myopia by reducing the mid-peripheral curvature of the cornea, and orthokeratology lenses16 are used to control myopia by changing the corneal curvature from peripheral hyperopic to myopic defocus. This is further evidence that corneal curvature is an important factor influencing peripheral refraction of the retina.
Our study provides reference values for peripheral retinal defocus in Chinese adults with varying myopia ranges. However, it did have some limitations. First, all patients in this study were candidates for refractive surgery, and there were sex differences among the three groups; therefore, there may have been some selection bias. Second, due to the difficulty of sample collection, this study was unable to include an emmetropic group as a control group for comparative analyses, which may limit our comprehensive understanding and in-depth exploration of the peripheral defocus studies in Chinese adults with different refractive states. Third, the wide age range may have masked different trends in retinal peripheral refraction across age intervals, and the effect of age on the development of retinal peripheral refraction remains unclear. Fourth, the partial correlation results in Figs. 4, 5, 6 and 7 show limited correlation between variables. This low level of correlation may have been caused by a number of factors, including the specific characteristics of the sample, precision of the definition of the variables, and limitations of the measurement tools. This low correlation may have some impact on the explanatory power and scope of application of this study findings. Nonetheless, these results have important research value because they reveal the complexity of the relationships among variables and prompt us to rethink existing theoretical frameworks and assumptions. In future studies, the inclusion of an emmetropic population as a control group should be considered to refine the study of peripheral defocus in adults with different refractive status, and different age ranges should be grouped to investigate the effects of age on peripheral refraction better, and further basic and cohort studies will help explore the causal relationship between peripheral refraction and myopia.
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
Holden, B. A. et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology . 123, 1036–1042 (2016).
Qi, L. S. et al. Relative peripheral refraction and its role in myopia onset in teenage students. Int. J. Ophthalmol. 15, 1108–1115 (2022).
Smith, E. L., Hung, L. F. & Huang, J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vis. Res. 49, 2386–2392 (2009).
Chiang, S. T., Phillips, J. R. & Backhouse, S. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol. Opt. 35, 405–413 (2015).
Rosén, R., Lundström, L. & Unsbo, P. Influence of optical defocus on peripheral vision. Invest. Ophthalmol. Vis. Sci. 52, 318–323 (2011).
Stoor, K., Karvonen, E., Liinamaa, J. & Saarela, V. Evaluating refraction and visual acuity with the Nidek autorefractometer AR-360A in a randomized population-based screening study in. Acta Ophthalmol. 96, 384–389 (2018).
Lu, W. et al. Agreement and repeatability of central and peripheral refraction by one novel multispectral-based refractor. Front. Med. (Lausanne). 8, 777685 (2021).
Liao, Y. et al. A quantitative comparison of multispectral refraction topography and autorefractometer in young adults. Front. Med. (Lausanne). 8, 715640 (2021).
Rosén, R. et al. Have we misinterpreted the study of Hoogerheide et al. (1971)? Optom. Vis. Sci. 8, 1235–1237 (2012).
Schippert, R. & Schaeffel, F. Peripheral defocus does not necessarily affect central refractive development. Vis. Res. 46, 3935–3940 (2006).
Chen, X. et al. Characteristics of peripheral refractive errors of myopic and non-myopic Chinese eyes. Vis. Res. 50, 31–35 (2010).
Mutti, D. O. et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest. Ophthalmol. Vis. Sci. 48, 2510–2519 (2007).
Smith, E. L., Kee, C. S., Ramamirtham, R., Qiao-Grider, Y. & Hung, L. F. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest. Ophthalmol. Vis. Sci. 46, 3965–3972 (2005).
Wallman, J. & Winawer, J. Homeostasis of eye growth and the question of myopia. Neuron . 43, 447–468 (2004).
Mutti, D. O., Sholtz, R. I., Friedman, N. E. & Zadnik, K. Peripheral refraction and ocular shape in children. Invest. Ophthalmol. Vis. Sci. 41, 1022–1030 (2000).
Ni, N. J. et al. Novel application of multispectral refraction topography in the observation of myopic control effect by orthokeratology lens in adolescents. World J. Clin. Cases. 9, 8985–8998 (2021).
Chen, Y. et al. Comparison of visual performance between peripheral gradient high-addition multifocal soft contact lenses and orthokeratology. Ophthalmic Physiol. Opt. 43, 874–884 (2023).
Erdinest, N. et al. Peripheral defocus and myopia management: a mini-review. Korean J. Ophthalmol. 37, 70–81 (2023).
Wetton, M. A. et al. The development and validation of two complementary measures of drivers’ hazard perception ability. Accid. Anal. Prev. 42, 1232–1239 (2010).
Patino, C. M. et al. Central and peripheral visual impairment and the risk of falls and falls with injury. Ophthalmology. 117, 199–206 (2010).
Zheng, X. et al. Relationship between peripheral refraction in different retinal regions and myopia development of young Chinese people. Front. Med. (Lausanne). 8, 802706 (2021).
Zhao, Q. et al. Quantitative analysis of peripheral retinal defocus checked by multispectral refraction topography in myopia among youth. Chin. Med. J. (Engl). 136, 476–478 (2023).
Xiaoli, L. et al. Comparative study of relative peripheral refraction in children with different degrees of myopia. Front. Med. (Lausanne). 9, 800653 (2022).
Li, Z. et al. Relative peripheral refraction characteristics and their relationship with retinal microvasculature in young adults: using a novel quantitative approach. Photodiagnis Photodyn Ther. 38, 102750 (2022).
Allon, G., Machluf, Y., Mezer, E. & Chaiter, Y. Screening for myopia-related retinal changes among teenagers. Ophthalmic Surg. Lasers Imaging Retina. 50, e311–e319 (2019).
Atchison, D. A., Pritchard, N. & Schmid, K. L. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vis. Res. 46, 1450–1458 (2006).
Jonas, J. B. & Panda-Jonas, S. [Epidemiology and anatomy of myopia]. Ophthalmologe . 116, 499–508 (2019).
Jonas, J. B., Wang, Y. X., Dong, L. & Guo, Y. Panda-Jonas, S. Advances in myopia research anatomical findings in highly myopic eyes. Eye Vis. (Lond). 7, 45 (2020).
Huang, Y. et al. Defocus-induced spatial changes in choroidal thickness of chicks observed by wide-field swept-source OCT. Exp. Eye Res. 233, 109564 (2023).
Delshad, S., Collins, M. J., Read, S. A. & Vincent, S. J. The time course of the onset and recovery of axial length changes in response to imposed defocus. Sci. Rep. 10, 8322 (2020).
Chang, L. et al. Myopia-related fundus changes in Singapore adults with high myopia. Am. J. Ophthalmol. 155, 991–999e1 (2013).
Jin, P. et al. Development of the retina and its relation with myopic shift varies from childhood to adolescence. Br. J. Ophthalmol. 106, 825–830 (2022).
Verkicharla, P. K., Mathur, A., Mallen, E. A., Pope, J. M. & Atchison, D. A. Eye shape and retinal shape, and their relation to peripheral refraction. Ophthalmic Physiol. Opt. 32, 184–199 (2012).
Atchison, D. A. et al. Eye shape in emmetropia and myopia. Invest. Ophthalmol. Vis. Sci. 45, 3380–3386 (2004).
Atchison, D. A. et al. Shape of the retinal surface in emmetropia and myopia. Invest. Ophthalmol. Vis. Sci. 46, 2698–2707 (2005).
Du, Y., Zhou, Y., Ding, M., Zhang, M. & Guo, Y. Changes in relative peripheral refraction and optical quality in Chinese myopic patients after small incision lenticule extraction surgery. PLoS One 18, e0291681 (2023).
Lin, Z., Lu, Y., Artal, P., Yang, Z. & Lan, W. Two-dimensional peripheral refraction and image quality for four types of refractive surgeries. J. Refract. Surg. 39, 40–47 (2023).
Funding
This research did not receive any specific grant from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Contributions
YD, Writing - original draft, Investigation, Methodology, Writing - review & editing, and Data curation; MZ, Formal analysis and Visualization; YZ, Project administration and Resources; SL: Investigation; YT: Investigation; and XM: Investigation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, Y., Zhang, M., Zhou, Y. et al. Observations on relative peripheral refraction in Chinese adults with myopia based on a novel application of multispectral refraction topography. Sci Rep 14, 25854 (2024). https://doi.org/10.1038/s41598-024-77289-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77289-7